Abstract
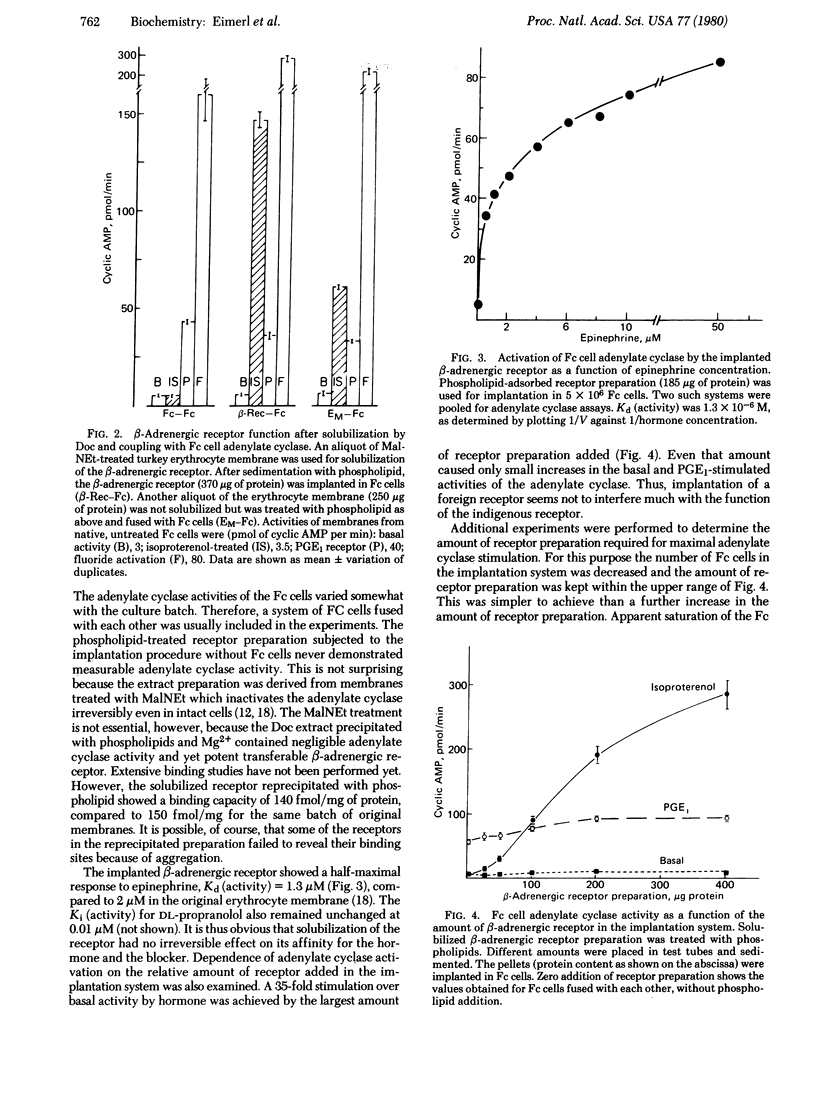
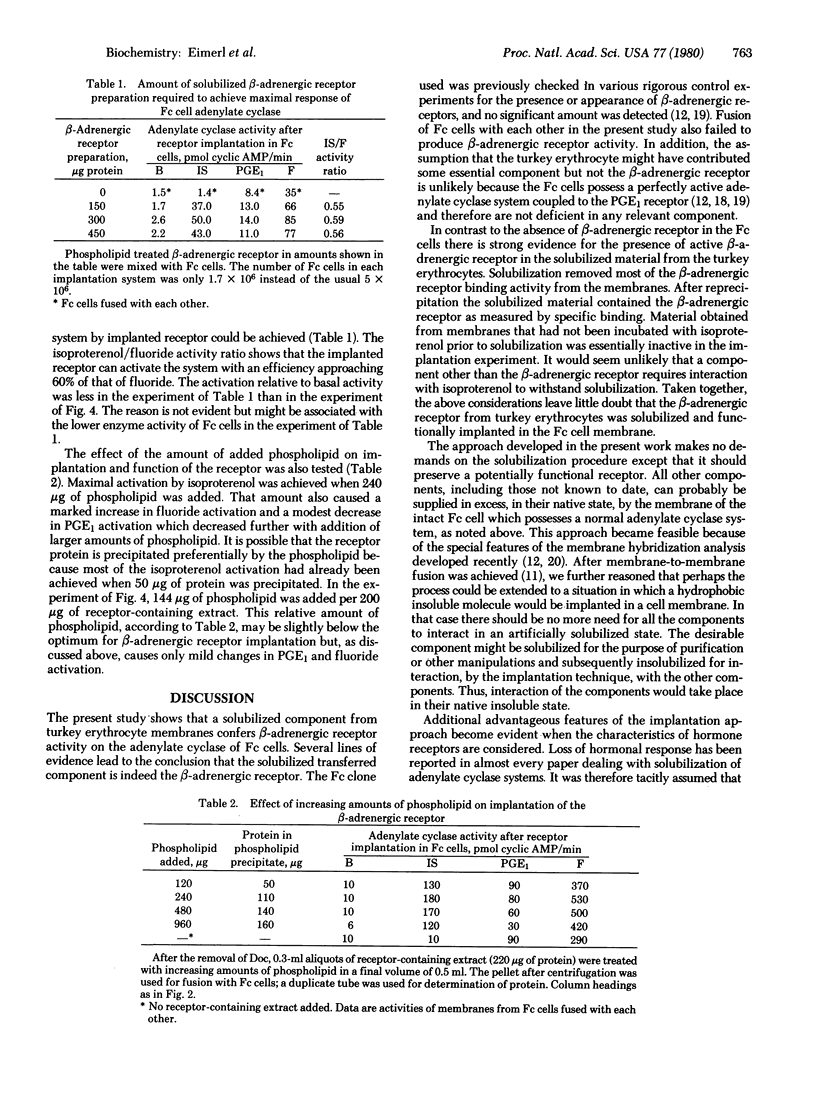
When the β-adrenergic receptor of turkey erythrocytes was solubilized by deoxycholate, it retained its potential to activate an adenylate cyclase system. Electron microscopy showed that true solubilization had apparently been achieved; no residual membrane or vesicle structure was found. After removal of deoxycholate and addition of phospholipid, the reprecipitated β-adrenergic receptor was implanted in the cell membrane of Friend erythroleukemia cells by using a chemical fusion method recently developed. Membranes prepared from the cells demonstrated 30-fold stimulation of the Friend cell adenylate cyclase by the implanted β-adrenergic receptor. The function of the indigenous prostaglandin E1 receptor of the Friend cells was not much affected by the implantation of large amounts of the foreign receptor. Activity mediated by the β-adrenergic receptor reached 60% of the activity obtained with fluoride. The implanted receptor is therefore considered to be efficiently coupled to the adenylate cyclase system. The major difficulties hitherto preventing solubilization of hormone receptors and subsequent reconstitution of their function have been overcome by the approach developed in the present work. Conditions of solubilization need preserve only the receptor because all other components, even those unidentified as yet, can be supplied in excess by the adenylate cyclase system of the cell in which the receptor will be implanted. Subsequent recoupling of the receptor to the adenylate cyclase is performed in the native insoluble state of these molecules. Thus, the components need not be subjected to the hazards of solubilization in a common detergent as is usually required in reconstitution procedures. The importance of using implantation as an assay for a functional receptor in the course of purification and the likelihood that the procedure can be adapted to other receptors for hormones and neurotransmitters are discussed.
Keywords: receptor-coupled adenylate cyclase, cyclic AMP, membrane fusion, hybridization of membrane components, hormone and neurotransmitter action
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cori C. F., Garland R. C., Chang H. W. Purification of particulate glucose-6-phosphatase. Biochemistry. 1973 Jul 31;12(16):3126–3130. doi: 10.1021/bi00740a029. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Hayashi K., Sala G., Baukal A., Catt K. J. Gonadal luteinizing hormone receptors and adenylate cyclase: transfer of functional ovarian luteinizing hormone receptors to adrenal fasciculata cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4769–4773. doi: 10.1073/pnas.75.10.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga T., Haga K., Gilman A. G. Hydrodynamic properties of the beta-adrenergic receptor and adenylate cyclase from wild type and varient S49 lymphoma cells. J Biol Chem. 1977 Aug 25;252(16):5776–5782. [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Structural and functional properties of the acetylcholine receptor protein in its purified and membrane-bound states. Annu Rev Biochem. 1978;47:317–357. doi: 10.1146/annurev.bi.47.070178.001533. [DOI] [PubMed] [Google Scholar]
- Hoffmann F. M. Solubilization and reconstitution of dopamine-sensitive adenylate cyclase from bovine caudate nucleus. J Biol Chem. 1979 Jan 25;254(2):255–258. [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Resolution of beta-adrenergic receptor binding and adenylate cyclase activity by gel exclusion chromatography. J Biol Chem. 1977 Jan 25;252(2):799–802. [PubMed] [Google Scholar]
- Maguire M. E., Wiklund R. A., Anderson H. J., Gilman A. G. Binding of (125I)iodohydroxybenzylpindolol to putative beta-adrenergic receptors of rat glioma cells and other cell clones. J Biol Chem. 1976 Mar 10;251(5):1221–1231. [PubMed] [Google Scholar]
- Meissner G., Fleischer S. Dissociation and reconstitution of functional sarcoplasmic reticulum vesicles. J Biol Chem. 1974 Jan 10;249(1):302–309. [PubMed] [Google Scholar]
- Neer E. J. Multiple forms of adenylate cyclase. Adv Cyclic Nucleotide Res. 1978;9:69–83. [PubMed] [Google Scholar]
- Orly J., Schramm M. Coupling of catecholamine receptor from one cell with adenylate cyclase from another cell by cell fusion. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4410–4414. doi: 10.1073/pnas.73.12.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schramm M., Orly J., Eimerl S., Korner M. Coupling of hormone receptors to adenylate cyclase of different cells by cell fusion. Nature. 1977 Jul 28;268(5618):310–313. doi: 10.1038/268310a0. [DOI] [PubMed] [Google Scholar]
- Schramm M., Rodbell M. A persistent active state of the adenylate cyclase system produced by the combined actions of isoproterenol and guanylyl imidodiphosphate in frog erythrocyte membranes. J Biol Chem. 1975 Mar 25;250(6):2232–2237. [PubMed] [Google Scholar]
- Schramm M. Transfer of glucagon receptor from liver membranes to a foreign adenylate cyclase by a membrane fusion procedure. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1174–1178. doi: 10.1073/pnas.76.3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulster D., Orly J., Seidel G., Schramm M. Intracellular cyclic AMP production enhanced by a hormone receptor transferred from a different cell. beta-adrenergic responses in cultured cells conferred by fusion with turkey erythrocytes. J Biol Chem. 1978 Feb 25;253(4):1201–1206. [PubMed] [Google Scholar]
- Welton A. F., Lad P. M., Newby A. C., Yamamura H., Nicosia S., Rodbell M. Solubilization and separation of the glucagon receptor and adenylate cyclase in guanine nucleotide-sensitive states. J Biol Chem. 1977 Sep 10;252(17):5947–5950. [PubMed] [Google Scholar]




