Abstract
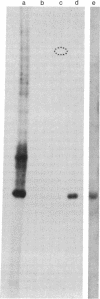
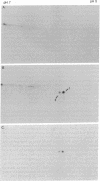
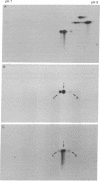
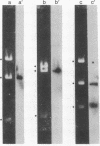
In order to investigate the regulation of actin gene transcription during early sea urchin development, a specific hybridization probe for actin sequences is required. Such a probe was produced by cloning cDNA transcribed from a sea urchin poly(A)-containing mRNA preparation enriched for actin message. Double-stranded DNA was ligated into the BamHI restriction site of plasmid pBR322, and the resulting hybrid molecules were used to transform the Escherichia coli strain ML100. After preliminary screening of bacterial colonies by antibiotic sensitivity and hybridization back to the original cDNA, clones containing sea urchin DNA were further characterized by a positive translation assay in which total sea urchin mRNA was hybridized to plasmid, and the hybridized message then was eluted and translated in a reticulocyte cell-free protein-synthesizing system. In this way, one clone (pSA38) was found to hybridize selectively to sea urchin mRNA coding for a protein of 43,000 daltons. This protein was identified as actin by three criteria: electrophoretic migration in two-dimensional polyacrylamide gels, affinity for DNase I, and peptide mapping. Restriction endonuclease and heteroduplex mapping of pSA38 indicate that it contains a 1.5-kilobase-pair insert and is therefore likely to contain a large portion of the actin coding sequence. By using pSA38 as a hybridization probe, it has been found that the level of actin-specific RNA sequences increases dramatically during early sea urchin development.
Keywords: recombinant plasmid, positive hybridization-translation, restriction mapping, Southern hybridization, heteroduplex analysis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J., Kinney T., Thompson S., Rubin L., Helling R. B. Frequency-Dependent Selection for Plasmid-Containing Cells of ESCHERICHIA COLI. Genetics. 1979 Apr;91(4):627–637. doi: 10.1093/genetics/91.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Harpold M. M., Dobner P. R., Evans R. M., Bancroft F. C. Construction and identification by positive hybridization-translation of a bacterial plasmid containing a rat growth hormone structural gene sequence. Nucleic Acids Res. 1978 Jun;5(6):2039–2053. doi: 10.1093/nar/5.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries P., Coggins L. W., Old R. W., Mitchell G. J., Coleclough C., Paul J. Structure of recombinant plasmids containing synthetic human foetal globin gene sequences. Mol Gen Genet. 1978 Sep 20;165(1):65–71. doi: 10.1007/BF00270377. [DOI] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Revel J. P. The molecular basis of cell movement. Sci Am. 1979 May;240(5):100–113. doi: 10.1038/scientificamerican0579-100. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Nakamura F., Bloom A., Konotey-Ahulu F., Bank A. Changes in restricted human cellular DNA fragments containing globin gene sequences in thalassemias and related disorders. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1222–1226. doi: 10.1073/pnas.75.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino G. T., Chamberlain J. P., Kleinsmith L. J. Effects of deciliation of tubulin messenger RNA activity in sea urchin embryos. J Biol Chem. 1978 Oct 10;253(19):7078–7085. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pemberton R. E., Liberti P., Baglioni C. Isolation of messenger RNA from polysomes by chromatography on oligo(dT)-cellulose. Anal Biochem. 1975 May 26;66(1):18–28. doi: 10.1016/0003-2697(75)90720-4. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shine J., Seeburg P. H., Martial J. A., Baxter J. D., Goodman H. M. Construction and analysis of recombinant DNA for human chorionic somatomammotropin. Nature. 1977 Dec 8;270(5637):494–499. doi: 10.1038/270494a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The amino acid sequence of Physarum actin. Nature. 1978 Dec 14;276(5689):720–721. doi: 10.1038/276720a0. [DOI] [PubMed] [Google Scholar]
- Weiss B., Live T. R., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. V. End group labeling and analysis of deoxyribonucleic acid containing single straned breaks. J Biol Chem. 1968 Sep 10;243(17):4530–4542. [PubMed] [Google Scholar]