Abstract
Binding of the beta-adrenergic agonist [3H]hydroxybenzylisoproterenol to the beta-adrenergic receptor of rat reticulocyte membranes results in the coupling of the receptor to the guanine nucleotide regulatory protein associated with the adenylate cyclase system. This regulatory component, referred to as the G-protein, was identified by its specific [32P]-ADP-ribosylation catalyzed by cholera toxin. Incubation of [32P]ADP-ribosylated rat reticulocyte membranes with the [3H]hydroxybenzylisoproterenol agonist prior to membrane solubilization and gel exclusion chromatography resulted in the coelution of the 42,000 Mr [32P]ADP-ribosylated G-proteins with the agonist-occupied beta-adrenergic receptors. The receptor-G-protein complex was not formed when receptors were unoccupied or occupied with antagonists at the time of solubilization. Incubation of rat reticulocyte membranes with [3H]hydroxybenzylisoproterenol in the presence of guanine nucleotides reversed or prevented the formation of this receptor-G-protein complex. These data provide direct evidence for the molecular interactions promoted by agonist occupancy of beta-adrenergic receptors. It is probable that the formation of a receptor-G-protein complex is crucial for catecholamine stimulation of the adenylate cyclase enzyme and, hence, transmembrane information transfer.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilezikian J. P. Dissociation of beta-adrenergic receptors from hormone responsiveness during maturation of the rat reticulocyte. Biochim Biophys Acta. 1978 Aug 17;542(2):263–273. doi: 10.1016/0304-4165(78)90022-3. [DOI] [PubMed] [Google Scholar]
- Bilezikian J. P., Spiegel A. M., Brown E. M., Aurbach G. D. Identification and persistence of beta adrenergic receptors during maturation of the rat reticulocyte. Mol Pharmacol. 1977 Sep;13(5):775–785. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto K., Gill D. M. Requirement for guanosine triphosphate in the activation of adenylate cyclase by cholera toxin. J Supramol Struct. 1979;10(1):51–60. doi: 10.1002/jss.400100106. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Kaiser G., Wiemer G., Kremer G., Dietz J., Hellwich M., Palm D. Correlation between isoprenaline-stimulated synthesis of cyclic AMP and occurrence of beta-adrenoreceptors in immature erythrocytes from rats. Eur J Pharmacol. 1978 Apr 1;48(3):255–262. doi: 10.1016/0014-2999(78)90084-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Caron M. G. Regulation of beta-adrenergic receptors by guanyl-5'-yl imidodiphosphate and other purine nucleotides. J Biol Chem. 1976 Aug 10;251(15):4686–4692. [PubMed] [Google Scholar]
- Lefkowitz R. J., Williams L. T. Catecholamine binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1977 Feb;74(2):515–519. doi: 10.1073/pnas.74.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E., Hickey A. R., Lefkowitz R. J. The molecular size of adenylate cyclase in the absence and presence of nucleotide and hormone effectors. J Cyclic Nucleotide Res. 1979;5(3):251–259. [PubMed] [Google Scholar]
- Limbird L. E., Hickey A. R., Lefkowitz R. J. Unique uncoupling of the frog erythrocyte adenylate cyclase system by manganese. Loss of hormone and guanine nucleotide-sensitive enzyme activities without loss of nucleotide-sensitive, high affinity agonist binding. J Biol Chem. 1979 Apr 25;254(8):2677–2683. [PubMed] [Google Scholar]
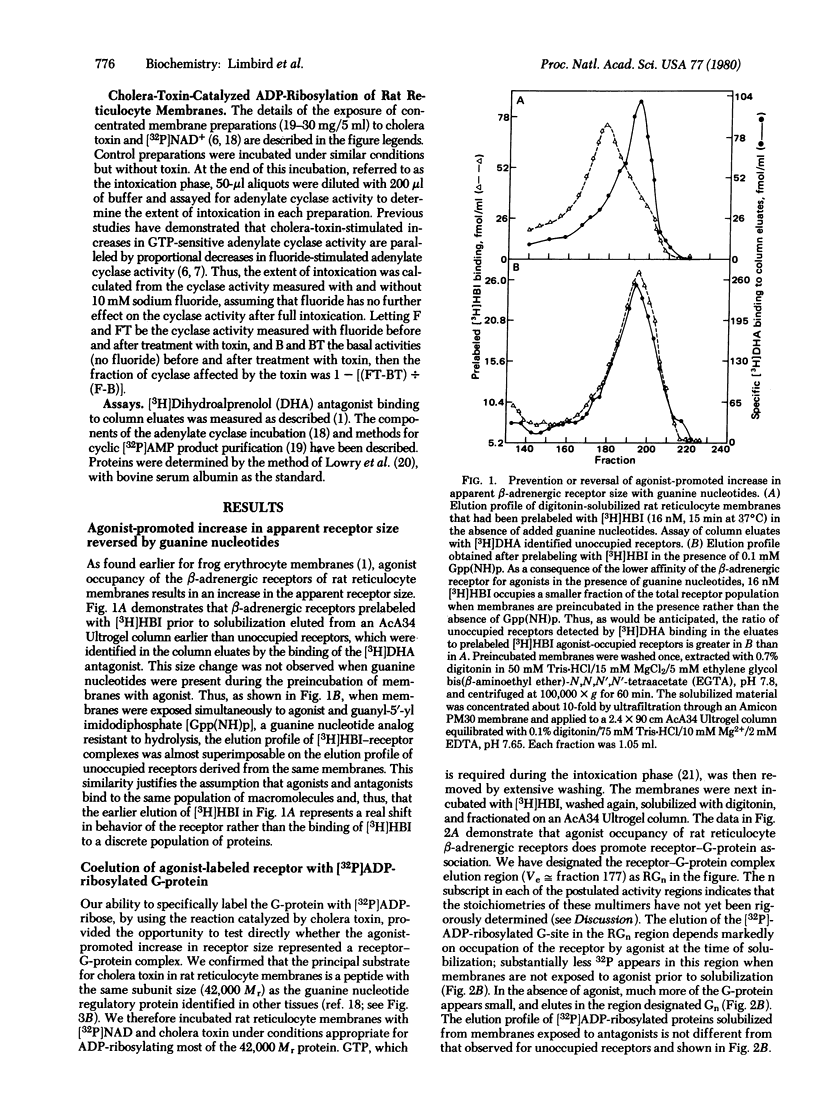
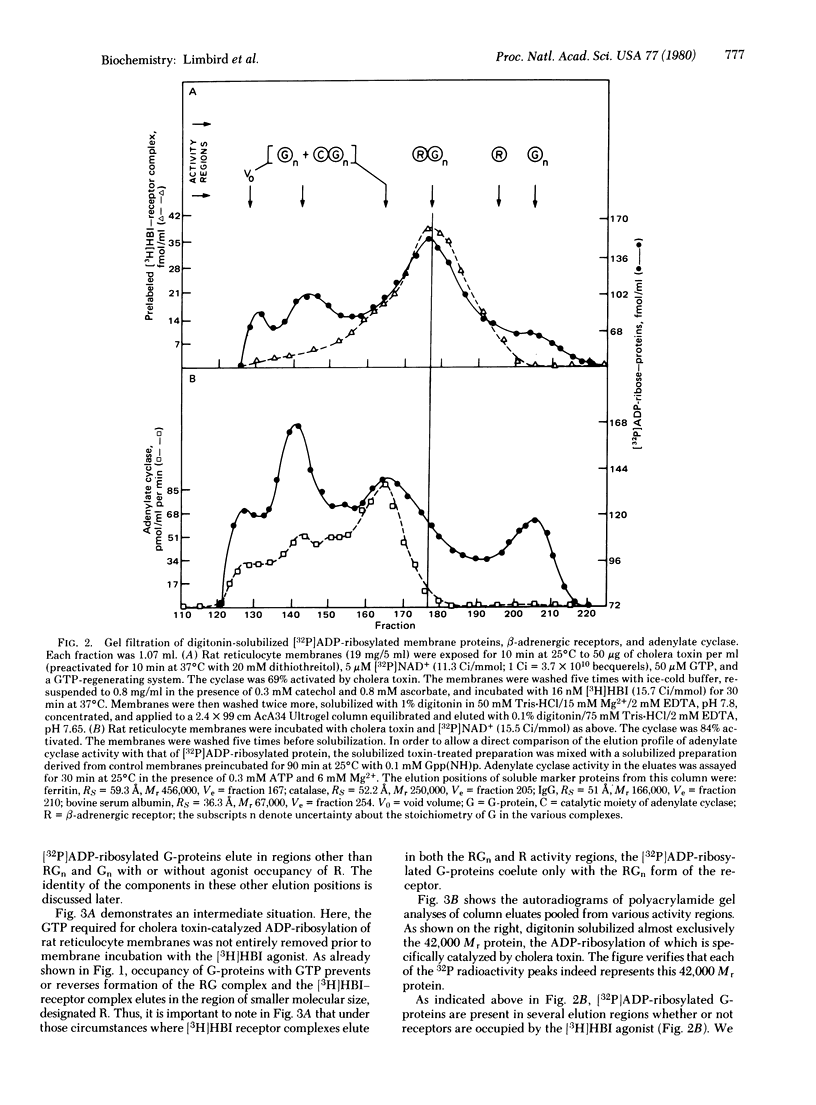
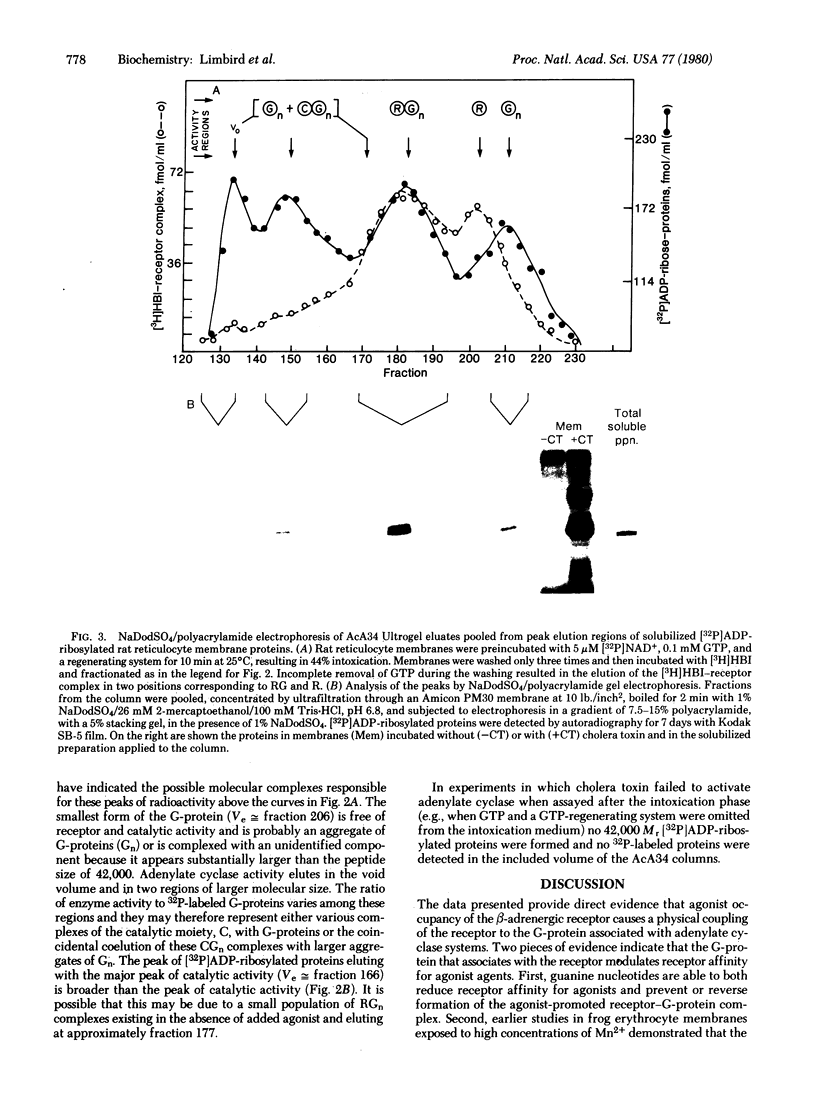
- Limbird L. E., Lefkowitz R. J. Agonist-induced increase in apparent beta-adrenergic receptor size. Proc Natl Acad Sci U S A. 1978 Jan;75(1):228–232. doi: 10.1073/pnas.75.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M. E., Van Arsdale P. M., Gilman A. G. An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. Mol Pharmacol. 1976 Mar;12(2):335–339. [PubMed] [Google Scholar]
- Pfeuffer T. Guanine nucleotide-controlled interactions between components of adenylate cyclase. FEBS Lett. 1979 May 1;101(1):85–89. [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J. Slowly reversible binding of catecholamine to a nucleotide-sensitive state of the beta-adrenergic receptor. J Biol Chem. 1977 Oct 25;252(20):7207–7213. [PubMed] [Google Scholar]




