Abstract
Preeclampsia, (PE) new onset hypertension with proteinuria during pregnancy, is associated with increased reactive oxygen species, the vasoactive peptide ET-1, T and B lymphocytes, soluble antiangiogenic factors sFlt-1 and sEndoglin (sFlt-1 and sEng) and agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA). One important area of investigation for our laboratory has been to determine what role AT1-AA play in the pathophysiology associated with PE. To achieve this goal we examined the effect of AT1-AA suppression on hypertension in response to placental ischemia as well as the effect of AT1-AA to increase blood pressure, ET-1, ROS, and sFlt-1 in normal pregnant rats (NP). We have demonstrated reductions in uterine perfusion pressure (RUPP) to be a stimulus for AT1-AA during pregnancy. We utilized the technique of B cell depletion to suppress circulating AT1-AA in RUPP rats and found that AT1-AA suppression in RUPP rats was associated with lower blood pressure and ET-1 activation. To determine a role for AT1-AA to mediate hypertension during pregnancy we have chronically infused purified rat AT1-AA (1:50) into NP rats and analyzed blood pressure and soluble factors. We have consistently shown that AT1-AA infused rats significantly increased AT1-AA and blood pressure above NP rats. This hypertension is associated with significantly increased ET-1 in renal cortices (11-fold) and placenta (4-fold), and approximately 2 to 3 fold increase in placental oxidative stress. Furthermore, antiangiogenic factors sFlt-1 and sEng were significantly increased in AT1-AA induced hypertensive group compared to the NP controls. Collectively, these data indicate an important role for AT1-AA stimulated in response to placental ischemia to cause hypertension during pregnancy.
Introduction
Preeclampsia is estimated to affect 5% to 7% of all pregnancies in the United States.1–3 Despite being one of the leading causes of maternal death and maternal and perinatal morbidity, the mechanisms underlying the pathogenesis of preeclampsia remain unclear. The initiating event in preeclampsia is postulated to involve Reduced Utero Placental Perfusion (RUPP) that leads to hypertension by mechanisms not yet elucidated.4–8 Recent developments in preeclamptic research confirm the initial speculations that this disease is an immunological disorder during pregnancy.1–4 In recent years we have learned that preeclamptic women display characteristics similar to various chronic inflammatory diseases such as elevated inflammatory cytokines, activated circulating immune cells, autoantibodies and most recently autoimmune associated T cells and cytokines (T helper 17 and IL-17, respectively) .3–13 Alterations in the renin angiotensin system plays an important role in the development of hypertension and preeclamptic women have long been known to have increased vascular sensitivity to angiotensin II without elevated angiotensin II or plasma renin activity. Recently activating autoantibodies to the angiotensin II type I receptor (AT1-AA) were found to be present in the serum of preeclamptic women at much higher levels than sera from non pregnant women or pregnant women that went on to have normal pregnancies. Therefore, in recent years much research has been performed to determine the role AT1-AA to mediate much of the pathophysiology associated with preeclampsia.12–21
The AT1-AA binds to and activates the AT1-receptor and induces signaling in vascular cells, including activating protein 1, calcineurin, and nuclear factor kappa-β activation, which can be blocked by an AT1 receptor antagonist.11–17 This signaling results in increased reactive oxygen species, sFlt-1 production and plasminogen activator inhibitor-1 all of which have been implicated in preeclampsia.14–17 In addition to being elevated during preeclampsia, the AT1-AA has also been reported to be increased in postpartum women. Hubel and colleagues demonstrated that the AT1-AA does not regress completely after delivery and that the increase in AT1-AA correlated with insulin resistance and sFlt-118. Although these autoantibodies have been linked to poor placentation and abnormal renal function, their role in the hypertensive state of preeclampsia have yet to be elucidated. Furthermore, the importance of AT1-AA after preeclampsia, especially in the context of increased cardiovascular risk, remains to be determined.The standard for measuring AT1-AA is by bioassay. Our research utilizes a bioassay employing rat neonatal cardiomyocytes. When the AT1-AA binds to the AT1 R on the cardiomyocyte it stimulates chronotropic events, similar to ANGII. The increase chronotropic event is expressed as an increase in beats per minutes (BPM) and is indicative of increased AT1-AA in a purified IgG preparation of serum. The AT1-AA is an IgG type 3 class antibody produced by mature B cells. For B cell maturation and IgG production, several co-stimulatory signals must be occur between the antibody producing B lymphocyte and CD4+T helper cells.19, 20 One of these includes stimulation of the CD20 receptor on the surface of the B cell.21,22 This recognition stimulates the B cell to enter the circulation and mass produce specific immunoglobulin. Inhibiting B lymphocytes from entering the circulation is a technique coined B cell depletion and is one way to attenuate or blunt antibody secretion and the deleterious effect seen in various autoimmune diseases .21–23 Most recently we utilized a new chemotherapeutic agent, rituximab, blocking the CD20 co-stimulatory molecule in our RUPP rat model of preeclampsia. 21,22,23 By administering rituximab we demonstrated an important role for endogenous AT1-AA to mediate blood pressure increases in response to placental ischemia. This rat model mimicking preeclampsia is induced by reductions in uterine perfusion pressure (RUPP) in pregnant rats (Fig. 1).24 Following a midline incision, the lower abdominal aorta is isolated and a silver clip (0.230mm ID) placed around the aorta above the iliac bifurcation. Branches of both the right and left ovarian arteries clipped using a silver clip (0.100mm ID). Rats are also surgically instrumented with a carotid catheter for subsequent arterial pressure measurement on day 18. At day 19 of gestation arterial pressure were recorded and blood samples collected. We have demonstrated that hypertension in the RUPP model is associated with increased circulating levels of the AT1-AA, endothelin-1 (ET-1), ROS, sFlt-1, sEng and elevated inflammatory cytokines TNF alpha and IL-6.25,26
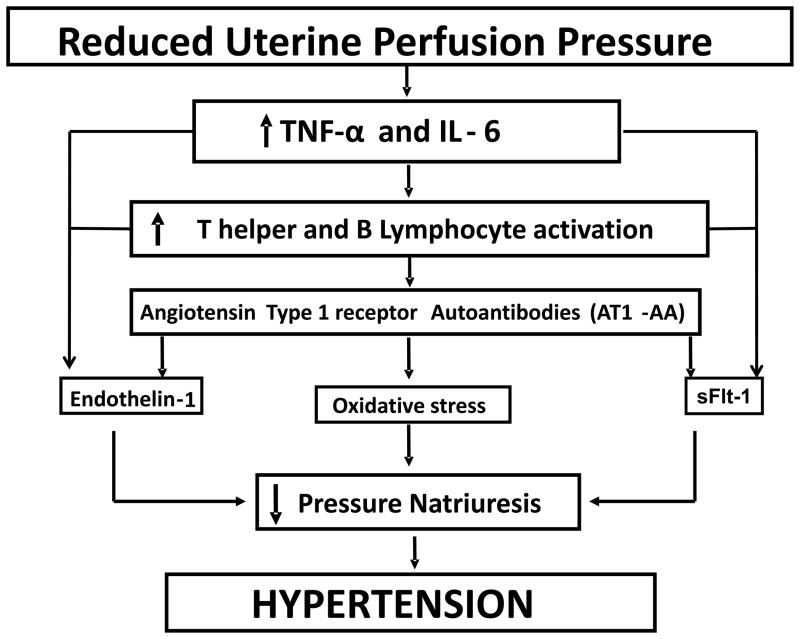
Figure 1.
Potential role for AT1-AA in mediating the pathophysiology of hypertension during preeclampsia. Inflammatory cytokines, TNF alpha and IL-6, stimulated in response to placental ischemia play an important role in the generation of ROS, sFlt-1 and enhanced ET-1 senthesis. One potential mechanism is stimulation of T helper 1 and B lymphocyte maturation and secretion of AT1-AA. AT1-AA has been shown by ours and other laboratories to mediate activation of ET-1, oxidative stress pathways, and sFlt-1 thereby contributing to the development of hypertension during pregnancy.
In addition we have demonstrated chronic infusion of inflammatory cytokines, either TNF alpha or IL-6, to stimulate hypertension and production of the AT1-AA in normal pregnant rats.25,27,28 Furthermore we have shown that infusion of the rat form of AT1-AA into normal pregnant rats during the later stages of gestation, increases blood pressure, the antiangiogenic factors sFlt-1 and sEng and placental oxidative stress and increased local transcription of the potent vasoconstrictor peptide, Endothelin-1 (Figure 1). 29–31 Therefore the focus of this review is to highlight previous studies demonstrating a role for the AT1-AA to mediate hypertension during pregnancy by activating several mechanisms we know to contribute to the phenotype of preeclampsia.
A role for AT1-AA to mediate hypertension during pregnancy
One important endothelial-derived factor elevated in the plasma of preeclamptic women and thought to play a role in preeclampsia is the vasoconstrictor peptide, endothelin-1 (ET-1). We have recently reported that infusion of purified rat AT1-AA, into normal pregnant rats increased serum AT1-AA, blood pressure, and tissue levels of preproendothelin.30 Preproendothelin is the first transcribed message of ET-1, and is the standard for measuring tissues levels of ET-1 utilizing real time pcr. Chronic infusion of purified rat AT1-AA into pregnant rats resulted in significant increases in mean arterial pressure, (MAP) from 99 ± 1 mmHg in NP controls to 119 ± 2 mmHg (P<0.001) in AT1-AA treated pregnant rats. In contrast, chronic infusion of a control IgG into pregnant rats had no effect on MAP compared to normal pregnant rats (102 ± 2 vs 100 ± 2 mmHg). In response to chronic AT1-AA infusion into NP rats, levels of AT1-AA increased from 0.88 +/− 5 beats per minute in normal pregnant controls to 14 +/− 1 beats per minute (AT1-AA infused) (P<0.01). Real time PCR was used to measure preproendothelin in the placenta and kidney and indicated that preproendothelin increased 11 fold in the renal cortices and 4 fold in the placentas of AT1-AA infused rats compared to control pregnant rats. Furthermore, AT1-AA induced hypertensive pregnant rats displayed renal vascular dysfunction.32 Renal endothelial function was tested using isolated renal interlobar arteries in a pressure myograph system. Vasodilatory responses to the endothelial dependent agonist acetylcholine (10–8 M) were impaired in AT1-AA rats (74±10%) compared to NP controls (95±5%, p<0.05). The response to sodium nitroprusside was not different.
To determine a role for endothelin activation and endothelial dysfunction in AT1-AA induced hypertension, a selective ETA receptor antagonist was administered for 5 days to normal pregnant control rats and pregnant rats chronically treated with AT1-AA. Normal pregnant rats treated with ETA receptor antagonist alone served as controls. Administration of an ETA receptor antagonist attenuated AT1-AA induced hypertension (100 ± 1 mmHg in AT1-AA + ETA pregnant rats vs 98 ± 2 mmHg in ETA rats). Furthermore, ETA receptor antagonist completely attenuated the vascular dysfunction observed in AT1-AA induced hypertensive pregnant rats. These data indicate the importance of the AT1-AA to cause hypertension by stimulating ET-1 and causing renal vascular endothelial dysfunction during pregnancy.
Clinical studies indicate that both plasma and amniotic fluid concentrations as well as placental sFlt-1 mRNA are increased in preeclamptic patients. 33–35 Moreover, increases in plasma levels of sFlt-1 in pregnant rodent models lead to phathophysiological alterations that mimic many of the characteristics observed in women with preeclampsia.36,37 Previous studies by Xia and Kellems et al demonstrated AT1-AA from preeclamptic women induces sFlt-1 production via AT1R and calcineurin/nuclear factor of activated T-cells signaling38. The authors demonstrated by injecting the IgG or affinity-purified AT1-AA from women into pregnant mice caused hypertension, proteinuria, glomerular endotheliosis, placental abnormalities, IUGR and elevated sFlt-112,38 . The onset of these symptoms were prevented by AT1R antagonist or an AT1-AA neutralizing seven-amino-acid epitope binding peptide12. In agreement with the Xia laboratory, we have confirmed that AT1-AA infusion increased blood pressure and plasma sFlt-1 in pregnant rats29. sFlt-1 was significantly increased in the AT1-AA induced hypertensive group (3920 +/− 800 pg/mL) compared to the NP control group (246 +/−38). In addition, sEng increased from 30 +/− 2.7 to 44 +/− 3.3 pg/ml. Placental explants from NP and AT1-AA infused pregnant rats were cultured overnight and sFlt-1 concentrations were determined from cell culture media. At three hours incubation basal sFlt-1 is measured from the cell culture media. Placental sFlt-1 secretion into the media was2480 +/− 257 (NP) which increased to 3421 +/− 125 pg/mL (AT1-AA). After 22 hours cultivation , secreted sFlt into the cell culture media , from NP placentas was 2189 +/− 221 compared to 3369+/−152 pg/mL from placental explants from AT1-AA induced hypertensive pregnant rats. Interestingly, there was no difference in sEng secretion from placental explants from NP and AT1-AA infused pregnant rats. Furthermore, in a later study, Xia and colleagues clearly demonstrate that the titer of AT1-AA not only correlate to the severity of the disease but that there was a strong correlation between AT1-AA activity to sFlt-1 in severe preeclamptics 39. Together, these data support the theory that immune activation and or AT 1 receptor activation, potentially due to AT1-AA, provides a link between antiangiogenic factors, sFlt-1 and sEng, and the progression of preeclampsia.
Reactive oxygen species are important immune signaling molecules that may mediate endothelial cell dysfunction and contribute to the pathophysiology of preeclampsia40. Placentas from women with preeclampsia contain more reactive oxygen species (ROS), such as lipid peroxides and malondialdehyde, than women with normal pregnancies. In addition, 8-isoPGF2α, the free form of isoprostane, is also increased in preeclamptic women. There is evidence of increased pro-oxidant activity formation along with decreased anti-oxidant protection during preeclampsia. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases are an important source of superoxide in neutrophils, vascular endothelial cells, and cytotrophoblast41. Increased expression of NAD(P)H oxidase subunits have been reported in both trophoblast and placental vascular smooth muscle cells in placental tissue of women with preeclampsia40. Moreover, higher placental NADPH oxidase activity has been reported in women with early-onset preeclampsia as compared with those with late-onset of disease which is consistent with the concept that early-onset preeclampsia is more dependent on placental dysfunction than the later-onset disease 31,40, 41. Thus, there is considerable evidence to suggest that activation of NADPH oxidase plays an important role in the placental oxidative stress associated with preeclampsia.
Recently we have shown that oxidative stress is important in mediating hypertension in the RUPP rat model of preeclampsia. 42 In this study we demonstrated that like preeclamptic women placental levels of 8-isoprostane and malondialdehyde are significant increased in RUPP rats compared to normal pregnant controls. In contrast, renal cortical SOD activity was decreased in RUPP rats compared to normal pregnant rats. Furthermore administration of Tempol, an SOD mimetic to RUPP rats lowered the blood pressure response to placental ischemia compared to control RUPP rats 42.
When evaluating the effects of AT1-AA on ROS, others have previously demonstrated that the AT1-AA stimulates ROS through NADPH oxidase in vitro from vascular smooth muscle cells.43 In the AT1-AA induced hypertensive pregnant rats we observed a significant increase in basal placental production of ROS in the AT1-AA induced hypertensive pregnant rats 159 +/− 29 RLUs compared to normal pregnant controls; 29 +/− 6 RLUs 31. We observed further increased placental ROS production in the setting of NADPH stimulation in AT1-AA infused rats (287 +/− 60 RLUs) compared to normal pregnant control rats (92 +/− 10 RLUs). In the group of AT1-AA exposed rats that were treated with tempol, we observed decreased basal and NADPH stimulated placental production of ROS (121 +/−13; 262 +/−21 RLUs) compared to our tempol treated controls (69 +/− 24; 141 +/− 33 RLUs). Similarly we observed attenuation of hypertension in the MAP of the AT1-AA exposed rats treated with tempol compared to normal pregnant control rats treated with tempol (AT1-AA + T rats was 109 +/− 3 vs 109 +/− 2 mmHg). These observations and those from other laboratories indicate that AT1-AA produced in response to placental ischemia is an important mediator of placental oxidative stress and hypertension during pregnancy.
A role for AT1-AA to mediate hypertension in response to placental ischemia
Although, we have recently reported that infusion of purified rat AT1-AA, into normal pregnant rats increased serum AT1-AA, blood pressure, and tissue levels of preproendothelin. sFlt-1 and ROS29,30,31,32, (Figure 1) the role of endogenous AT1-AA to mediate hypertension in response to placental ischemia remained unchallenged. To answer this question, we recently utilized the technique of B cell depletion to suppress lymphocyte entry into the circulation and subsequent antibody secretion23. RUPP rats treated with Rutiximab and having suppressed circulating B lymphocytes and AT1-AA exhibited less blood pressure increase in response to induced placental ischemia. Blood pressure was 103 +/− 1 mmHg in NP;103 +/− 3 mmHg in NP+R vs 133 +/−2 mmHg in RUPP rats and 118 +/−2 mmHg in RUPP+R. Furthermore, B cell depleted RUPP rats had lower tissue ET-1 transcript in renal cortices and placentas compared to RUPP control rats. Importantly, circulating B lymphocytes and AT1-AA decreased significantly in RUPP rats treated with Rituximab. Furthermore, ET-1 decreased 1.5 fold in kidneys and 4 fold in placenta of RUPP+R vs RUPP controls23. In addition, we had previously shown that circulating factors in serum from placental ischemic rats causes endothelial cell activation as measured by ET-1 secretion from vascular endothelial cells in culture44. This response is attenuated by AT1-receptor blockade, thus suggesting a role for the AT1-AA in response to placental ischemia to induce endothelial cell activation.44 Therefore, we examined our hypothesis by exposing endothelial cells to serum from B cell depleted, AT1-AA suppressed RUPP rats and determined media ET-1 excretion from endothelial cells. ET-1 from endothelial cells treated with RUPP serum increased 14% above that treated with NP serum and was the same as NP levels in response to B cell depleted RUPP serum.
Summary
There is growing evidence to suggest that dysregulation of the tissue based and circulating renin-angiotensin system (RAS) is involved in the pathophysiology of preeclampsia45–49. One mechanism by which this system may exert its effects has been suggested to occur through the excess production of an agonistic autoantibody to the angiotensin II type 1 receptor (AT1-AA). This autoantibody is thought to be a central mediator of several pathways in preeclampsia. (Figure 1) However, both the specific mechanisms that lead to excess production and all the mechanisms whereby AT1-AA increases blood pressure during pregnancy remain under investigation. However, we have recently provided evidence that placental ischemic induced hypertension in pregnant rats is associated with increased circulating levels of the AT1-AA25. In addition, we have demonstrated that chronic elevation of TNF alpha or IL-6 in pregnant rats is also associated with increased production of the AT1-AA25,28. Moreover, we discovered that the hypertension in response to placental ischemia in pregnant rats and in response to chronic infusion of TNF alpha or IL-6 in pregnant rats was markedly attenuated by antagonism of the AT1 receptor 25,28. Collectively, these novel findings indicate that placental ischemia, TNF alpha and IL-6 are important stimuli of AT1-AA production during pregnancy and that activation of the AT1 receptor appears to play an important role in the hypertension produced by placental ischemia and inflammatory cytokines in pregnant rats.
We have demonstrated that one important mechanism of AT1-AA induced hypertension to be stimulation of the endothelin-1 system leading to an eventual state of renal endothelial dysfunction and a resultant preeclamptic state 30,32. We were able to confirm the presence of this mechanism by demonstrating that increasing levels of AT1-AA to levels observed in preeclamptic women and in placental ischemic rats, led to increased blood pressure in pregnant rats by activation of the endothelin system and decreased vasodilation at the level of the renal interlobar artery in those rats exposed to the AT1-AA.32
Another pathway stimulated by excess AT1-AA during pregnancy is the sFlt-1 activation. We and others have demonstrated a role of AT1 receptor activation mediated by elevated AT1-AA as a potential stimulus for excess sFlt-1 and sEng during pregnancy29. We found both circulating sFlt-1 and sEng to be significantly elevated in AT1-AA-induced hypertensive pregnant rats and one potential source for this excess is the placenta.
Another important mechanism by which the AT1-AA exerts its hypertensive effects in pregnant rats is the increase of reactive oxygen species in the placenta31. We discovered that treatment with the superoxide dismutase mimetic, tempol, markedly attenuated the hypertension induced by chronic AT1-AA excess in pregnant rats. In addition to blunting the hypertensive response, placental production of ROS was decreased in the presence tempol.
Finally we have demonstrated that inhibiting secretion of the AT1-AA in placental ischemic rats blunted TNF alpha, hypertension and ET-1, all known to be important players in the pathophysiology of preeclampsia. B cell depletion in RUPP rats clearly demonstrated a role of endogenously produced AT1-AA to mediate hypertension and activation of ET-1 and TNF alpha secretion during pregnancy. Therefore, collectively these data indicate the importance of AT1-AA to mediate much of the pathophysiology associated with hypertension during pregnancy illustrating the importance of identifying the stimulus and mechanisms of production in the area of future preeclamptic research.
Acknowledgments
Sources of Funding
This work was supported by AHA SDG0835472N; NIH grants HL78147 and HL51971. RD is supported by the German Research Foundation (DFG 631/7-1).
Abbreviations
- MAP
Mean arterial pressure
- RUPP
Reductions in uterine perfusion pressure
- ET-1
endothelin-1
- sFlt-1
soluble fms like tyrosine kinase-1
- sEng
sEndoglin
- RAS
renin angiotensin system
- AT1-AA
agonistic autoantibodies to the angiotensin II type I receptor
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States. Am J Obste Gynecol. 1990;163:460–465. doi: 10.1016/0002-9378(90)91176-d. [DOI] [PubMed] [Google Scholar]
- 2.August P, Lindheimer MD. Pathophysiology of preeclampsia. Hypertension. 1995;142:2407–2426. [Google Scholar]
- 3.Chesley LC. Hypertensive Disorders in Pregnancy. New York, NY: Appleton-Century-Crofts; 1978. pp. 777–822. [Google Scholar]
- 4.Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 6.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 7.Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek MH, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;10:1113–1152. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 9.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Invest. 2003;10:82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 11.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 12.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, III, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110:1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 13.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 14.Bobst SM, Day MC, Gilstrap LC, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. Am J Hypertens. 2005;18:330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Dechend R, Homuth V, Wallukat G, Müller DN, Krause M, Dudenhausen J, Haller H, Luft FC. Agonistic antibodies directed at the angiotensin II, AT1 receptor in preeclampsia. J Soc Gynecol Investig. 2006;13:79–86. doi: 10.1016/j.jsgi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Dechend R, Müller DN, Wallukat G, Homuth V, Krause M, Dudenhausen J, Luft FC. Activating auto-antibodies against the AT1 receptor in preeclampsia. Autoimmun Rev. 2005;4:61–65. doi: 10.1016/j.autrev.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007;50:269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, Markovic N, Thadhani R, Luft FC, Dechend R. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007 Mar;49(3):612–7. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 19.Abbus A. In: Cellular and Molecular Immunology. Lichtman A, editor. Vol. 5. Philadelphia, Pennsylvania: Elsevier; pp. 189–215. [Google Scholar]
- 20.Panayi GS. B cells: a fundamental role in the pathogenesis of rheumatoid arthritis? Rheumatology (Oxford) 2005;44(Suppl 2):ii3–ii7. doi: 10.1093/rheumatology/keh616. [DOI] [PubMed] [Google Scholar]
- 21.Cianchini G, Corona R, Frezzolini A, Ruffelli M, Didona B, Puddu P. Treatment of severe pemphigus with rituximab: report of 12 cases and a review of the literature. Arch Dermatol. 2007;143:1033–1038. doi: 10.1001/archderm.143.8.1033. [DOI] [PubMed] [Google Scholar]
- 22.Cianchini G, Masini C, Lupi F, Corona R, De PO, Puddu P. Severe persistent pemphigoid gestationis: long-term remission with rituximab. Br J Dermatol. 2007;157:388–389. doi: 10.1111/j.1365-2133.2007.07982.x. [DOI] [PubMed] [Google Scholar]
- 23.LaMarca B, Wallace K, Herse F, Wallukat G, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: Role of agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA) Hypertension. 2011;57(4):865–871. doi: 10.1161/HYPERTENSIONAHA.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 25.LaMarca Babbette, Wallukat Gerd, Llinas Mayte, Herse Florian, Dechend Ralf, Joey P, Granger Autoantibodies to the Angiotensin Type I Receptor in Response to Placental Ischemia and Tumor Necrosis Factor-α in Pregnant Rats. Hypertension. 2008;52:1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaMarca BD, Gilbert J, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension. 2008 Apr;51(4):982–8. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005 Oct;46(4):1022–5. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 28.LaMarca B, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, Granger J. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. Inter. J of interferon, cytokine, and mediator res. 2011 Nov;3:65–70. doi: 10.2147/IJICMR.S22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens. 2010 Aug;23(8):911–6. doi: 10.1038/ajh.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamarca BB, Parrish MR, Ray LF, Murphy SR, Roberts, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in Response to Autoantibodies to the Angiotensin II Type I Receptor (AT1-AA) in Pregnant Rats: Role of Endothelin-1. Hypertension. 2009;54:905–90. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parrish MR, Wallace K, Tam Tam KB, Herse F, Weimer A, Wenzel K, Wallukat G, Ray LF, Arany M, Cockrell K, Martin JN, Dechend R, LaMarca B. Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension. Am J Hypertens. 2011 Jul;24(7):835–40. doi: 10.1038/ajh.2011.62. Epub 2011 Apr 7. [DOI] [PubMed] [Google Scholar]
- 32.Parrish MR, Ryan MJ, Glover P, Brewer J, Ray L, Dechend R, Martin JN, LaMarca B. Angiotensin II Type 1 Autoantibody Induced Hypertension during Pregnancy is associated with Renal Endothelial Dysfunction. Gen Med. 2011 Jun;8(3):184–8. doi: 10.1016/j.genm.2011.04.003. Epub 2011 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and egg” question. Endocrinology. 2004;145:4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 34.Karumanchi SA, Maynard SE, Stillman IE, Epstein FH, Sukhatme VP. Preeclampsia: a renal perspective. Kidney Int. 2005;67:2101–2113. doi: 10.1111/j.1523-1755.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 35.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 37.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou C, Ahmad S, Mi T, Abbasi S, Xia L, Day M, Ramin SM, Ahmed A, Kellems RE, Xia Y. Autoantibody From Women With Preeclampsia Induces Soluble Fms-Like Tyrosine Kinase-1 Production via Angiotensin Type 1 Receptor and Calcineurin/Nuclear Factor of Activated T-Cells Signaling. Hypertension. 2008;51:1010–1019. doi: 10.1161/HYPERTENSIONAHA.107.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension. 2010 Feb;55(2):386–93. doi: 10.1161/HYPERTENSIONAHA.109.140061. Epub 2009 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raijmakers MTM, Dechend R, Poston L. Oxidative Stress and Preeclampsia: Rationale for Antioxidant Clinical Trials. Hypertension. 2004;44:374–380. doi: 10.1161/01.HYP.0000141085.98320.01. [DOI] [PubMed] [Google Scholar]
- 41.Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16:93–104. doi: 10.1055/s-2007-1016256. [DOI] [PubMed] [Google Scholar]
- 42.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008 Oct;21(10):1152–6. doi: 10.1038/ajh.2008.239. Epub 2008 Jul 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 44.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 45.Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am J Physiol Renal Physiol. 2005;288:F614–F625. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 46.Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007;50:269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herse F, Dechend R, Harsem NK, Wallukat G, Janke J, Qadri F, Hering L, Muller DN, Luft FC, Staff AC. Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension. 2007;49:604–611. doi: 10.1161/01.HYP.0000257797.49289.71. [DOI] [PubMed] [Google Scholar]
- 48.Levesque S, Moutquin JM, Lindsay C, Roy MC, Rousseau F. Implication of an AGT haplotype in a multigene association study with pregnancy hypertension. Hypertension. 2004;43:71–78. doi: 10.1161/01.HYP.0000104525.76016.77. [DOI] [PubMed] [Google Scholar]
- 49.Mello G, Parretti E, Fatini C, Riviello C, Gensini F, Marchionni M, Scarselli GF, Gensini GF, Abbate R. Low-molecular-weight heparin lowers the recurrence rate of preeclampsia and restores the physiological vascular changes in angiotensin-converting enzyme DD women. Hypertension. 2005a;45:86–91. doi: 10.1161/01.HYP.0000149950.05182.a3. [DOI] [PubMed] [Google Scholar]