Abstract
A possible function for the alternative (nonphosphorylating) pathway is to stabilize the reduction state of the ubiquinone pool (Qr/Qt), thereby avoiding an increase in free radical production. If the Qr/Qt were stabilized by the alternative pathway, then Qr/Qt should be less stable when the alternative pathway is blocked. Qr/Qt increased when we exposed roots of Poa annua (L.) to increasing concentrations of KCN (an inhibitor of the cytochrome pathway). However, when salicylhydroxamic acid, an inhibitor of the alternative pathway, was added at the same time, Qr/Qt increased significantly more. Therefore, we conclude that the alternative pathway stabilizes Qr/Qt. Salicylhydroxamic acid increasingly inhibited respiration with increasing concentrations of KCN. In the experiments described here the alternative oxidase protein was invariably in its reduced (high-activity) state. Therefore, changes in the reduction state of the alternative oxidase cannot account for an increase in activity of the alternative pathway upon titration with KCN. The pyruvate concentration in intact roots increased only after the alternative pathway was blocked or the cytochrome pathway was severely inhibited. The significance of the pyruvate concentration and Qr/Qt on the activity of the alternative pathway in intact roots is discussed.
The Cyt pathway and the alternative pathway constitute the respiratory electron-transport pathways of plant mitochondria. In contrast to the Cyt pathway, beyond the branch point (ubiquinone), the alternative pathway does not contribute to the generation of a proton-motive force. The AOX protein is found in every examined plant species and in every plant organ, and the genes encoding AOX have regions that are very conserved (Vanlerberghe and McIntosh, 1997), suggesting that the alternative pathway plays a vital role in plant functioning. However, a clearly identified function for the alternative pathway has been documented only once to our knowledge (in thermogenic flowers; Meeuse, 1975).
Purvis and Shewfelt (1993) and Wagner and Wagner (1995) speculated that the alternative pathway helps to stabilize Qr/Qt. Qr is a common substrate for both respiratory pathways. It has been suggested that high Qr/Qt levels promote free radical formation when the Cyt pathway is inhibited or restricted; respiration via the alternative pathway might then help to maintain Qr/Qt at a low level.
Although there is a linear relationship between the rate of mitochondrial respiration and the rate of radical formation (Puntelarulo et al., 1991; Leprince et al., 1994), radical formation is not directly connected to O2 consumption, because uncouplers increase radical formation only to a minor extent (Chance et al., 1977; Leprince et al., 1994) and may even decrease it (Liu and Huang, 1996). Rather, radical formation is linked to the relative reduction state of the respiratory chain (Forman and Boveris, 1982). The addition of uncoupler enhances respiration but not Qr/Qt (Wagner and Wagner, 1995). Radical formation increases if the appropriate inhibitors (Purvis et al., 1995) are used to block one or more respiratory pathways (Chance et al., 1977; Forman and Boveris, 1982, and refs. therein; Rich and Bonner, 1987). However, when the transmembrane potential increases, the production of radicals and H2O2 increase as well (Liu and Huang, 1996), so it is reasonable to assume that the formation of radicals increases with an increase in Qr/Qt.
If Qr/Qt is stabilized by the alternative pathway, then the Qr/Qt should be less stable if the alternative pathway is blocked (with SHAM) than when it is not blocked. To determine if Qr/Qt is stabilized by the alternative pathway in vivo, we titrated root respiration of Poa annua (L.) with KCN (an inhibitor of the Cyt pathway) in the absence or presence of SHAM. We used a range of KCN concentrations to achieve no inhibition, a small inhibition, or full inhibition of the Cyt pathway.
On the basis of data on isolated mitochondria and kinetic modeling (Wagner and Krab, 1995) it can be expected that the alternative pathway stabilizes Qr/Qt in vivo; however, this hypothesis remains to be proven.
In the recent past our understanding of the mechanisms that account for activation of the alternative pathway in isolated mitochondria has increased dramatically. We now know that the alternative pathway is more active when the AOX protein becomes reduced or when specific organic acids, e.g. pyruvate, are present in sufficiently high concentrations (Umbach and Siedow, 1993; Umbach et al., 1994; Hoefnagel et al., 1995; Millar et al., 1996). If and how the activity of the alternative pathway is controlled in vivo is still entirely unknown.
To determine the activation state of the alternative pathway in intact roots, we measured the concentration of the activator pyruvate and the reduction state of the AOX protein in the roots that were used in the titration experiments.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Roots of 6- to 7-week-old Poa annua (L.) plants were used for the measurements. Seeds were germinated on moistened filter paper for 1 week and then transferred to sand for 1 week, after which time they were placed in 30-L containers (24 plants per container) and grown on an aerated nutrient solution (as described by Poorter and Remkes [1990], with the exception that Fe concentration was doubled). The nutrient solution was replaced every week and the pH was adjusted every 2nd d to 5.8. The growth conditions were 20°C, 60% RH, 14-h day length, and 450 μmol m−2 s−1 PAR.
Respiration of Intact Roots
Roots (1.5–2.0 g FM) were severed and transferred to an airtight cuvette containing nutrient solution without Fe, and respiration was measured as the decrease in the O2 concentration using a Clark-type electrode (Yellow Springs Instrument Co., Yellow Springs, OH). The alternative pathway was inhibited with 3 mm SHAM (1 m stock solution in methoxyethanol). To inhibit the Cyt pathway, KCN was used in a wide range of concentrations (0–400 μm; stock solutions were made in 20 mm Hepes, pH 8.0). The respiration 10 to 15 min after addition of the inhibitors was used to calculate the percent inhibition.
Measurements of Pyruvate, Ethanol, and Lactate in Intact Roots
Pyruvate, ethanol, and lactate concentrations in intact roots were measured enzymatically according to the product protocol of Boehringer Mannheim. About 1 g of fresh root material was used for every measurement. To reduce the background extinction, an extra purification step was included by mixing active carbon (approximately 30 mg per 1.5-mL sample) to the sample mixture, followed by filtration. The recovery was 101% ± 4.5% (n = 3), 81% ± 1.5%, and 99% ± 6.6% (n = 3) for pyruvate, ethanol, and lactate, respectively.
AOX Protein
Root extracts were prepared from 100 mg FM of frozen root material that was ground in liquid N2 using a mortar and pestle and then suspended in a total volume of 400 μL of protein sample mix (62.5 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 0.001% bromophenol blue), and boiled for 5 min. After centrifugation for 10 min at 16,000g in an Eppendorf centrifuge to precipitate cell debris, the proteins were separated by SDS-PAGE according to the method of Laemmli (1970), and then electrotransferred to nitrocellulose filters using blot-transfer buffer (25 mm Tris, 192 mm Gly, 20% [v/v] methanol). Immunodetection of the AOX protein was carried out according to the product protocol of the AOX monoclonal antibody (GTMA, Lincoln, NE). Antibodies were kindly provided by Dr. T.E. Elthon (Elthon et al., 1989) and used as a primary antibody (1:50). Anti-mouse IgG Fab fragments conjugated to peroxidase (Boehringer Mannheim) were used as a secondary antibody (1:25,000), using a chemiluminescent substrate (SuperSignal Ultra, Pierce) according to the product-usage protocol supplied by the manufacturer. To quantify the bands in the autoradiograms, an image-analysis system (IBAS, Kontron/Zeiss) was used. Scanning was performed with a black-and-white CCD camera (WC-CD50, Panasonic), digitized four times, and averaged to improve the signal-to-noise ratio (frame size, 640 × 512 pixels; 256 gray levels). The bands were corrected for the background.
Measurement of Ubiquinone Reduction Levels in Intact Roots
The ubiquinone assays were based on the method of Wagner and Wagner (1995). Root extracts were prepared from 1 g of fresh root material that was ground in liquid N2 using a mortar and pestle, and then suspended in a total volume of 15 mL of methanol and 15 mL of petroleum ether (boiling point, 40–60°C) and vortexed for 30 s. The mixture was centrifuged at 1500g for 1 min and the upper petroleum ether phase was removed, transferred to a test tube, and evaporated to dryness under a flow of N2. Another 15 mL of petroleum ether was added to the lower phase, and the vortex and centrifugation steps were repeated. The upper phase was added to the one previously obtained.
The extracted ubiquinones were resuspended with a glass rod in 75 μL of N2-purged ethanol and analyzed by HPLC (HP 1050 series, Hewlett-Packard, Amstelveen, the Netherlands). A reversed-phase Lichrosorb 5 RP 18 column (Chrompack, Bergen op Zoom, The Netherlands) with an ethanol-methanol mixture (starting with 10 min in 20% ethanol, and then through a gradient to 70% ethanol at 40 min as the mobile phase at 1 mL min−1) was used. Detection was performed at 290 and 275 nm for Qr and oxidized ubiquinone, respectively. Commercially obtained Q10 and Q9 were used as standards (Sigma and Fluka). The extinction of Qr measured at 290 nm was multiplied by 3.56 according to the method of Crane (1963) because of the lower extinction coefficient for Qr compared with oxidized ubiquinone. The ubiquinone measurements were made with a recovery for Q10 of 93% (n = 4); the Q10 was added to the sample just after grinding.
Isolation of Mitochondria from Roots
One gram FM of roots was used for a fast isolation procedure to obtain mitochondria. Roots were ground using an ice-cold mortar and pestle with sand, and suspended in a total volume of 5 mL of buffer (0.05 m Mops, pH 7.4, 0.4 m mannitol, 0.25% BSA [m/v]). After centrifugation at 4,000g for 3 min at 2°C, the supernatant was centrifuged at 19,100g for 7 min at 2°C. The pellet was suspended in 5 mL of buffer and centrifuged again at 19,100g for 7 min at 2°C. The pellet was suspended in 200 μL of protein sample mix.
RESULTS
To study the effects of a (partial) inhibition of the Cyt pathway on the levels of Qr/Qt with or without the alternative pathway operating, we first examined the KCN concentrations at which the Cyt pathway is inhibited and electrons are diverted to the alternative pathway.
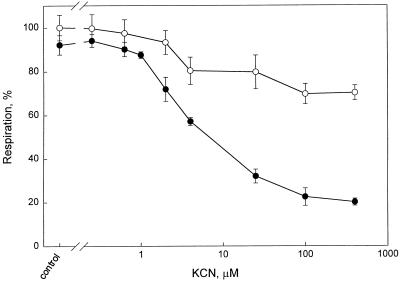
O2 uptake in intact roots was measured at a range of KCN concentrations in the absence or presence of SHAM. The rate of root respiration in the absence of SHAM was unaffected by KCN concentrations lower than 2 μm and was 4.4 nmol O2 g−1 FM s−1 (Fig. 1). The respiration decreased by 35% in the presence of 100 to 400 μm KCN. SHAM (3 mm) alone significantly decreased the rate of respiration by 11% (P = 0.013). In the presence of SHAM, KCN concentrations between 0.6 and 25 μm inhibited respiration significantly more, up to 75%. Therefore, in the absence of SHAM, the alternative pathway apparently takes over an increasing number of electrons from the increasingly inhibited Cyt pathway.
Figure 1.
O2 consumption (percent of control) by intact roots (detached from the plant) of P. annua plotted against the KCN concentration (note the logarithmic scale). The following KCN concentrations were used: 0 (control), 0.25, 0.63, 1, 2, 4, 25, 40, and 400 μm. The error bars indicate the sd. ○, Measurements made in the absence of SHAM; •, measurements made in the presence of SHAM (3 mm). The number of replicates was at least three and these were from different plants and plant batches. The control respiration was 4.4 nmol O2 g−1 FM s−1.
At KCN concentrations exceeding 25 μm, the difference in inhibition of respiration with and without SHAM was constant, with an average of 49%.
To determine if the presence of an alternative pathway acting as an overflow for an inhibited Cyt pathway can stabilize Qr/Qt, we determined Qr/Qt at different KCN concentrations in the absence and presence of SHAM.
In intact roots of P. annua we found mainly Q9 (67%), as has been found for many other species (Threlfall and Whistance, 1970), some Q8 (23%), and almost no Q10 (<10%). The present results for Qr/Qt are for Q9 only, which had an average concentration of 4.4 ± 0.6 nmol g−1 FM. Ribas-Carbo et al. (1995) showed that different ubiquinones have the same redox behavior in isolated mitochondria.
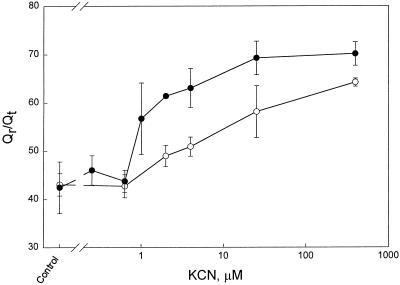
To determine if the alternative pathway stabilizes Qr/Qt, we looked for an extra increase in Qr/Qt, which would be expected when the alternative pathway is inhibited by SHAM. By measuring Qr/Qt at different KCN concentrations, we determined if an increase in Qr/Qt coincided with an increase in activity of the alternative pathway. Qr/Qt was constant (44% ± 3%) up to a KCN concentration of 0.63 μm with or without SHAM (Fig. 2). Between 0.63 and 2 μm KCN, Qr/Qt increased much more in the presence of SHAM than in its absence. Between 2 and 25 μm KCN, the increase in Qr/Qt was the same (12%) in the absence and presence of SHAM. With increasing inhibition of the Cyt pathway, the alternative pathway became increasingly engaged in respiration. An increase in the activity of the alternative pathway might be the result of an increased substrate concentration (Qr). However, AOX might also be activated when the Cyt pathway is inhibited. Such an activation could be the result of an increase in pyruvate concentration and/or in the reduction state of the protein.
Figure 2.
Qr/Qt (percent) in intact roots (detached from the plant) of P. annua plotted against the KCN concentration (note the logarithmic scale). The following KCN concentrations were used: 0 (control), 0.25, 0.63, 1, 2, 4, 25, 40, and 400 μm. The error bars indicate the sd. ○, Measurements made in the absence of SHAM; •, measurements made in the presence of SHAM (3 mm). The number of replicates was at least three and these were from different plants and plant batches.
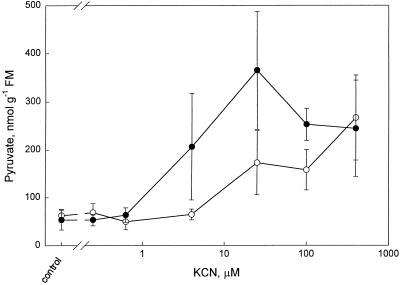
The pyruvate concentration in the roots was constant (58 ± 7 nmol g−1 FM) up to a KCN concentration of 0.63 μm with or without SHAM (Fig. 3). Up to a KCN concentration of 4 μm (without SHAM), the pyruvate concentration did not change, whereas after the addition of SHAM, it increased significantly (P < 0.05) to 206 nmol g−1 FM. At the two highest KCN concentrations there was no further effect of SHAM on the pyruvate concentration.
Figure 3.
Pyruvate concentration (nanomoles per gram FM) in intact roots (detached from the plant) of P. annua 10 to 15 min after the inhibitors were applied plotted against the KCN concentration (note the logarithmic scale). The following KCN concentrations were used: 0 (control), 0.25, 0.63, 1, 2, 4, 25, 40, and 400 μm. The error bars indicate the sd. ○, Measurements made in the absence of SHAM; •, measurements made in the presence of SHAM (3 mm). The number of replicates was at least three and these were from different plants and plant batches.
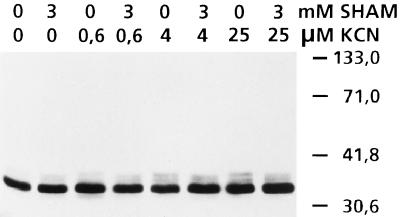
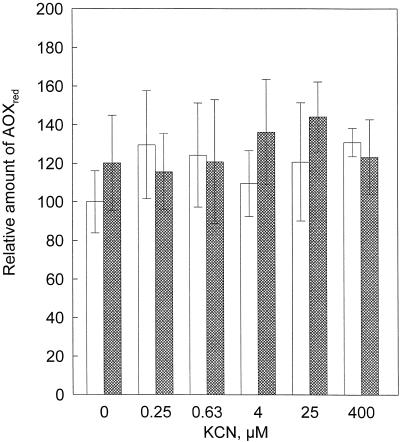
We also determined the reduction state of the AOX protein in vivo in a tissue extract without the intermediate step of isolating mitochondria. No changes in the reduction state of the protein upon addition of KCN or SHAM were observed. The protein was invariably almost completely in its reduced (higher-activity) state (Figs. 4 and 5). The reduced AOX protein gave one prominent and three minor bands around 35 kD. The oxidized dimer gave two bands around 66 kD. The difference between the intensity of the reduced (monomer) and oxidized (dimer) bands was at least 50-fold. However, when we first isolated mitochondria from the roots and then assayed AOX, the oxidized bands were more abundant than the reduced forms. In lanes with a mixture of isolated mitochondria and whole-root extract the oxidized bands were clearly detectable (data not shown). We conclude that the absence of visible oxidized bands of AOX on our gels was not an artifact, but was caused by the virtual absence of oxidized protein in intact P. annua roots.
Figure 4.
Western blot of AOX detected with monoclonal antibodies of whole-root extracts of P. annua at different concentrations of KCN in the presence or absence of SHAM (3 mm). Lane 1, 0 μm KCN without SHAM; lane 2, 0 μm KCN + SHAM; lane 3, 0.63 μm KCN without SHAM; lane 4, 0.63 μm KCN + SHAM; lane 5, 4 μm KCN without SHAM; lane 6, 4 μm KCN + SHAM; lane 7, 25 μm KCN without SHAM; lane 8, 25 μm KCN + SHAM.
Figure 5.
Relative levels of reduced AOX protein from whole-root extracts of P. annua at different concentrations of KCN in the presence (cross-hatched columns) or absence (open columns) of SHAM (3 mm), with the control (0 KCN without SHAM) as 100%. The error bars indicate the sd. The number of replicates was at least three and these were from different plants and plant batches.
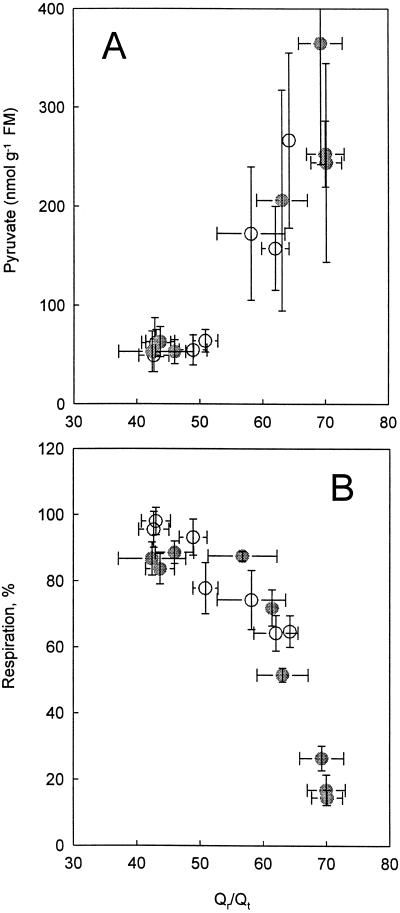
Figure 6 shows the root respiration in the absence and presence of SHAM (Fig. 6A) and pyruvate concentrations (Fig. 6B) plotted against Qr/Qt, calculated from Figures 1–3. Both O2 uptake and pyruvate concentration were dependent on Qr/Qt and were unaffected by the presence of SHAM. The increase in Qr/Qt was obtained because the respiration was increasingly blocked by inhibitors. Because at every point a steady state was reached at which the rate of ubiquinone oxidation by definition equaled the rate of ubiquinone reduction, the curve shown in Figure 6B represents the kinetics of the combined actions of the several dehydrogenases operating in vivo: when Qr/Qt increases, the dehydrogenases are less able to donate electrons to the Qr/Qt pool. With the dehydrogenases becoming less active, the concentration of respiratory substrates (pyruvate) increases (compare Fig. 6, A and B).
Figure 6.
Respiration (A; percent of control) and pyruvate concentration (B; nanomoles per gram FM) plotted against Qr/Qt in roots of P. annua. ○, Measurements made in the absence of SHAM (3 mm); •, measurements made in the presence of SHAM. The error bars indicate the sd. The number of replicates was at least three. The data were extracted from Figures 1–3.
There was no significant change in the concentration of lactate with SHAM (235 ± 44, 240 ± 48, and 189 ± 87 nmol g−1 FM for 0, 4, and 100 μm KCN, respectively; mean values ± sd; n = 3) or ethanol (9.9 and 9.0 nmol g−1 FM for 0 and 100 μm KCN, respectively) 15 min after the addition of inhibitors.
DISCUSSION
A Role for the Alternative Pathway in the Stabilization of Qr/Qt in Intact Roots
Our data confirm a hypothesized physiological role for the alternative pathway to stabilize Qr/Qt, as proposed by Purvis and Shewfelt (1993) and Wagner and Wagner (1995). If the alternative pathway stabilizes Qr/Qt, then it should be expected that when respiration via the Cyt pathway is inhibited by KCN, Qr/Qt increases to a greater extent in the presence of SHAM than in its absence.
We conclude from the data presented in Figures 1 and 2 that, especially at low KCN concentrations (up to 4 μm), when respiration is inhibited by 20%, Qr/Qt increases by almost 20% when SHAM is present, but only by approximately 8% when the alternative pathway is able to accept electrons. In addition, O2 uptake proceeds at a faster rate when the alternative pathway participates in respiration. Because a high Qr/Qt favors the formation of free radicals, it is feasible that engaging the AOX when the Cyt pathway is inhibited is an important mechanism to prevent such a potentially harmful situation. With increasing KCN concentrations, Qr/Qt further increases, also in the absence of SHAM, suggesting that the AOX is not able to fully buffer a considerable inhibition of the Cyt pathway.
Activity of the Alternative Pathway in Intact Roots
At KCN concentrations between 0.63 and 25 μm there was an increasing effect of SHAM on the inhibition of respiration. That part of the Cyt pathway that was not inhibited by KCN increased in activity as a result of an increase in Qr/Qt. SHAM caused an extra increase in Qr/Qt compared with that in roots that were not exposed to SHAM. Therefore, SHAM inhibition of root respiration is an underestimation of the activity of the alternative pathway.
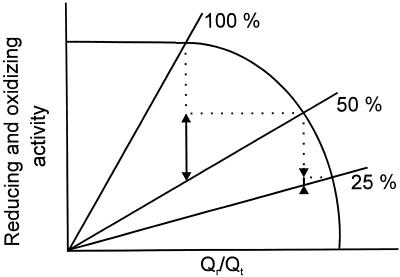
At KCN concentrations greater than 25 μm, SHAM inhibited O2 uptake, with an average inhibition of 48%, whereas Qr/Qt was similar in the absence and presence of SHAM. This is explained by the relationship between O2 uptake and Qr/Qt as plotted in Figure 6B. At a high Qr/Qt, a considerable decrease in respiration no longer results in a further increase in Qr/Qt (Fig. 7).
Figure 7.
Hypothetical reducing (curved line) and oxidizing (straight lines) pathway activities against Qr/Qt. In a steady state, the reducing activity (combined dehydrogenases) is by definition equal to the oxidizing activity (Cyt and alternative pathway). If 50% of the oxidizing pathways is inhibited, the other 50% will become more active (long arrow) because of the increase in Qr/Qt, and the inhibition will be underestimated. If subsequently 75% of the oxidizing pathways is inhibited, the 25% that is left becomes only slightly more active (short arrow) because of the steep slope of the reducing pathways and therefore the estimation of the inhibition becomes more accurate.
When the Cyt pathway is partially inhibited by low KCN concentrations (<0.60 μm), a small (11%) but significant inhibition of O2 uptake by SHAM was observed in intact roots of P. annua. If this inhibition represented the participation of the alternative pathway, it is expected that at any of those low KCN concentrations, Qr/Qt would be higher in the presence of SHAM than in its absence (Wagner and Krab, 1995). However, no difference was observed in Qr/Qt with or without SHAM, suggesting that the alternative pathway does not contribute to respiration under these conditions. If, on the other hand, the inhibition were caused by a nonspecific effect on the reducing side of ubiquinone, a change in Qr/Qt would be expected: a shift to a more oxidized situation. However, Qr/Qt remained unchanged, so SHAM probably inhibited a nonmitochondrial component of O2 uptake.
The Mechanisms Accounting for Increased AOX Activity
The data presented in Figures 1 and 2 clearly show that at KCN concentrations greater than 1 μm, the alternative pathway contributes to respiration (without SHAM) to an increasing extent, although the exact rates cannot be established.
In vitro the AOX can become more active by increasing the concentration of its substrate (Qr), by reduction of the AOX protein to its higher-activity configuration (Umbach and Siedow, 1993; Umbach et al., 1994), or by increasing the concentration of some α-keto acids, e.g. pyruvate (Umbach et al., 1994; Hoefnagel et al., 1995; Millar et al., 1996). It is unknown if and to what extent these mechanisms occur in intact tissues. In vivo the amount of AOX protein might also change over time; however, because our experiments lasted only 10 to 15 min, we can exclude such an effect of protein synthesis.
Activation of the AOX by the Reduction State of AOX
The slope of the kinetic curve of AOX activity (O2 uptake against Qr/Qt) is much steeper when the AOX protein is reduced (Umbach and Siedow, 1993; Umbach et al., 1994). If the reduction state of the AOX protein functions as a mechanism to increase respiration via the alternative pathway when the Cyt pathway in intact grass roots is inhibited, then the AOX protein should become more reduced at KCN concentrations greater than 0.6 μm. However, at all KCN concentrations and independent of the presence of SHAM, the AOX protein was mainly in its reduced state (a factor of 50 difference in intensity between the oxidized and reduced bands). We conclude, therefore, that the reduction state of the AOX protein has no regulatory function in the experiments described here. This does not necessarily mean that the reduction state of the AOX protein never changes to the oxidized (less-active) form in vivo under other circumstances, e.g. different developmental stages, different growth conditions, but this remains to be confirmed.
The AOX protein of P. annua roots was much more oxidized in the same experiments when mitochondria were isolated before the AOX protein measurements (data not shown). The oxidized form of the protein is clearly detectable in isolated mitochondria and in samples with a mixture of isolated mitochondria with whole-root extracts. This indicates that in our experiments, the primary antibodies recognized both the oxidized and the reduced forms of the protein, as has been found previously in comparable experiments (e.g. Umbach and Siedow, 1993). The only difference between the AOX protein measurements in intact plant material and those in isolated mitochondria was the isolation of the mitochondria before immunodetection. Therefore, our results show that the AOX protein becomes more oxidized during isolation of the mitochondria material, as observed previously by Umbach and Siedow (1997). Estimations of the reduction state of the AOX protein in vivo using isolated mitochondria are therefore not suitable for obtaining information on the reduction state of the protein in intact plants.
Activation of the AOX by Pyruvate
The kinetic curve of the AOX (O2 uptake against Qr/Qt) shifts to the left upon the addition of pyruvate or other α-keto acids in isolated mitochondria, so α-keto acids increase the activity of the AOX (Umbach et al., 1994; Hoefnagel et al., 1995; Hoefnagel and Wiskich, 1996; Millar et al., 1996). If pyruvate activated the alternative pathway in the present experiments, the pyruvate concentration would be expected to increase at KCN concentrations greater than 1 μm. However, assuming that our results on concentrations in whole tissue reflect the intramitochondrial pyruvate concentration, then these results do not indicate that the alternative pathway is activated by an increase in the pyruvate concentration. At those KCN concentrations at which the activity of the alternative pathway increased (without SHAM) the pyruvate concentration did not increase.
If the alternative pathway were activated by increased concentrations of pyruvate, the concentrations inside the mitochondria (Umbach and Siedow, 1996) should change in a range around the half-maximum pyruvate stimulation. Values for the half-maximum pyruvate stimulation vary between different studies, from 128 μm in mitochondria isolated from potato tubers (Wagner et al., 1995) to 500 μm in mitochondria isolated from tobacco leaves (Vanlerberghe et al., 1995). Values for half-maximum pyruvate stimulation were determined by adding pyruvate to intact mitochondria. The binding site of pyruvate on the AOX is at the matrix side of the mitochondria (Umbach and Siedow, 1996). The pyruvate concentration outside the mitochondria may not be representative of the concentration inside the mitochondria at the activation site because of a low capacity of the mitochondrial pyruvate transporter and the production of pyruvate by malic enzyme from malate inside the mitochondria, especially at pH values around 6.5 (Millar et al., 1996).
Zang et al. (1996) found a half-maximum pyruvate stimulation of 400 μm for purified AOX protein from Arum lily and soybean. However, in these studies the protein was isolated from the mitochondrial membranes, which does not necessary represent the situation when the protein is still in a membrane, e.g. due to conformational changes.
Millar et al. (1996) used inside-out mitochondrial particles to measure the half-maximum pyruvate stimulation and found a value of less then 4 μm (in sweet potato and soybean). Finnegan et al. (1997) found comparable half-maximum pyruvate stimulation: 4.5 μm in cotyledons and 51 μm in roots of soybean inside-out mitochondrial particles. This is presumably the most appropriate method for determining the half-maximum pyruvate stimulation, because it gives the pyruvate concentration at the site where it reacts with the AOX protein to stimulate its activity.
The measured pyruvate concentrations are values pertaining to the whole tissue and were 58 ± 7 nmol g−1 FM for the control plants and increased to 250 nmol g−1 FM at the highest KCN concentrations (Fig. 3). These concentrations are similar to those found for other plants (e.g. carrot roots, 39 nmol g−1 FM [Kato-Noguchi, 1996]; petunia cell suspensions, 100 nmol g−1 FM [Wagner and Wagner, 1997]; and 60, 32, and 67 nmol g−1 FM for roots of spinach, bean, and wheat, respectively [Day and Lambers, 1983]). During hypoxia, the pyruvate concentration in barley roots increases from 60 to 120 nmol g−1 FM (Good and Muench, 1993). In tobacco cell suspensions, the pyruvate concentration increases upon the addition of antimycin from 100 to 550 nmol g−1 FM (assuming a dry matter percentage of 10%) in less than 1 h (Vanlerberghe et al., 1997).
If we estimate the concentration of pyruvate in the cytoplasm we might be able to conclude if it is in the range of the half-maximum pyruvate stimulation. The mitochondrial volume is about 0.2% to 0.7% of the total cell volume (Douce, 1985). The volume of the mitochondria is small in comparison with that of the whole cell; therefore, it is impossible to separate the pyruvate concentration inside the mitochondria from whole-tissue data. If the pyruvate is equally distributed over the mitochondria, cytosol, and vacuole, then the concentration is 60 μm, which is 1.2 to 15 times as high as the half-maximum pyruvate stimulation of 50 and 4 μm found by Finnegan et al. (1997). If the pyruvate concentration in the vacuole is 0 (which it probably is), then the concentration in the cytoplasm (10% of cell volume) will be about 10 times as high, i.e. 600 μm (12–150 times as high as the half-maximum pyruvate stimulation of 50 and 4 μm). If the pyruvate concentration in the mitochondria is at least equal to that in the cytosol, then the AOX is always fully activated by pyruvate. The end product of glycolysis in plants is not only pyruvate but also a substantial amount of malate (Day and Hanson, 1977). Malate by itself cannot be the substrate for the citric acid cycle, but needs to be converted into pyruvate via malic enzyme. The pyruvate concentration inside the mitochondria may be even higher than that in the cytosol.
Increase in AOX Activity Caused by an Increase in Qr/Qt
The alternative pathway becomes more active if there is more of its substrate, Qr. The slope of the activity of the alternative pathway (respiration against Qr/Qt) determines the change in activity of the alternative pathway for a given change in Qr/Qt. If the slope is steep, then a small change in Qr/Qt has a major influence on the activity of the alternative pathway. In the present experiments Qr/Qt increased by 8% between 0.6 and 25 μm KCN (in the absence of SHAM), and under these conditions the activity of the alternative pathway increased (indicated by an inhibition of SHAM).
If we consider data from the literature that describe the relationship between Qr/Qt and O2 uptake via the alternative pathway, a nonlinear relationship is often found when the oxidized form is measured in the absence of pyruvate. If the AOX protein is reduced, the relationship often approaches linearity, except for the first approximately 10% of the O2 uptake (nanomoles of O2 per milligram of protein per minute). If a straight line is fitted through these points, an average slope of 143% ± 39% (with pyruvate) and 208% ± 96% (without pyruvate) AOX activity (% Qr/Qt)−1 is found (Umbach et al., 1994; Day et al., 1995; Hoefnagel et al., 1995; Hoefnagel and Wiskich, 1996; Millar et al., 1996, 1997). The first nonlinear part was ignored if necessary, and the values were recalculated to percentages to compare the different respiration rates. Millar et al. (1996) found even steeper slopes using inside-out submitochondrial particles, 476% AOX activity (% Qr/Qt)−1 in the presence of pyruvate.
If the Qr/Qt increases by 10% (on a 0%–100% scale), then the activity of the alternative pathway increases by 14%, and if the active ubiquinone pool is 50% of the Qt pool, then a change in Qr/Qt of 10% results in an increase of 28% in alternative pathway activity.
The alternative pathway can stabilize Qr/Qt because of its steep kinetics, even though Qr/Qt cannot be kept absolutely constant; if Qr/Qt increases marginally, the alternative pathway rapidly becomes more active and thereby prevents a further increase in Qr/Qt.
Kinetic Properties of Mitochondrial Dehydrogenases
All of the mitochondrial dehydrogenases have unique kinetic properties that may differ between the different types (van den Bergen et al., 1994). Because at steady state the Qr/Qt activity of ubiquinone-reducing pathways by definition equals the activity of ubiquinone-oxidizing pathways, the obtained rate at equilibrium also represents the rate of the combined dehydrogenase activity. By manipulating the oxidizing pathways with inhibitors, a relationship between activity of dehydrogenases and ubiquinone reduction can be obtained (van den Bergen et al., 1994; Millar et al., 1995; Wagner and Krab, 1995). Figure 6B shows the combined activity of all of the dehydrogenase acting in P. annua roots in vivo as a function of Qr/Qt. The resulting curve suggests that there is no large or sudden change in the use of different types of dehydrogenases either at the various levels of Qr/Qt or in the absence or presence of SHAM. If the activity of the combined dehydrogenases decreases at higher Qr/Qt values, and if glycolysis is not decreased to the same extent, the concentration of the end products of glycolysis will increase. Figure 6A shows an increase in the concentration of pyruvate when the combined dehydrogenases become less active as a result of the increase in Qr/Qt.
Vanlerberghe et al. (1997) showed that if there is an imbalance between oxidation of organic acids (production of NADH) and the activity of the electron-transport pathway (production of NAD+), pyruvate will accumulate, which may result in fermentation under aerobic conditions. In roots of P. annua there was no accumulation of ethanol or lactate, and therefore fermentation did not occur, probably because the respiration measurements lasted only 10 to 15 min. In roots of maize (Wignarajah and Greenway, 1976), barley, and rice (Wignarajah et al., 1976), the maximum activity of alcohol dehydrogenase and especially pyruvate decarboxylase is low and increases upon anoxia. These enzymes probably have to be synthesized first in the roots of P. annua before lactate/ethanol accumulates, and that takes longer than 15 min.
The alternative pathway can avoid fermentation because it can prevent an increase in Qr/Qt and therefore prevent the dehydrogenases from becoming less active, so pyruvate accumulates.
CONCLUDING REMARKS
Our results show that the alternative pathway can stabilize Qr/Qt in roots of P. annua (Figs. 1 and 2) when the Cyt pathway is restricted by KCN. By stabilizing Qr/Qt, an increase in the production of radicals and fermentation products can be prevented. In this way potential cell damage is avoided.
The increased activity of the alternative pathway as a result of KCN inhibition of the Cyt pathway is not caused by a further reduction of the AOX protein (Fig. 5); almost all of the AOX is already in its reduced state in the intact P. annua roots in the absence of inhibitors. A small change in Qr/Qt has a large effect on the activity of the alternative pathway. Therefore, the alternative pathway stabilizes Qr/Qt. The role of pyruvate in the increased activity of the alternative pathway is not entirely clear from our results, but the pyruvate concentration always seemed to be higher than the half-maximum pyruvate stimulation.
Abbreviations:
- AOX
alternative oxidase
- FM
fresh mass
- Qr
reduced ubiquinone
- Qr/Qt
reduction state of the ubiquinone pool
- Qt
total ubiquinone
- SHAM
salicylhydroxamic acid
LITERATURE CITED
- Atkin OK, Villar R, Lambers H. Partitioning of electrons between the cytochrome and alternative pathways in intact roots. Plant Physiol. 1995;108:1179–1183. doi: 10.1104/pp.108.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Boveris A, Oshino N (1977) Alcohol and Aldehyde Metabolizing Systems. Academic Press, New York, pp 261–274
- Crane FL. The coenzyme Q group (ubiquinones) Prog Chem Fats Other Lipids. 1963;7:267–289. [Google Scholar]
- Day DA, Hanson JB. Pyruvate and malate transport and oxidation in corn mitochondria. Plant Physiol. 1977;59:630–635. doi: 10.1104/pp.59.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Krab K, Lambers H, Moore AL, Siedow JN, Wagner AM, Wiskich JT. The cyanide-resistant oxidase. To inhibit or not to inhibit, that is the question. Plant Physiol. 1996;110:1–2. doi: 10.1104/pp.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Lambers H. The regulation of glycolysis and electron transport in roots. Plant Physiol. 1983;58:155–160. [Google Scholar]
- Day DA, Whelan J, Millar AH, Siedow JN, Wiskich JT. Regulation of the alternative oxidase in plants and fungi. Aust J Plant Physiol. 1995;22:497–509. [Google Scholar]
- Douce R (1985) Mitochondria in Higher Plants: Structure, Function, and Biogenesis. Academic Press, New York, pp 4–7
- Elthon TE, Nickels RL, McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 1989;89:1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan PM, Whelan J, Millar AH, Zhang Q, Smith MK, Wiskich JT, Day DA. Differential expression of the multigene family encoding the soybean mitochondrial alternative oxidase. Plant Physiol. 1997;114:455–466. doi: 10.1104/pp.114.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Boveris A (1982) Superoxide radical and hydrogen peroxide in mitochondria. In Free Radicals in Biology, Vol V. Academic Press, New York, pp 65–90
- Good AG, Muench DG. Long-term anaerobic metabolism in root tissue. Plant Physiol. 1993;101:1163–1168. doi: 10.1104/pp.101.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel MHN, Millar AH, Wiskich JT, Day DA. Cytochrome and alternative respiratory pathways compete for electrons in the presence of pyruvate in soybean mitochondria. Arch Biochem Biophys. 1995;318:394–400. doi: 10.1006/abbi.1995.1245. [DOI] [PubMed] [Google Scholar]
- Hoefnagel MHN, Wiskich JT. Alternative oxidase activity and the ubiquinone redox level in soybean cotyledon and Arum spadix mitochondria during NADH and succinate oxidation. Plant Physiol. 1996;110:1329–1335. doi: 10.1104/pp.110.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Noguchi H. Effects of citrate on respiratory gas exchange and metabolism in carrot root tissues. Phytochemistry. 1996;45:225–227. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leprince O, Atherton NM, Deltour R, Hendry GAF. The involvement of respiration in free radical processes during loss of desiccation tolerance in germinating Zea mays L. Plant Physiol. 1994;104:1333–1339. doi: 10.1104/pp.104.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS, Huang JP (1996) Coexistence of a “reactive oxygen cycle” with the “Q-cycle” in the respiratory chain: a hypothesis for generating, partitioning, and function of superoxide in mitochondria. In Proceedings of International Symposium on Natural Antioxidants: Molecular Mechanisms and Health Effects. AOCS Press, Champaign, IL, pp 513–529
- Meeuse BJD. Thermogenic respiration in aroids. Annu Rev Plant Physiol. 1975;26:117–126. [Google Scholar]
- Millar AH, Atkin OK, Lambers H, Wiskich JT, Day DA. A critique of the use of inhibitors to estimate partitioning of electrons between mitochondrial respiratory pathways in plants. Physiol Plant. 1995;95:523–532. [Google Scholar]
- Millar AH, Finnegan PM, Whelan J, Drevon JJ, Day DA. Expression and kinetics of the mitochondrial alternative oxidase in nitrogen-fixing nodules of soybean roots. Plant Cell Environ. 1997;20:1273–1282. [Google Scholar]
- Millar AH, Hoefnagel MHN, Day DA, Wiskich JT. Specificity of the organic acid activation on the alternative oxidase in plant mitochondria. Plant Physiol. 1996;111:613–618. doi: 10.1104/pp.111.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Remkes C. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia. 1990;83:553–559. doi: 10.1007/BF00317209. [DOI] [PubMed] [Google Scholar]
- Puntelarulo S, Galleano M, Sanchez RA, Boveris A. Superoxide anion and hydrogen peroxide metabolism in soybean embryonic axes during germination. Biochim Biophys Acta. 1991;1074:277–283. doi: 10.1016/0304-4165(91)90164-c. [DOI] [PubMed] [Google Scholar]
- Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant tissue? Physiol Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Purvis AC, Shewfelt RL, Gegogeine JW. Superoxide production by mitochondria isolated from green bell pepper fruit. Physiol Plant. 1995;94:743–749. [Google Scholar]
- Ribas-Carbo M, Wiskich JT, Berry JA, Siedow JN. Ubiquinone redox behavior in plant mitochondria during electron transport. Arch Biochem Biophys. 1995;317:156–160. doi: 10.1006/abbi.1995.1148. [DOI] [PubMed] [Google Scholar]
- Rich PR, Bonner WD. The sites of superoxide anion generation in higher plant mitochondria. Arch Biochem Biophys. 1987;188:206–213. doi: 10.1016/0003-9861(78)90373-9. [DOI] [PubMed] [Google Scholar]
- Threlfall DR, Whistance GR. Biosynthesis of ubiquinone: a search for polyprenyl phenol and quinone precursors. Phytochemistry. 1970;9:355–359. [Google Scholar]
- Umbach AL, Siedow JN. Covalent and noncovalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol. 1993;103:845–854. doi: 10.1104/pp.103.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. The reaction of the soybean cotyledon mitochondrial cyanide-resistant oxides with sulfhydryl reagents suggests that α-keto acid activation involves the formation of a thiohemiacetal. J Biol Chem. 1996;271:25019–25026. doi: 10.1074/jbc.271.40.25019. [DOI] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. Changes in the redox state of the alternative oxidase regulator sulfhydryl/disulfide system during mitochondrial isolation: implications for inferences of activity in vivo. Plant Sci. 1997;123:19–28. [Google Scholar]
- Umbach AL, Wiskich JT, Siedow JN. Regulation of alternative oxidase kinetics by pyruvate and intermolecular disulfide bond redox status in soybean seedling mitochondria. FEBS Lett. 1994;348:181–184. doi: 10.1016/0014-5793(94)00600-8. [DOI] [PubMed] [Google Scholar]
- van den Bergen CWM, Wagner AM, Krab K, Moore AL. The relationship between electron flux and the redox poise of the quinone pool in plant mitochondria: interplay between quinol-oxidizing and quinone-reducing pathways. Eur J Biochem. 1994;226:1071–1078. doi: 10.1111/j.1432-1033.1994.01071.x. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, Day DA, Wiskich JT, Vanlerberghe AE, McIntosh L. Alternative oxidase activity in tobacco leaf mitochondria. Plant Physiol. 1995;109:353–361. doi: 10.1104/pp.109.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, Vanlerberghe AE, McIntosh L. Molecular genetic evidence of the ability of alternative oxidase to support respiratory carbon metabolism. Plant Physiol. 1997;113:657–661. doi: 10.1104/pp.113.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Bergen van CWM, Wincencjusz H. Stimulation of the alternative pathway by succinate and malate. Plant Physiol. 1995;108:1035–1042. doi: 10.1104/pp.108.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Krab K. The alternative respiration pathway in plants. Role and regulation. Plant Physiol. 1995;95:318–325. [Google Scholar]
- Wagner AM, Wagner MJ. Measurements of in vivo ubiquinone reduction levels in plant cells. Plant Physiol. 1995;108:277–283. doi: 10.1104/pp.108.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Wagner MJ. Changes in mitochondrial respiratory chain components of petunia cells during culture in the presence of antimycin A. Plant Physiol. 1997;115:617–622. doi: 10.1104/pp.115.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignarajah K, Greenway H. Effect of anaerobiosis on activities of alcohol dehydrogenase and pyruvate decarboxylase in roots of Zea mays. New Phytol. 1976;77:575–584. [Google Scholar]
- Wignarajah K, Greenway H, John CD. Effect of waterlogging on growth and activity of alcohol dehydrogenase in barley and rice. New Phytol. 1976;77:585–592. [Google Scholar]
- Zhang Q, Hoefnagel MHN, Wiskich JT. Alternative oxidase from Arum and soybean: its stabilization during purification. Physiol Plant. 1996;96:551–558. [Google Scholar]