Abstract
We have defined functions of MEK in regulating gliogenesis in developing cerebral cortex using loss and gain of function mouse genetics. Radial progenitors deficient in both Mek1 and Mek2 fail to transition to the gliogenic mode in late embryogenesis, and astrocyte and oligodendroglial precursors fail to appear. In exploring mechanisms, we found that the key cytokine regulated gliogenic pathway is attenuated. Further, the Ets transcription family member Etv5/Erm is strongly regulated by MEK and Erm overexpression can rescue the gliogenic potential of Mek deleted progenitors. Remarkably, Mek1/2 deleted mice surviving postnatally exhibit cortices almost devoid of astrocytes and oligodendroglia and exhibit neurodegeneration. Conversely, expression of constitutively active MEK1 leads to a major increase in numbers of astrocytes in the adult brain. We conclude that MEK is essential for acquisition of gliogenic competence by radial progenitors, and that levels of MEK activity regulate gliogenesis in the developing cortex.
Keywords: Radial glia, MAPK signaling, glial progenitor, cytokines, Ets transcription factors
Introduction
The RAF/MEK/ERK pathway is among the most studied signaling cascades in biology by virtue of its critical, conserved functions in mediating the effects of extracellular factors on cell proliferation, differentiation, and function (Cargnello and Roux, 2011; Johnson and Lapadat, 2002). Importantly, genetic mutations in core pathway components including Mek1 (MAP2K1) and Mek2 (MAP2K2) cause cardiac, craniofacial and cutaneous abnormalities (CFC syndrome) in humans that are invariably associated with severe cognitive impairment (Rodriguez-Viciana et al., 2006; Samuels et al., 2009; Tidyman and Rauen, 2009). Nonetheless, many of the critical functions of MEK in brain development have yet to be defined.
Due to the broad availability of inhibitors of MEK, a key node in the pathway, the requirement for RAF/MEK/ERK signaling has been extensively studied in reduced preparations. However, despite a myriad of effects attributed to MEK inhibition, the functions mediated by MEK during mammalian development in vivo remain largely uncharacterized. Recently the generation of null and floxed alleles have provided the tools for decisive studies of the requirement of RAF/MEK/ERK signaling in key neurodevelopmental events in mice (Fyffe-Maricich et al., 2011; Galabova-Kovacs et al., 2008; Newbern et al., 2008; Newbern et al., 2011; Pucilowska et al., 2012; Samuels et al., 2008; Satoh et al., 2011; Zhong et al., 2007). However, interpretation of many of the analyses published so far has been complicated by the possibility of redundant functions of multiple family members at each level of the cascade and early death of many of the mutant lines.
Here we have determined the requirement for MEK in regulating gliogenesis in the developing cortex by deleting both Mek1 and Mek2 (Mek1/2) or overexpressing constitutively active Mek1 (caMek1) in radial progenitors at mid-embryogenesis. Radial progenitors are a self-renewing stem cell population, giving rise to both neurons and glia (Kriegstein and Alvarez-Buylla, 2009). Several lines of evidence have suggested key roles for the MEK/ERK signaling cascade in the regulation of neurogenesis. An upstream regulator of the pathway, SHP-2, is reported to be required for the proliferation of neural progenitors and neurogenesis (Gauthier et al., 2007; Ke et al., 2007). Further, a recent study showed a requirement for ERK2 in regulating the proliferation of neurogenic precursors (Pucilowska et al., 2012). Finally, neurotrophin-induced differentiation of cortical neuronal progenitors is critically dependent on a MEK-C/EBP pathway (Menard et al., 2002).
Consistent with the idea that MEK is required for neurogenesis, some studies have suggested that MEK/ERK signaling suppresses astrocytic differentiation (Menard et al., 2002; Paquin et al., 2005). On the other hand, in vitro studies show that FGF2, a powerful activator of MEK/ERK signaling, induces glial fate specification and enhances differentiation of glia induced by gliogenic signals (Morrow et al., 2001; Song and Ghosh, 2004). Moreover, analyses of Fgfr1 null mice demonstrate that FGF signaling is required for radial glia somal translocation and the formation of specialized astroglial populations required for commissure development (Smith et al., 2006). However, it remains unclear whether the effects of FGF signaling on glial development in mammalian brain are mediated by MEK/ERK, PI3K or other pathways downstream of FGF receptors. Interestingly, in Drosophila, glial differentiation in the developing eye requires FGF/Rolled (Drosophila MAPK) signaling acting via the Drosophila Ets transcription factor, Pointed (Franzdottir et al., 2009). Finally, a recent study of cortical astrocytic development showed proliferation of mature-appearing astrocytes in upper cortical layers, raising the possibility that FGFs or other growth factors might act at more than one stage in regulating the astrocytic lineage (Ge et al., 2012).
Genetic manipulation of MEK specifically in radial progenitors can address decisively the role of MEK/ERK MAPK signaling in cortical gliogenesis. To achieve this goal, we conditionally deleted Mek1/2 specifically in radial progenitors using NestinCre, hGFAPCre, and in utero electroporation (IUE) of Cre, and assessed gain of function by introducing caMek1 using similar methodologies. We have found that Mek1/2 deletion severely compromises radial progenitor fate transition into a gliogenic state. Our results show a striking reduction of glial progenitors in Mek1/2 deleted cortices and a failure of gliogenesis. Conversely we demonstrate that caMEK1 promotes precocious glial progenitor specification and that the effect is cell autonomous. In exploring the mechanism of the glial specification defect, we found the key cytokine regulated gliogenic pathway is attenuated. We further find that the Ets transcription family member Etv5/Erm is strongly regulated by MEK, has an expression pattern restricted to the ventricular zone (VZ) at E14, and rescues the gliogenic potential of Mek deleted progenitors. Finally, examination of brains postnatally in loss and gain of function mutant animals shows that numbers of glial cells in the cortex are strongly and persistently under the control of MEK signaling. We conclude that MEK is a key regulator of gliogenesis in the developing brain.
Results
Mek1/2 deletion leads to loss of radial glial properties
To study the function of MEK1/2 in cortical development, we bred Mek1 exon-3 floxed and Mek2−/− mice with a NestinCre line (see supplemental references). Three-allele deletion mutants, Mek1fl/+Mek2−/−NesCre and Mek1fl/flMek2+/−NesCre are viable and breed, though the latter are smaller than controls. The viability of mutants with a single wild type allele of either Mek1 or Mek2 suggests that MEK1 and MEK2 can significantly compensate for one another in the nervous system and that deletion of four alleles is necessary for complete elimination of pathway function.
In contrast, Mek1fl/flMek2−/−NesCre conditional mutants (referred to as Mek1,2\Nes) fail to acquire milk and die shortly after birth. Western blots show that levels of total and phosphorylated MEK1 protein are strongly reduced in mutant dorsal telencephalon lysates by E11.5 (Figure S1A). To our surprise, Mek1,2\Nes mutant brains did not exhibit gross morphological abnormalities at P0 (Figure S1B).
We assessed radial progenitor development at two stages, E13.5 and E17.5. Staining for the radial progenitor marker, Nestin, or the neural stem cell marker, Sox2, or proliferation as assessed by Brdu incorporation showed no major difference between E13.5 Mek1,2\Nes and WT cortices (Figure S1C–E′). However, a conclusion that MEK is dispensable for the initial behavior of radial progenitors should be tempered by the possible persistence of low levels of MEK1 protein within the cells at E13.5.
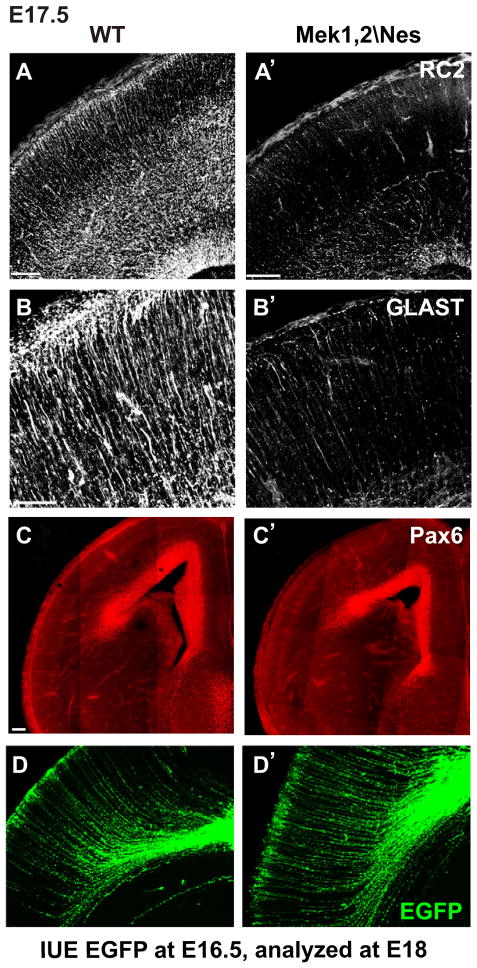
By late embryogenesis, mutant radial progenitors showed striking reduction in glial-like biochemical properties. Thus, we found dramatic reductions in the expression of RC2 and glial glutamate transporter (GLAST) in E17.5 mutant dorsal cortices (Figure 1A–B′). These marker reductions were not due to loss of the radial progenitor pool since immunostaining for the transcription factor Pax6, which labels progenitor nuclei, revealed a relatively normal pattern (Figure 1C–C′). Furthermore, electroporation of CAG (chick β-actin promoter/CMV enhancer) driven ires-EGFP plasmid (pCAG-EGFP) into WT and mutant cortices labeled a roughly comparable number of radial glia with grossly normal morphology including processes reaching the pial surface (Figure 1D–D′). Finally, we did not observe major changes in proliferation or survival as assessed by immunostaining of E17.5 cortices for phosphorylated histone-3 and activated caspase-3 (data not shown). Indeed mutant radial progenitors continued to generate neurons (See below). In summary, our studies indicate that Mek1/2 inactivation leads to a failure in the maintenance of glial-like properties of radial progenitors at late embryonic stages.
Figure 1. Radial glial properties are not maintained in the absence of Mek1/2.
(A–B′) The expression of radial progenitor markers, RC2 and GLAST, is strongly reduced in E17.5 Mek1,2\Nes mutant radial progenitors (n=3). In A–A′, scale bar=100μm. In B–B′, scale bar=50 μm. (C–C′) Pax6 immunostaining of radial progenitor nuclei is not obviously different between E17.5 Mek1,2\Nes and WT cortices. Scale bar=100 μm. (D–D′) EGFP labeled radial progenitors at E18 after in utero electroporation of pCAG-EGFP into E16.5 Mek1,2\Nes and WT dorsal cortices. Radial progenitors in Mek1,2\Nes animals exhibited a normal morphological appearance. See also Figure S1.
Mek1/2 deletion blocks glial progenitor specification
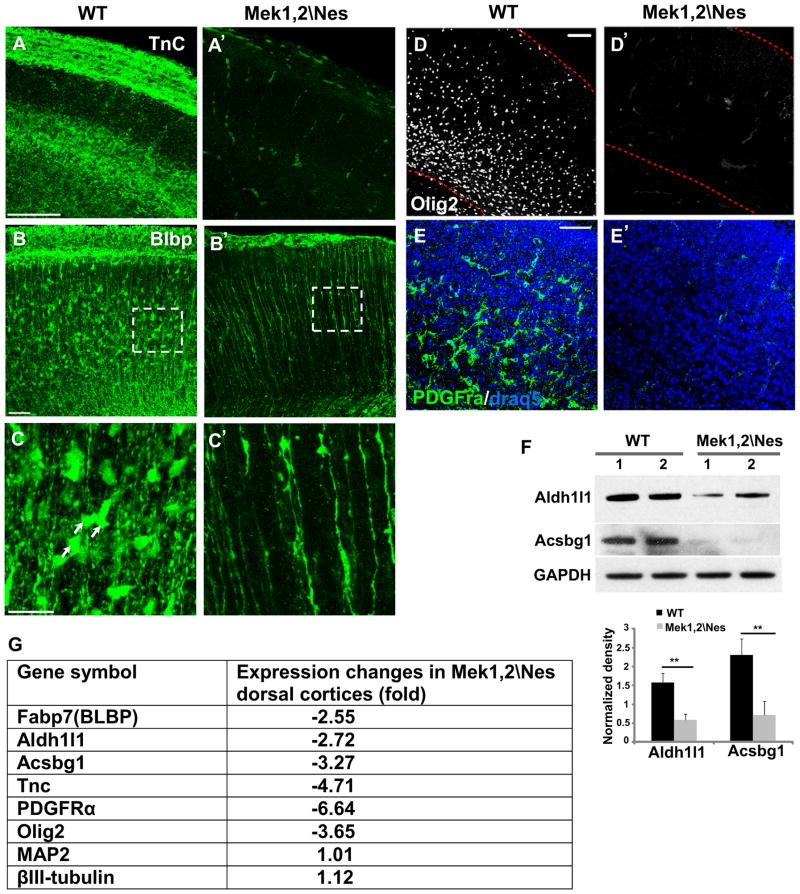
During late embryogenesis, radial progenitors undergo a transition from a neurogenic to a gliogenic mode. Since MEK clearly regulated glial characteristics of late embryonic radial progenitors, we tested whether the production of astrocyte and oligodendrocyte progenitors was affected. We analyzed the expression of multiple glial progenitor markers in E18.5-P0 brains. Tenascin C, an extracellular matrix glycoprotein secreted by astrocytes, was found to be dramatically reduced in E18.5 Mek1,2\Nes cortex (Figure 2A–A′). In P0 WT cortices, brain lipid binding protein (BLBP) immunolabeling revealed numerous astrocytic precursors in the developing dorsal cortical wall. In Mek1,2\Nes dorsal cortices, these BLBP+ soma were almost entirely absent, further documenting the failure in formation of astrocytic precursors (Figure 2B–C′). We confirmed the loss of astrocyte precursors by western blotting for the pan-cortical astrocyte marker aldehyde dehydrogenase 1 family member L1 (Aldh1l1) and grey matter astrocyte marker Acyl CoA Synthetase bubblegum family member 1(Acsbg1)(Cahoy et al., 2008) in lysates of P0 control and mutant cortices (Figure 2F).
Figure 2. Mek1/2 deletion disrupts glial progenitor specification.
(A–A′) Expression of Tenascin C, a glycoprotein secreted by astrocyte precursor cells, is strongly reduced in the E18.5 Mek1,2\Nes cortex. (B–B′) BLBP immunostaining revealed a dramatic reduction in astrocyte precursor number in mutant cortex. Scale bar=100 μm. (C–C′) High magnification images of BLBP+ cells from delineated areas in B and B′. BLBP+ cell somas, which represent astrocytes precursors, were nearly absent in the mutant cortex at P0. Scale bars=50μm. (n=3). (D–E′) The numbers of Olig2+ and PDGFRα+ oligodendrocyte progenitor cells are profoundly reduced in the E19.5 mutant dorsal cortex. (n=3). Scale bar=100μm. (F) Western blotting confirms reduced Aldh1l1 and Acsbg1 expression in Mek1,2\Nes dorsal cortex compared to WT. 1 and 2 indicate duplicate protein samples. (mean ± s.e.m; ** = p-value < 0.01, paired t-test). (G) Microarray analysis shows dramatic reductions of mRNAs expressed specifically in glial progenitors in Mek1,2\Nes dorsal cortex, while expression of the neuronal genes shown was not altered. See also Figure S2.
MEK was also required for the appearance of oligodendrocyte progenitor cells (OPCs). Thus, immunostaining for Olig2 and PDGFRα showed nearly complete loss of OPCs in Mek mutant dorsal cortices (Figure 2D–E′). Finally, microarray analyses of E18.5 WT and Mek1,2\Nes dorsal cortices further confirmed marked and specific decreases in a number of genes expressed in astrocyte precursors and OPCs (Figure 2G and Table 1). Besides glial related markers, we also found a dramatic reduction of Egfr mRNA in the mutant cortices (Table 1). EGFR was previously shown to play a critical role in determining progenitor gliogenic fate and gliogenesis (Sun et al., 2005; Viti et al., 2003). In contrast, expression of neuronal genes MAP2 and βIII-tubulin was not altered. These results demonstrate that MEK is necessary for radial progenitors to transition to the gliogenic mode.
Table 1. Comparison of mRNA expression profile between WT and Mek1/2 deleted brain.
Microarray assay of E18.5 WT and Mek1,2\Nes mutant dorsal cortices revealed strong reductions in markers of radial, astrocyte and oligodendrocyte progenitors.
| Gene Symbol | Gene Name | Fold changes |
|---|---|---|
|
Radial glia markers
| ||
| Fabp7(BLBP) | fatty acid binding protein 7, brain | −2.55 |
| Slc1a3(GLAST) | solute carrier family 1 (glial high affinity glutamate transporter), member 3 | −2.88 |
|
| ||
| Genes related to astrocyte development | ||
|
| ||
| EGFR | epidermal growth factor receptor | −2.36 |
| Aldh1l1 | aldehyde dehydrogenase 1 family, member L1 | −2.72 |
| Aldoc | Aldolase C | −2.88 |
| Acsbg1 | acyl-CoA synthetase bubblegum family member 1 | −3.27 |
| Tnc | tenascin C | −4.71 |
|
| ||
| Genes related to oligodendrocyte development | ||
|
| ||
| PDGFRα | platelet derived growth factor receptor, alpha polypeptide | −6.64 |
| Olig1 | oligodendrocyte transcription factor 1 | −4.47 |
| Olig2 | oligodendrocyte transcription factor 2 | −3.65 |
|
| ||
|
Transcription factors
| ||
| Nr2f2 | nuclear receptor subfamily 2, group F, member 2, Coup TFII | −2.50 |
| Etv5 | Ets translocation variant 5, ERM | −2.94 |
Since Mek1/2 mutant radial progenitors failed to differentiate into glial progenitors, we reasoned that mutant progenitors may remain in the neurogenic mode and continue to produce neurons. To test this hypothesis, we performed Brdu birthdating analysis at E17.5 and found an increase in neurogenesis in the mutant cortex. Co-labeling of Brdu and Cux1 showed a 42% increase in upper layer neuron production in E17.5 Mek1,2\Nes cortex (Figure S2A–E). These data suggest that MEK signaling is required for radial progenitors to exit the neurogenic mode. We also noticed a delayed migration of these late born neurons. Quantification showed 70% of neurons born in E17.5 WT cortex migrate to upper cortical layers (bin1–2) by P0, while only 54% of E17.5-born neurons migrate to the upper cortical layers in the mutant mice (Figure S2F). This migration delay could be secondary to the delayed birthday of the neurons or to a failure in the maintenance of important RG properties.
MEK functions are cell autonomous and instructive
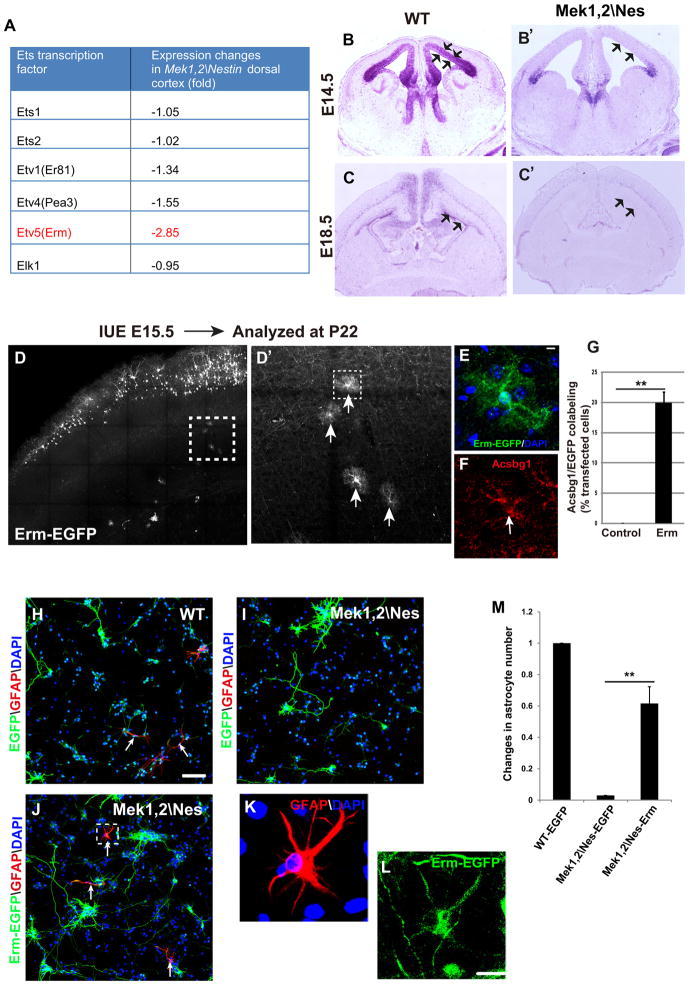
To determine if the failure of glial progenitor specification is cell autonomous, we performed mosaic loss of MEK function by electroporating pCAG-EGFP or pCAG-Cre-EGFP plasmids into E15.5 Mek1fl/fl Mek2−/− radial progenitors, followed by organotypic cortical slice culture for 4 days. We then assessed the proportion of transfected cells that coexpressed the astrocyte precursor marker, BLBP. After transfection of EGFP, we found that 6.13±0.44% of transfected cells expressed BLBP. In contrast, after transfection of Cre, only 1.15±0.29% of transfected cells were BLBP+ (Figure S3A–C). This result suggests that MEK plays a cell autonomous role in regulating glial progenitor specification.
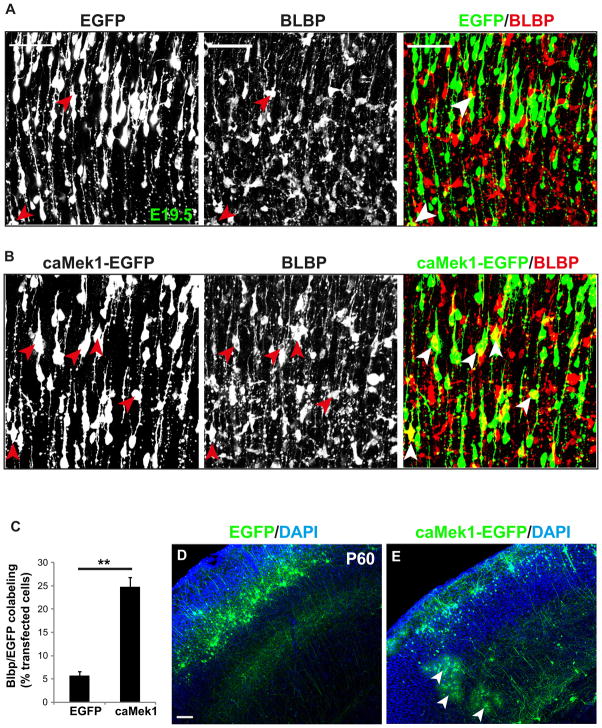
Our results strongly indicated that MEK is required for gliogenesis, however, it was unclear whether overactivation of MEK was sufficient to drive gliogenesis. We tested this by introducing pCAG-caMek1-EGFP into E15.5 WT radial progenitors by IUE and quantifying the proportion of transfected cells that express BLBP at E19.5. In EGFP transfected cortices, 5.68±0.94% of transfected radial progenitors became astrocyte precursors. In caMek1 transfected cortices, the proportion of radial progenitors that became astrocyte precursors increased robustly to 24.78±2.07% (Figure 3A–C).
Figure 3. Mosaic analysis of Mek gain of function in glial progenitor specification.
(A–B) E15.5 dorsal cortices were electroporated with EGFP (A) or caMek1-EGFP (B) and subsequently immunostained with BLBP to label astrocyte precursors at E19.5 (arrow head). Scale bar=50μm. (C) Quantification showed that 5.7% of EGFP transfected cells are BLBP+, while overexpression of caMek1 significantly increased the proportion of BLBP co-labeled cells to 24.8% (mean ± s.e.m; ** = p-value < 0.01, paired t-test). N=400–500 transfected cells from three independent trials. Arrowheads indicate BLBP+ transfected cells. (D–E) Overexpression of caMek1 in E15.5 radial progenitors induced formation of large numbers of mature astrocytes in P60 dorsal cortices (E), while no astrocytes were labeled in EGFP electroporated cortices (D). Arrowheads indicate astrocytes that express caMek1. Scale bar=100μm. n=5. See also Figure S3.
In additional experiments, we assessed the ability of caMek1 to convert radial progenitors into mature astrocytes by allowing mice electroporated at E15.5 to survive until P60. Normally, electroporation of EGFP at E15.5 results in robust labeling of neurons in layer II–III with virtually no astrocytes labeled. In striking contrast, transfection of caMek1 results in multiple mature astrocytes labeled in every section (Figure 3D–E). The astrocyte identity is confirmed by Acsbg1 immunostaining (Figure S3D–E). Overall, approximately 50% of labeled cells were astrocytes. Intriguingly, labeled neurons occupied a very superficial position in layer II consistent with overactive MEK stimulating accelerated progression of radial progenitor development (see below).
Taken together, these data suggest that the regulation of glial progenitor specification by MEK is cell autonomous and demonstrate that MEK is both necessary and instructive for glial specification.
Deletion of Mek1/2 impairs gliogenic signaling
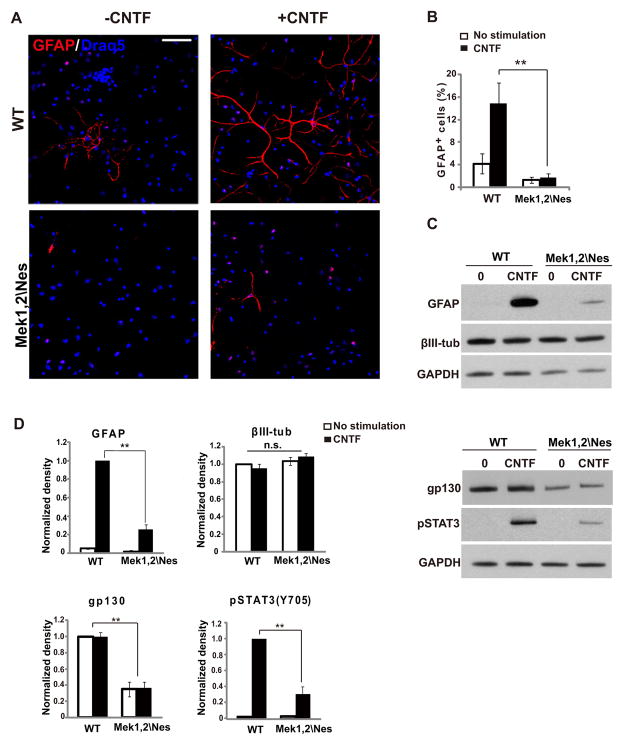
To assess the mechanisms by which MEK regulates glial progenitor specification, we examined the response of Mek deleted cortical cells to a gliogenic stimulus in vitro. Cytokine signaling through the LIFRβ/gp130-JAK-STAT pathway is a critical stimulus for astrocyte differentiation. We therefore induced astrocyte differentiation with CNTF (100ng/ml) in WT or Mek1,2\Nes E17.5 cortical cultures. In WT cultures without CNTF, 4% of the cells differentiated into GFAP+ astrocytes after 5 days. Adding CNTF increased astrocyte differentiation as expected to 15%. In mutant cultures, only 1% of cells differentiated into astrocytes without CNTF stimulation, which increased to only 2% after stimulation with CNTF (Figure 4A–B). Confirming the cell counting, western blotting showed that the level of GFAP protein in Mek1,2\Nes samples was only 20% of controls, while levels of the neuronal marker βIII-tubulin were comparable to controls (Figure 4C–D).
Figure 4. Gliogenic signaling is impaired in Mek1/2 deleted progenitor cultures.
(A) Representative images of cortical progenitor cultures derived from the E17.5 Mek1,2\Nes mutant and WT dorsal cortices. Cultures were stimulated with CNTF for 5 days and immunolabeled for GFAP (red) and Draq5 (blue). CNTF did not induce substantial levels of astrocyte differentiation in mutant cultures. Scale bar= 50um. (B) Quantification of the percentage of GFAP+ cells after 5 days of culture (mean ± s.e.m; n=4(>500 cells); ** = p-value < 0.01, paired t-test). (C) Upper panel: Western blotting revealed strong reductions in GFAP expression, while expression of the neural marker, βIII-tubulin, was not affected in mutant cultures when compared to WT. Progenitors were cultured for 3 days with CNTF as indicated. Lower panel: Further analysis of CNTF stimulated progenitor cultures by Western blotting demonstrated that gp130 expression and phosphorylated STAT3 levels in E17.5 Mek1,2\Nes mutant culture were profoundly reduced when compared to WT. Progenitors were cultured for 3 days prior to treatment with CNTF for 15 minutes as indicated. (D) Quantification for levels of GFAP, βIII-tubulin, gp130 and pSTAT3 in (C). (Mean ± s.e.m; n=3; ** = p-value < 0.01; n.s.: not statistically significant; paired t-test).
The STAT3 Tyrosine 705 site is directly phosphorylated by the upstream kinase JAK following CNTF stimulation (Liu et al., 1998). Importantly, STAT3 phosphorylation on Tyrosine 705 was dramatically reduced in mutant cultures, indicating that the CNTF gliogenic pathway is impaired (Figure 4C–D). The reduction of tyrosine phosphorylation is not likely due to a direct link between MEK signaling and STAT3. To better understand the interaction between MEK and CNTF signaling, we examined the expression levels of the major components in CNTF pathway. We found that the expression of gp130, the co-receptor for CNTF, was dramatically attenuated, thus hindering gliogenic signaling at the first step of the cascade (Figure 4C–D). These results demonstrate that the requirement for MEK is cell autonomous and that Mek mutant progenitors fail to acquire gliogenic competence.
Erm promotes glial progenitor specification
We interrogated our microarray data set from E18.5 Mek1,2\Nes cortices to identify candidate transcription factors downstream of MEK that may mediate glial progenitor specification. We noted a profound decrease in the expression of the Ets transcription factor family member-Ets related molecule (Etv5/Erm) (Figure 5A). Erm is a promising candidate to regulate glial development as PEA-3 family member transcription factors are known FGF/MAPK targets with multiple roles in the regulation of nervous system development.
Figure 5. Erm promotes glial progenitor specification and rescues gliogenesis of Mek deleted progenitors in vitro.
(A) Microarray analysis of E18.5 WT and Mek1,2\Nes mutant cortices showed that Etv5 (Erm) mRNA expression was strongly reduced in mutants, while other Ets family members were changed to a lesser extent. (B–B′) In situ hybridization revealed that Erm is predominantly expressed in VZ of E14.5 WT cortex (arrows); however, Erm expression in Mek1,2\Nes VZ was profoundly reduced. (C–C′) Erm is expressed broadly in E18.5 WT dorsal cortex VZ (arrows), hippocampus, and deep cortical layers. In mutant brains, Erm expression was dramatically downregulated in the VZ but not in deep cortical layers. (D–D′) Overexpression of Erm-EGFP in E15.5 radial progenitors promoted a dramatic increase in the number of mature astrocytes in postnatal day 22 dorsal cortices. D′ is the delineated areas in D. Arrows in D′ indicate EGFP labeled astrocytes. (E–F) High magnification images of the astrocyte from the delineated area in D′ that expresses Erm-EGFP and Acsbg1. (G) The proportion of EGFP+ astrocytes as quantified in EGFP or Erm-EGFP transfected cortices. (Mean ± s.e.m; ** = p-value < 0.01; paired t-test, n=4). (H–J) Representative images showing electroporation of radial progenitors ex vivo at E14.5 followed by dissociation and CNTF stimulation for 5 days. Some WT progenitors differentiated into astrocytes (arrows in H). However, EGFP transfected Mek1,2\Nes mutant progenitors did not become astrocytes (I). Electroporation of Erm-EGFP into mutant progenitors rescued astrocyte differentiation (arrows in J). Scale bar=100μm. (K–L) High magnification images from delineated area in (J) clearly show that Erm-EGFP transfected cells expressed GFAP. Scale bar= 20μm. (M) Quantification shows that in Erm transfected mutant cultures, astrocyte number reached 60% that of the EGFP transfected WT cultures. (Mean ± s.e.m; N=4. **=p value<0.01, paired t test). See also Figure S4.
In-situ hybridization was performed to visualize Erm expression in WT and mutant brains. Remarkably, we found that Erm is intensely expressed in the WT VZ at E14.5 (Figure 5B, arrows), which correlates with the enriched phosphorylated-ERK1/2 in the VZ at this stage (Pucilowska et al., 2012; Seuntjens et al., 2009). At E18.5, Erm continues to be expressed in the WT VZ (Figure 5C, arrows) and is also expressed in deep cortical layers. Strikingly, Erm expression in the VZ was profoundly reduced in both E14.5 and E18.5 Mek1,2\Nes cortices (Figure 5B′ and 5C′). Interestingly, Erm expression was maintained in the deep cortical layers of mutant cortices, suggesting that MEK regulation of Erm expression is specific to radial progenitors.
To test whether Erm plays a role in glial progenitor specification, we overexpressed Erm by IUE of a pCAG-Erm-GFP plasmid into E15.5 dorsal cortical radial progenitors. The proportion of EGFP and GLAST co-expressing astrocyte precursors was then assessed at E19.5. We found overexpression of Erm led to a 3.4 fold increase in the proportion of cells that became GLAST+ astrocyte precursors when compared to cells transfected with EGFP alone (Figure S4A–B). In addition, many more Erm expressing cells were present in the VZ/SVZ, possibly due to impaired neurogenesis.
These results indicate that Erm is instructive for the specification of astrocyte precursors. To assess whether these precursors overexpressing Erm further differentiate into mature astrocytes, we allowed some animals to survive until P22. In contrast to cortices electroporated with pCAG-EGFP at E15, which display no labeled astrocytes (Figure S4C), Erm overexpression induced the formation of large numbers of astrocytes (20% of transfected cells) (Figure 5D–D′, 5G and Figure S4C′). Further, colabeling with Acsbg1 confirmed the astrocyte identity (Figure 5E–F).
To explore whether Erm is a major transcriptional mediator of MEK signaling on gliogenesis, we expressed Erm in Mek1,2\Nes mutant progenitors to determine whether Erm is able to rescue the gliogenic defect. Because Mek mutant mice die at early postnatal stages, electroporations were performed ex vivo and cortices dissociated so that astrogenesis could be induced by CNTF. We introduced pCAG-Erm-EGFP into mutant progenitors at E14.5, the same time point at which expression was dramatically downregulated in vivo in Mek mutants. Although induction of astrogenesis by CNTF is less efficient at this early stage than at E17.5, numerous GFAP positive cells can be observed in WT cultures 5 days after addition of CNTF (100ng/ml) (Figure 5H). Consistent with the lack of gliogenesis in E17.5 Mek1,2\Nes mutant cultures (Figure 4), E14.5 mutant progenitors did not differentiate into astrocytes in the presence of CNTF stimulation (Figure 5I). Strikingly, expression of Erm largely rescued astrocyte number in the mutant cultures (Figure 5J–M). This result demonstrates that Erm mediates MEK regulation of CNTF-induced astrogenesis.
To further test whether Erm is required for MEK mediated gliogenesis, we co-electroporated dominant negative Erm (DN-Erm) with caMek1-EGFP into E14.5-E15.5 WT progenitors to explore whether DN-Erm could inhibit caMEK1 induced astrocyte differentiation. The DN-Erm plasmid contains the Ets domain of Erm but lacks the transcription activation domain (Hasegawa et al., 2004). Consistent with our in vivo results (Figure 3E), caMek1 overexpression dramatically increased astrocyte number to 2.5 fold that in EGFP-transfected cultures. Strikingly, expression of DN-Erm and caMek1 together abolished the ability of caMEK1 to induce astrogenesis (Figure S4D–G). Western blotting of GFAP protein confirmed that DN-Erm blocked caMEK1 induced astrocyte differentiation (Figure S4H).
In conclusion, our results demonstrate that MEK regulates Erm expression in radial progenitors and that Erm is an important transcriptional mediator of MEK regulated gliogenesis.
Mek1/2 deletion disrupts the generation of key midline glial populations
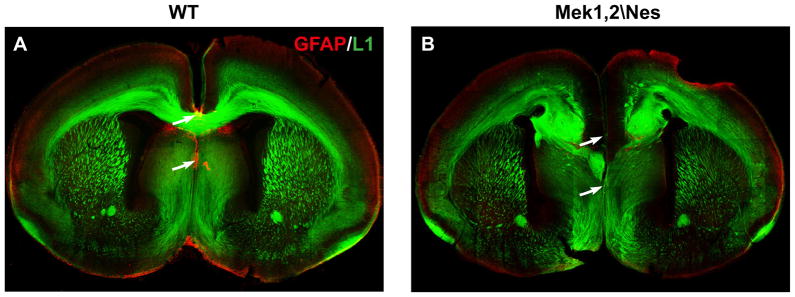
To confirm that the loss of MEK signaling in radial progenitors leads to a failure in the appearance of mature glia, we analyzed the development of specific early appearing glial populations in vivo. Though Mek1,2\Nes mutant mice die before the main wave of astrogenesis begins, we were able to analyze the formation of earlier born astrocytes along the cortical midline. In WT brains, GFAP staining labels three populations of midline astroglia at P0; the astroglia-indusium griesium (IG), the glial wedge and midline zipper glia (MZG) (Figure 6A). Strikingly, astroglia cells in IG and MZG were completely missing (arrows) in mutant cortex and the glial wedge did not form normally (Figure 6B).
Figure 6. Disrupted midline astroglia and absence of corpus callosum in Mek1/2 deleted cortex.
GFAP and L1 double immunostaining labels midline astroglial populations and the corpus callosum in P0 WT and Mek1,2\Nes mutant cortices (n=5). Note that in mutants (B), axons in the corpus callosum form Probst bundles, a hallmark of callosal agenesis. Arrows indicate the indusium gresium (IG) and midline zipper glia (MZG) in WT cortex (A), which were missing in Mek1,2\Nes mutant cortex (B). See also Figure S5.
As it is known that midline astroglia are critical for commissural axons to cross midline (Paul et al., 2007), we performed L1 (N-CAM) immunostaining to visualize the major axon tracts in the brains of P0 mice. We observed that two dorsal telencephalic commissures, the corpus callosum and hippocampal commissure, did not cross the midline between the two hemispheres in the Mek1,2\Nes brains (Figure 6B and data not shown). Instead the callosal axons formed Probst bundles, a hallmark of callosal agenesis. This phenotype exhibited complete penetrance. These data provide additional evidence for a glial specification defect in Mek deficient dorsal cortices.
Mek1/2 deletion results in a persistent failure of gliogenesis
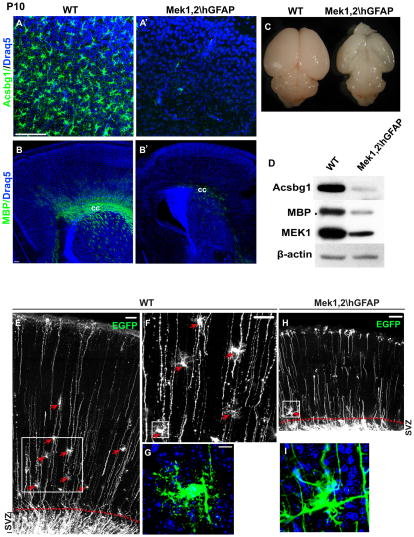
The early lethality of Mek1,2\Nes mice did not allow us to test the possibility that gliogenesis was simply delayed rather than prevented. To generate a mutant model that survives into the postnatal period, we utilized the hGFAPCre line, which recombines later (E12.5) than NesCre in dorsal telencephalic progenitors (Anthony and Heintz, 2008). Importantly, Mek1,2\hGFAP mutants survive until P10. The mutant brains appear grossly normal at birth but were dramatically smaller than controls by P10 (Figure 7C). Consistent with findings in Mek1,2\Nes mutants, the generation of BLBP+ astrocyte precursors and PDGFRα+ OPCs were severely suppressed in E19.5 Mek1,2\hGFAP dorsal cortices (Figure S5A–B′). GFAP strongly labels astrocytes in the white matter at postnatal stages, while Acsbg1 staining labels gray matter astrocytes. In P3 mutant dorsal cortices, both Acsbg1+ and GFAP+ astrocytes were nearly absent (Figure S5C–C′). At P10, the loss of Acsbg1+ astrocytes was clearly persistent in mutant dorsal cortices and there was a profound decrease in MBP labeling in the corpus callosum (Figure 7A′ and 7B′). Western blotting at P10 further confirmed dramatic and persistent reductions of Acsbg1 and MBP protein in mutant dorsal cortices (Figure 7D).
Figure 7. Deletion of Mek1/2 results in persistent disruption of gliogenesis.
(A–B′) In the P10 mutant brain, the number of Acsbg1+ astrocytes and MBP+ oligodendrocytes in dorsal cortices were dramatically reduced. cc: corpus callosum. (C) Mek1,2\hGFAP brains are smaller and appear transparent at P10. (D) Western blots confirmed the dramatic reductions of Acsbg1 and MBP expression in Mek1,2\hGFAP dorsal cortices. (E–G) EGFP was electroporated at P1 to follow maturation of radial progenitors in control and Mek deleted animals. EGFP-expressing mature appearing astrocytes were readily apparent in P8 WT dorsal cortices (arrows in E and F), F is the delineated area in E, G is the delineated area in F. Scale bars=100 μm in E and F, 10 μm in G. (H–I) EGFP transfected Mek1,2\hGFAP cortices. Note that no mature-appearing astrocytes are observed. I is the delineated area in H. EGFP expressing cells remained close to SVZ and did not exhibit typical astrocytic morphology (I). N=4. See also Figure S6.
Coincident with the persistent failure of gliogenesis, the mutant cortex was dramatically reduced in size (Figure 7C). Neurodegeneration was apparent and likely due to lack of glia support, as no degeneration was observed when Mek is specifically deleted in neurons (data not shown). These findings strongly suggest that gliogenesis is permanently blocked in the absence of MEK signaling and that cortical neurons require glial support for survival.
In order to rule out the possibility that deletion of Mek1/2 merely reduces glial marker expression without affecting glial specification, we electroporated pCAG-EGFP construct into radial progenitors at postnatal day 0–1 and assessed the cell fate over 7 days. As recently reported (Ge et al., 2012), EGFP-expressing cells with a clear astrocyte morphology that expressed Acsbg1, were readily observed in deeper cortical layers (Figure 7E–G and data not shown). In contrast, in Mek deleted cortices, mature astrocytes were not observed after electroporation at P0–1(Figure 7H–I). Many transfected cells in controls and Mek mutants remained in the SVZ. Among the proportion of cells that migrated out to the cortical plate, in controls 97% of transfected cells (total 115 cells) exhibited a clear astrocyte morphology, whereas in mutants, 80% of labeled cells (total 92 cells) were NeuN+ neurons which were rarely observed in WT (Figure 7E–G and Figure S6A–D). The remaining 19.6% in the mutant cortex were non-neuronal cells near the SVZ border that exhibited an abnormal morphology (Figure 7H–I).
To further assess cellular morphologies in Mek deleted brains, we injected an Adeno-associated virus expressing EGFP (AAV-EGFP (serotype 9)) intraventricularly at P0 to label astrocytes in vivo. We found that AAV9 labeled both neurons and astrocytes when delivered intraventricularly at an early postnatal stage. In WT cortices, AAV-EGFP labeled numerous astrocytes that co-expressed Acsbg1, while in Mek1,2\hGFAP cortices, virtually no cells with a typical astrocytic morphology were visualized (Figure S6E–E′). The few AAV-GFP labeled non-neuronal cells did not exhibit a typical cortical astrocyte morphology (Figure S6F–F′), failed to elaborate extensive processes, and resembled the aberrant non-neuronal cells labeled after electroporation at P0 (Figure 7I).
We also examined the effect of Erk1/2 deletion in gliogenesis. Loss of radial progenitor markers was noted previously in Erk1,2\NesCre mice (Imamura et al., 2010). Erk1,2\hGFAP mutants qualitatively phenocopy Mek1,2\hGFAP mutants in glial development as expected. Thus, we observed that Acsbg1+ staining was markedly reduced in P20 Erk1,2\hGFAP mutant brains compared to controls (Figure S6 G–G′). However, we consistently observed that Erk1,2\hGFAP survived roughly a week longer than Mek mutants. Further, some mutant phenotypes (e.g. absence of corpus collosum in NesCre deleted mutants, data not shown) were more variable than in Mek mutants. The milder phenotype exhibited by the Erk mutants may be due to a relatively delayed recombination of Erk2 floxed allele or delayed protein degradation in comparison to that observed in Mek mutant mice, although other explanations are possible (see discussion).
Levels of MEK activation regulate glial number
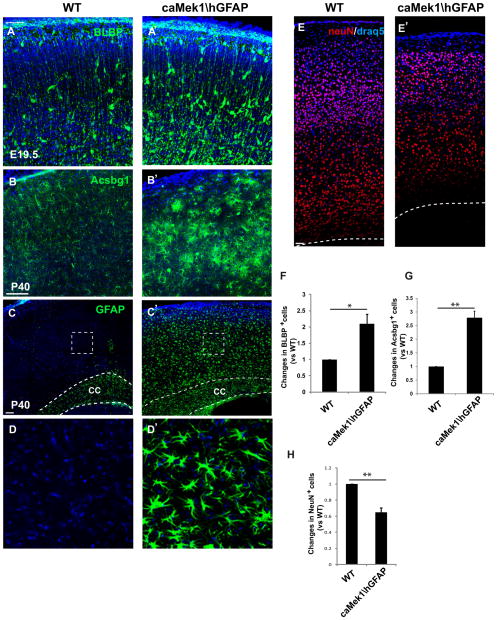
To assess whether enhanced MEK signaling might lead to increased number of glia in the postnatal brain, we crossed the CAG-loxpSTOPloxp-Mek1S218E,S222E line (Krenz et al., 2008) with hGFAPCre (referred to as caMek1\hGFAP) in order to hyperactivate MEK signaling in radial progenitors. Strikingly, MEK hyperactivation in radial progenitors leads to a marked increase in the production of astrocyte precursors and mature astrocytes. We found a more than two fold increase of BLBP+ astrocyte precursor number in caMek1\hGFAP dorsal cortex at E19.5 (Figure 8A–A′ and 8F). Coincident with the increased astrocyte precursor production, neuron numbers in caMek1\hGFAP dorsal cortex were significantly reduced (Figure 8E–E′ and 8H). This reduced neurogenesis is consistent with the idea that hyperactive MEK accelerates radial progenitor progression into a gliogenic mode and prematurely terminates neurogenesis.
Figure 8. Overactivation of MEK leads to excessive gliogenesis and reduced neurogenesis.
(A–A′) BLBP immunostaining showed a robust increase in astrocyte precursor numbers in E19.5 caMek1\hGFAP dorsal cortices compared to WT. (B–B′) Acsbg1 immunostaining showed a profound increase of astrocyte number in caMek1\hGFAP dorsal cortices compared to WT. (C–C′) GFAP+ labeling is typically restricted to white matter (corpus collosum) in WT at P40, while GFAP + cells fill the entire thickness of ca-Mek1\hGFAP dorsal cortex. (D–D′) Higher magnification images of GFAP+ cells from delineated areas in C and C′. (E–E′) Immunostaining of NeuN revealed a marked decrease of neuron number in caMek1\hGFAP dorsal cortices. (F–H) Quantification showed a significant increase of BLBP+ astrocytic precursor number in E19.5 caMek1\hGFAP dorsal cortices (F), a profound increase in the number of Acsbg1+ astrocytes (G) and a dramatic reduction of NeuN+ neuron in P40 caMek1\hGFAP dorsal cortices (H). n=3. Scale bars= 100 μm, except in E–E′ it represents 50 μm. N=3. *=p value <0.05, **=p value <0.01, paired t test. See also Figure S7.
We also observed a major increase in the number of astrocytes in mature brain as manifested by increases in the numbers of Acsbg1 and GFAP-labeled cells at P40 (Figure 8B–D′ and 8G). We further found that MBP staining was strikingly enhanced throughout all cortical layers in caMek1\hGFAP mice (Figure S 7A–B′), indicating changes in oligodendrocyte production (Fruttiger et al., 1999), MBP levels (Ishii et al., 2012) or both. The increase in GFAP-labeled cells was particularly remarkable. GFAP+ astrocytes are normally restricted to white matter in mature WT mice. In caMek1\hGFAP dorsal cortices, GFAP+ cells filled the entire cortex occupying both grey and white matter (Figure 8C–C′). Further, we noted a major increase in the number of Ki67+ astrocytes in postnatal day 10 cortices in the caMek1\hGFAP mice (Figure S 7D–E). Thus, the increased astrocyte number observed in mature mice is likely due to both an increase in the number of radial progenitors that committed to the astrocytic lineage and further proliferation of astrocyte precursors/astrocytes postnatally.
Discussion
We have demonstrated that MEK signaling strongly regulates the generation of glia from radial progenitors in developing cortex. This conclusion is based on multiple clear-cut in vivo findings in genetically-induced loss and gain of function models. First, glial-like properties of radial progenitors are not maintained in Mek deleted mice and glial specification is almost completely blocked. Second, expression of Cre or caMek1 in individual radial progenitors suggests that functions of MEK are cell autonomous and can be instructive. Third, Mek deletion leads to a persistent loss of gliogenic competence as Mek1,2\hGFAP mutants are nearly devoid of astrocytes and oligodendrocytes in the dorsal cortex at postnatal stages. Finally, expression of caMek1 in radial progenitors leads to a major increase in numbers of cortical astrocytes in mature mice. Our data establish MEK as a key regulator of gliogenesis in developing mammalian cortex.
MEK signaling regulates acquisition of gliogenic competence
In developing cortex, radial progenitors first generate neurons to form neuronal circuits, and then generate matching numbers of glial cells (Guillemot and Zimmer, 2011). Multiple studies have demonstrated that extrinsic factors such as Notch and BMP stimulate progenitors to become gliogenic (Gaiano and Fishell, 2002; Nakashima et al., 1999b; Rowitch and Kriegstein, 2010). Interestingly, many of these gliogenic cues are present at early neurogenic stages but do not induce gliogenesis (Molne et al., 2000; Takizawa et al., 2001). An idea that has emerged is that radial progenitors undergo a cell fate switch at the gliogenic stage making them competent to respond to gliogenic signals (Molne et al., 2000; Song and Ghosh, 2004; Viti et al., 2003). We suggest that MEK/ERK MAPK signaling mediates this switch from neurogenic to gliogenic competence.
ERK MAPK signaling has been implicated previously in cell fate switching. For example, FGF4 activation of the ERK pathway is the major differentiation signal for mouse ES cells (Kunath et al., 2007; Ying et al., 2008). Further, an important recent report demonstrated that FGF10 determines the timing of the transition from neural epithelium to radial progenitor, a key event in neurogenesis (Sahara and O’Leary, 2009). Our study does not assess the role of MEK in mediating the FGF10 effect since recombination in our study occurred after the neural epithelium stage. However, our finding that Mek deletion prevents an important step in radial progenitor fate transition in late embryogenesis clearly has parallels with the FGF10 result at an earlier stage in radial progenitor development. Importantly, FGFs have previously been implicated in glial development (Naruse et al., 2006; Seuntjens et al., 2009; Song and Ghosh, 2004). We suggest that FGF effects on regulating radial progenitor fate transitions are mediated via MEK/ERK signaling.
Our findings provide strong evidence for an important interaction between MEK signaling and the CNTF/LIFRβ-gp130/JAK-STAT cascade, a major cytokine signaling pathway that promotes astrogenesis (Bonni et al., 1997). It has been well established that an increase in the gp130 expression level in radial progenitors at mid-embryogenesis is vital for initiating astrogenesis (He et al., 2005; Nakashima et al., 1999a). Importantly, we have demonstrated here that MEK was required for the expression of gp130 by radial progenitors. Consequently, CNTF failed to induce STAT3 phosphorylation and GFAP expression efficiently in Mek1,2\Nes mutant progenitor cultures. Our data suggest MEK and CNTF/JAK-STAT pathways work contiguously in regulating astrogenesis. Thus, MEK signaling promotes gliogenic fate transition by radial progenitors, and then CNTF/JAK-STAT signaling induces further differentiation of astrocyte precursors.
Progression along the oligodendrocyte lineage was also profoundly affected by Mek deletion. In line with our results, partial inhibition of ERK/MAPK signaling by deletion of Braf or Erk2 has previously been shown to inhibit CNS myelination, though the effect on radial progenitor fate transition to OPC was unclear (Fyffe-Maricich et al., 2011; Galabova-Kovacs et al., 2008). Here, we have completely eliminated ERK/MAPK signaling by deleting Mek1/2 and show that the generation of OPCs in the developing cortex is almost completely blocked.
MEK regulation of neurogenesis was not definitively addressed in this study due to uncertainty about the timing of recombination in relation to the major phases of neurogenesis which occur earlier than gliogenesis. A recent study showed a requirement for ERK2 in regulating the proliferation of neurogenic precursors at E14, although results were complex in that some layer markers showed depletion after Erk2 deletion but other layer markers showed expansion (Pucilowska et al., 2012). Here, our data suggest prolonged neurogenesis at late embryonic stages in Mek deleted cortices. Further, we demonstrate fewer neurons, rather than more, in mature animals in the setting of Mek1 gain of function, consistent with enhancement of gliogenesis at the expense of neurogenesis. Reconciliation of these findings will require additional work with careful attention to timing of recombination in neurogenic precursors.
Interestingly, the phenotype of Mek-deleted brains was somewhat more severe than in Erk-deleted brains, an effect observed previously with other Cre lines (Newbern et al., 2008; Newbern et al., 2011). Whether these differences relate to timing of the disappearance of protein after Cre mediated recombination has yet to be determined. Although ERK is the only well-established downstream substrate of MEK (Morandell et al., 2010), it is interesting that a few studies outside the nervous system have reported kinase activity-independent MEK functions that do not require ERK (Scholl et al., 2004; Wang et al., 2009). Whether MEK may function independently of ERK in the mammalian brain remains to be explored.
Mechanisms of MEK regulation of gliogenesis
We have found that the Ets family transcription factor Etv5/Erm is strongly regulated by MEK. Erm is a member of the PEA3 subgroup which is comprised of Erm (Etv5), Er81 (Etv1) and Pea3 (Etv4). Ets transcription factors are well established as FGF targets and have been reported to be phosphorylated and transactivated by the MAPK pathway (Bertrand et al., 2003; Chen et al., 2005; Sharrocks, 2001). Several prior studies have implicated Ets family members in regulation of gliogenesis. The Drosophila gene pointed, which encodes an Ets transcription factor, is critical for directing glial differentiation in the developing CNS of Drosophila (Jacobs, 2000; Klaes et al., 1994). Indeed a recent study has demonstrated an important role for a FGF-Rolled (Drosophila MAPK)-Pointed signaling cascade in inducing glia differentiation in the Drosophila eye (Franzdottir et al., 2009). In Xenopus, both loss and gain of function studies demonstrated that RAS-MAPK signaling acts through Xenopus Ets-1 to regulate radial glia development (Kiyota et al., 2007). In the mammalian PNS, Erm has been implicated in glial cell fate decisions of neural crest progenitors (Hagedorn et al., 2000). Although a previous study of Erm null mice found no gross abnormality in the brain, the glial population was not assessed (Chen et al., 2005). As all three PEA3 subgroup members are expressed in progenitors and their sequences are highly homologous (Hasegawa et al., 2004), loss of one family member may not have a drastic effect in vivo. However, our data clearly demonstrate that MEK specifically regulates Erm expression in radial progenitors, that Erm overexpression in radial progenitors is instructive in inducing glial progenitor specification and astrocyte differentiation, and that Erm introduction into Mek-deleted radial progenitors ex vivo can restore CNTF-induced astrogenesis.
Additional mechanisms are likely to be at play. Another strongly regulated transcription factor is CoupTF-II (Table1). COUP TFs have previously been implicated in the neurogenic to gliogenic fate transition (Naka et al., 2008). Further, we found Egfr expression is strongly modulated by MEK signaling (Table 1). EGFR has been shown to increase its expression during late cortical development and promotes progenitor gliogenic competence (Viti et al., 2003). Finally, it is highly likely that MEK acts through epigenetic mechanisms to regulate transcription of multiple genes related to glial differentiation. Indeed a prior study strongly implicated modulation of H3 methylation as an important mechanism of FGF signaling in the cell fate switch that allowed glial differentiation (Song and Ghosh, 2004). Analysis of epigenetic regulation will be an important area for future investigation.
Importance of findings for neurodevelopmental disorders
Importantly, gliogenesis has been assessed in detail in mouse models of human syndromes due to RAF/MEK/ERK cascade overactivation. Work in a mouse model of neurofibromatosis type1 (NF1) has shown a dramatic increase in brain gliogenesis and decreased neurogenesis (Hegedus et al., 2007), findings that are very much in line with the results reported here. A study using a Costello syndrome H-RAS active mutant construct also showed a similar phenotype (Paquin et al., 2009). In both of these syndromes, gene mutations lead to overactivation of RAF/MEK/ERK signaling and the phenotypes are entirely consistent with our findings.
Another RAS/MAPK syndrome, Noonan’s syndrome, typically results from mutations in SHP-2, an upstream modifier of the RAF/MEK/ERK cascade. Our results are not in line with the concept that a SHP-2-MEK/ERK cascade is essential for neurogenesis and suppresses gliogenesis as has been reported previously (Gauthier et al., 2007; Ke et al., 2007). Although reasons for these differing interpretations are not entirely clear, it is important to note that SHP-2 regulates several signaling cascades in addition to the RAS-MAPK pathway. Additional effects of SHP-2 regulation include activation of PI3K-AKT pathway and inactivation of JAK-STAT3 pathway (Coskun et al., 2007; Feng, 2007; Neel et al., 2003). Thus, the reported effects in Shp-2 deficient animals may be due to abnormalities in several pathways. Whatever the explanation for divergent results related to SHP-2, our results are definitive as to the gliogenic functions mediated by MEK.
Importance of regulation of glial number in the postnatal brain
Astrocytes are thought to have critical functions in the postnatal brain related to neuronal support and synaptic function. However, few prior studies have produced brains where astrocyte number has been dramatically reduced during development. We have defined several important in vivo consequences of regulating glial number in our study. First, we noticed that Mek1,2\Nes mice are acallosal due to absence of midline astroglia. Interestingly, the Fgfr1f/f;NesCre conditional mutant shows a similar phenotype. FGFR1 is highly enriched in the telencephalic midline and is required for targeting specific groups of radial glia to dorsal midline to form the indusium gresium, an important structure for axon guidance (Smith et al., 2006).
Second, Mek1,2\hGFAP conditional nulls that survive through the first postnatal week display a dorsal cortex which is almost completely devoid of astrocytes and exhibit a major neurodegeneration phenotype. Although both neurons and glia lack MEK in these mice, results from neuron specific Mek deleted mice suggest that neurons can survive into adulthood in the absence of MEK (data not shown), indicating the degeneration in Mek1,2\hGFAP dorsal cortices is likely due to the lack of glial support. A similar situation holds in the periphery where MEK/ERK signaling is required for Schwann cell development and neurons deprived of Schwann cell support die massively during embryonic development (Newbern et al., 2011). Finally, subcortical dopamine neuron survival has also been shown to be critically dependent on the astrocyte derived trophic factors-conserved dopamine neurotrophic factor (CDNF) and mesencephalic astrocyte-derived neurotrophic factor (MANF) (Lindholm et al., 2007; Petrova et al., 2003). The nature of glial-derived survival signals for cortical neurons remains to be determined and should be a rich area for future investigation.
It is important to note that postnatal regulation is critical to establishing the number of astrocytes and oligodendroglia in the mature CNS. It has long been known that proliferation of OPCs postnatally is regulated by PDGF (Fruttiger et al., 1999). Very recently it has been demonstrated that mature-appearing astrocytes in upper cortical layers also proliferate in the postnatal period (Ge et al., 2012). Further recent studies demonstrate that oligodendrocyte proliferation in spinal cord is partially under ERK/MAPK control (Newbern et al., 2011) and that constitutively active B-Raf can drive proliferation of spinal cord astrocyte precursors (Tien et al., 2012). These results in combination with our results showing expansion of astrocytes in mice expressing caMek1, all strongly suggest that postnatal stages of glial development may also be regulated by MEK/ERK/MAPK signaling.
Lastly we note that astrocytes are now known to play critical roles in synapse formation, elimination and function (Allen and Barres, 2005; Christopherson et al., 2005; Stevens et al., 2007). However, the consequences of increasing astrocyte number for cortical neuronal physiology and behavior are unknown. Our MEK hyperactivation model may provide a unique approach to study the effects of changing the glia/neuron ratio on synapse formation and neuronal activity. Such studies may facilitate our understanding of the role of glia in the cognitive abnormalities observed in CFC syndrome patients.
Materials and Methods
Genetically modified mice
The Mek1f/f, Mek2−/−, Erk1−/, Erk2 f/f and CAG-loxpSTOPloxp-Mek1S218E,S222E (caMek1) mouse lines and associated genotyping procedures have been previously described (Krenz et al., 2008; Newbern et al., 2008) and see supplemental experimental procedures. All laboratory animal experiments were conducted in accordance with NIH guidelines and were approved by the UNC-Chapel Hill Institutional Animal Care and Use Committee.
In utero and postnatal electroporation
Mice were anesthetized using 2,2,2-Tribromoethanol (4mg/10g mouse) and embryos were gently exposed. Plasmids mixed with fast green were then microinjected into the lateral ventricle of embryos. Using 5mm paddle electrodes, embryos were electroporated with five, 50ms pulses at 30V with a 950ms interval and gently returned to the abdominal cavity. For postnatal electroporation, 1–2 μl of plasmid DNA was injected into the lateral ventricle of cryo-anesthetized pups and three, 100ms pulses at 100V with a 950 ms interval were administered.
In situ hybridization and Immunohistochemistry
Experiments were carried out using standard procedures. Details and a full list of primary antibodies are given in the Supplemental Experimental Procedures.
Cortical progenitor cultures
Methods associated cortical progenitor cultures and organotypic slice cultures are described in further detail in the Supplemental Experimental Procedures.
Additional methodological detail regarding quantification methods, laboratory animals, Brdu labeling, Western blotting, viral vector transduction and microarray analysis is provided in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We are grateful to G. Landreth (Case Western Reserve University) for providing us with Erk1−/− and Erk2fl/fl mutant mice, S. Arber (University of Basel, Switzerland) for Erm full length cDNA; C. Derr (UNC Lineberger Cancer Center) for the caMek1 construct; S. Gray and J. Samulski for the AAV9-EGFP virus; E. Anton, C. Birchmeier and T. Muller (Max-Delbrück-Centrum for Molecular Medicine, Germany) for BLBP antibody and E. Anton (UNC Neuroscience Center), Franck Polleux (Scripps Research Institute), and the members of the Snider lab for helpful discussions. This work was supported by NIH grant RO1 NS031768 to W.D.S.; F32NS061591 to J.M.N. and the Confocal and Multiphoton Imaging Core, Functional Genomics Core, and Expression Localization Core Facilities fundedby NINDS Center grant P30 NS045892.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. doi: 10.1016/j.conb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Heintz N. Genetic lineage tracing defines distinct neurogenic and gliogenic stages of ventral telencephalic radial glial development. Neural Dev. 2008;3:30. doi: 10.1186/1749-8104-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand V, Hudson C, Caillol D, Popovici C, Lemaire P. Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell. 2003;115:615–627. doi: 10.1016/s0092-8674(03)00928-0. [DOI] [PubMed] [Google Scholar]
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Coskun V, Zhao J, Sun YE. Neurons or glia? Can SHP2 know it all? Sci STKE. 2007;2007:pe58. doi: 10.1126/stke.4102007pe58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng GS. Shp2-mediated molecular signaling in control of embryonic stem cell self-renewal and differentiation. Cell Res. 2007;17:37–41. doi: 10.1038/sj.cr.7310140. [DOI] [PubMed] [Google Scholar]
- Franzdottir SR, Engelen D, Yuva-Aydemir Y, Schmidt I, Aho A, Klambt C. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–761. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Betsholtz C, et al. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Karlo JC, Landreth GE, Miller RH. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J Neurosci. 2011;31:843–850. doi: 10.1523/JNEUROSCI.3239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- Galabova-Kovacs G, Catalanotti F, Matzen D, Reyes GX, Zezula J, Herbst R, Silva A, Walter I, Baccarini M. Essential role of B-Raf in oligodendrocyte maturation and myelination during postnatal central nervous system development. J Cell Biol. 2008;180:947–955. doi: 10.1083/jcb.200709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron. 2007;54:245–262. doi: 10.1016/j.neuron.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012;484:376–380. doi: 10.1038/nature10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F, Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron. 2011;71:574–588. doi: 10.1016/j.neuron.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Hagedorn L, Paratore C, Brugnoli G, Baert JL, Mercader N, Suter U, Sommer L. The Ets domain transcription factor Erm distinguishes rat satellite glia from Schwann cells and is regulated in satellite cells by neuregulin signaling. Dev Biol. 2000;219:44–58. doi: 10.1006/dbio.1999.9595. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Ashigaki S, Takamatsu M, Suzuki-Migishima R, Ohbayashi N, Itoh N, Takada S, Tanabe Y. Laminar patterning in the developing neocortex by temporally coordinated fibroblast growth factor signaling. J Neurosci. 2004;24:8711–8719. doi: 10.1523/JNEUROSCI.3070-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, Wu H, Castro D, Guillemot F, Fan G, et al. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci. 2005;8:616–625. doi: 10.1038/nn1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Imamura O, Pages G, Pouyssegur J, Endo S, Takishima K. ERK1 and ERK2 are required for radial glial maintenance and cortical lamination. Genes Cells. 2010;15:1072–1088. doi: 10.1111/j.1365-2443.2010.01444.x. [DOI] [PubMed] [Google Scholar]
- Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, Bansal R. ERK1/ERK2 MAPK Signaling is Required to Increase Myelin Thickness Independent of Oligodendrocyte Differentiation and Initiation of Myelination. J Neurosci. 2012;32:8855–8864. doi: 10.1523/JNEUROSCI.0137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JR. The midline glia of Drosophila: a molecular genetic model for the developmental functions of glia. Prog Neurobiol. 2000;62:475–508. doi: 10.1016/s0301-0082(00)00016-2. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Ke Y, Zhang EE, Hagihara K, Wu D, Pang Y, Klein R, Curran T, Ranscht B, Feng GS. Deletion of Shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality. Mol Cell Biol. 2007;27:6706–6717. doi: 10.1128/MCB.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Kato A, Kato Y. Ets-1 regulates radial glia formation during vertebrate embryogenesis. Organogenesis. 2007;3:93–101. doi: 10.4161/org.3.2.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaes A, Menne T, Stollewerk A, Scholz H, Klambt C. The Ets transcription factors encoded by the Drosophila gene pointed direct glial cell differentiation in the embryonic CNS. Cell. 1994;78:149–160. doi: 10.1016/0092-8674(94)90581-9. [DOI] [PubMed] [Google Scholar]
- Krenz M, Gulick J, Osinska HE, Colbert MC, Molkentin JD, Robbins J. Role of ERK1/2 signaling in congenital valve malformations in Noonan syndrome. Proc Natl Acad Sci U S A. 2008;105:18930–18935. doi: 10.1073/pnas.0806556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Voutilainen MH, Lauren J, Peranen J, Leppanen VM, Andressoo JO, Lindahl M, Janhunen S, Kalkkinen N, Timmusk T, et al. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature. 2007;448:73–77. doi: 10.1038/nature05957. [DOI] [PubMed] [Google Scholar]
- Liu KD, Gaffen SL, Goldsmith MA. JAK/STAT signaling by cytokine receptors. Curr Opin Immunol. 1998;10:271–278. doi: 10.1016/s0952-7915(98)80165-9. [DOI] [PubMed] [Google Scholar]
- Menard C, Hein P, Paquin A, Savelson A, Yang XM, Lederfein D, Barnabe-Heider F, Mir AA, Sterneck E, Peterson AC, et al. An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron. 2002;36:597–610. doi: 10.1016/s0896-6273(02)01026-7. [DOI] [PubMed] [Google Scholar]
- Molne M, Studer L, Tabar V, Ting YT, Eiden MV, McKay RD. Early cortical precursors do not undergo LIF-mediated astrocytic differentiation. J Neurosci Res. 2000;59:301–311. doi: 10.1002/(sici)1097-4547(20000201)59:3<301::aid-jnr3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Morandell S, Grosstessner-Hain K, Roitinger E, Hudecz O, Lindhorst T, Teis D, Wrulich OA, Mazanek M, Taus T, Ueberall F, et al. QIKS--Quantitative identification of kinase substrates. Proteomics. 2010;10:2015–2025. doi: 10.1002/pmic.200900749. [DOI] [PubMed] [Google Scholar]
- Morrow T, Song MR, Ghosh A. Sequential specification of neurons and glia by developmentally regulated extracellular factors. Development. 2001;128:3585–3594. doi: 10.1242/dev.128.18.3585. [DOI] [PubMed] [Google Scholar]
- Naka H, Nakamura S, Shimazaki T, Okano H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008;11:1014–1023. doi: 10.1038/nn.2168. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Yoshida K, Kishimoto T, Sendtner M, Taga T. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci. 1999a;19:5429–5434. doi: 10.1523/JNEUROSCI.19-13-05429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999b;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- Naruse M, Nakahira E, Miyata T, Hitoshi S, Ikenaka K, Bansal R. Induction of oligodendrocyte progenitors in dorsal forebrain by intraventricular microinjection of FGF-2. Dev Biol. 2006;297:262–273. doi: 10.1016/j.ydbio.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Newbern J, Zhong J, Wickramasinghe RS, Li X, Wu Y, Samuels I, Cherosky N, Karlo JC, O’Loughlin B, Wikenheiser J, et al. Mouse and human phenotypes indicate a critical conserved role for ERK2 signaling in neural crest development. Proc Natl Acad Sci U S A. 2008;105:17115–17120. doi: 10.1073/pnas.0805239105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, et al. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69:91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin A, Barnabe-Heider F, Kageyama R, Miller FD. CCAAT/enhancer-binding protein phosphorylation biases cortical precursors to generate neurons rather than astrocytes in vivo. J Neurosci. 2005;25:10747–10758. doi: 10.1523/JNEUROSCI.2662-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin A, Hordo C, Kaplan DR, Miller FD. Costello syndrome H-Ras alleles regulate cortical development. Dev Biol. 2009;330:440–451. doi: 10.1016/j.ydbio.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, et al. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003;20:173–188. doi: 10.1385/jmn:20:2:173. [DOI] [PubMed] [Google Scholar]
- Pucilowska J, Puzerey PA, Karlo JC, Galan RF, Landreth GE. Disrupted ERK Signaling during Cortical Development Leads to Abnormal Progenitor Proliferation, Neuronal and Network Excitability and Behavior, Modeling Human Neuro-Cardio-Facial-Cutaneous and Related Syndromes. J Neurosci. 2012;32:8663–8677. doi: 10.1523/JNEUROSCI.1107-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, Cruz MS, McCormick F, Rauen KA. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311:1287–1290. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- Sahara S, O’Leary DD. Fgf10 regulates transition period of cortical stem cell differentiation to radial glia controlling generation of neurons and basal progenitors. Neuron. 2009;63:48–62. doi: 10.1016/j.neuron.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, Saitta SC, Landreth GE. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J Neurosci. 2008;28:6983–6995. doi: 10.1523/JNEUROSCI.0679-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Saitta SC, Landreth GE. MAP’ing CNS development and cognition: an ERKsome process. Neuron. 2009;61:160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh Y, Kobayashi Y, Takeuchi A, Pages G, Pouyssegur J, Kazama T. Deletion of ERK1 and ERK2 in the CNS causes cortical abnormalities and neonatal lethality: Erk1 deficiency enhances the impairment of neurogenesis in Erk2-deficient mice. J Neurosci. 2011;31:1149–1155. doi: 10.1523/JNEUROSCI.2243-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl FA, Dumesic PA, Khavari PA. Mek1 alters epidermal growth and differentiation. Cancer Res. 2004;64:6035–6040. doi: 10.1158/0008-5472.CAN-04-0017. [DOI] [PubMed] [Google Scholar]
- Seuntjens E, Nityanandam A, Miquelajauregui A, Debruyn J, Stryjewska A, Goebbels S, Nave KA, Huylebroeck D, Tarabykin V. Sip1 regulates sequential fate decisions by feedback signaling from postmitotic neurons to progenitors. Nat Neurosci. 2009;12:1373–1380. doi: 10.1038/nn.2409. [DOI] [PubMed] [Google Scholar]
- Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- Smith KM, Ohkubo Y, Maragnoli ME, Rasin MR, Schwartz ML, Sestan N, Vaccarino FM. Midline radial glia translocation and corpus callosum formation require FGF signaling. Nat Neurosci. 2006;9:787–797. doi: 10.1038/nn1705. [DOI] [PubMed] [Google Scholar]
- Song MR, Ghosh A. FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat Neurosci. 2004;7:229–235. doi: 10.1038/nn1192. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Sun Y, Goderie SK, Temple S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron. 2005;45:873–886. doi: 10.1016/j.neuron.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M, Taga T. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell. 2001;1:749–758. doi: 10.1016/s1534-5807(01)00101-0. [DOI] [PubMed] [Google Scholar]
- Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19:230–236. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien AC, Tsai HH, Molofsky AV, McMahon M, Foo LC, Kaul A, Dougherty JD, Heintz N, Gutmann DH, Barres BA, et al. Regulated temporal-spatial astrocyte precursor cell proliferation involves BRAF signalling in mammalian spinal cord. Development. 2012;139:2477–2487. doi: 10.1242/dev.077214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viti J, Feathers A, Phillips J, Lillien L. Epidermal growth factor receptors control competence to interpret leukemia inhibitory factor as an astrocyte inducer in developing cortex. J Neurosci. 2003;23:3385–3393. doi: 10.1523/JNEUROSCI.23-08-03385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Whiteman MW, Lian H, Wang G, Singh A, Huang D, Denmark T. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J Biol Chem. 2009;284:21412–21424. doi: 10.1074/jbc.M109.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Li X, McNamee C, Chen AP, Baccarini M, Snider WD. Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci. 2007;10:598–607. doi: 10.1038/nn1898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.