Abstract
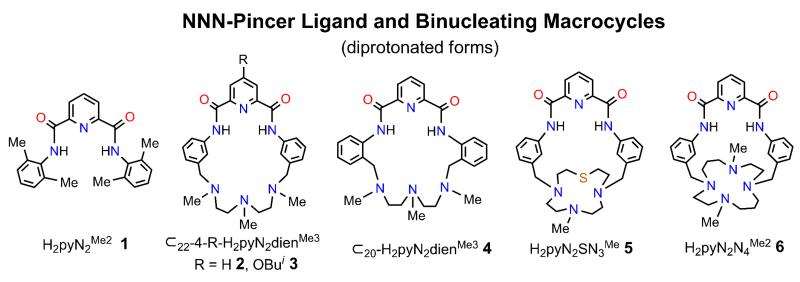
The planar NNN-pincer complexes [MII(pyN Me2)(OH)]1− (MII = Ni, Cu) fix CO2 in η1-OCO2H complexes; results for the copper system are described. MnII, FeII, CoII, and ZnII behave differently, forming [MII(pyN2Me)2]2− with N4O2 coordination. Incorporation of the NiII pincer into binucleating macrocycle 2 containing a triamino MII locus connected by two 1,3-biphenylene groups affords proximal NiII and MII sites for investigation of the synthesis, structure, and reactivity of Ni-X-M bridge units. This ligand structure is taken as a reference for variations in MII atoms and binding sites and bridges X = OH− and CN− to produce additional members of the macrocyclic family with improved properties. Macrocycle 2 with a 22-membered ring is shown to bind MII = Mn, Fe, and Cu with hydroxo bridges. Introduction of the 4-BuiO group (macrocycle 3) improves the solubility of neutral complexes such as those with NiII-OH-CuII and NiII-CN-FeII bridges. The syntheses of macrocycle 5 with a 7-Me-[12]aneSN3 and macrocycle 6 with a 1,8-Me2-[14]aneN4 MII binding site are described to together with hydoxo-bridged Ni-Cu and cyano-bridged Ni-Fe complexes. This work was motivated by the presence of a Ni⋯(HO)-Fe bridge grouping in a reactive state of carbon monoxide dehydrogenase. Attempted decrease in Ni-(OH)-M distances (3.70-3.87 Å) to smaller values observed in the enzyme by use of macrocycle 4 having 1,2-biphenylene connectors led to a mononuclear octahedral NiII complex. Bridge structural units are summarized and the structures of fourteen macrocyclic complexes including eight with bridges are described.
INTRODUCTION
The NiII⋯(HO)-FeII bridge grouping is a component of the catalytic site (C-cluster) in the Ni-Fe-S enzyme carbon monooxide dehydrogenase which catalyzes the reaction CO + H2O ⇌ CO2 + 2H+ + 2e−.1-4 The site consists of a NiFe3S4 cubanoid cluster to which is bound via a C3-S atom an exo FeII atom that is part of the bridge. While the cluster portion has been chemically synthesized,5,6 attainment of the complete assembly NiFe3S4-Feexo has proven elusive. Consequently, more recent research has concentrated on a different approach which departs from a Ni-Fe-S support platform but allows an investigation of the chemistry of NiII and FeII in combination. Suitable binucleating macrocyclic ligands allow specific binding of the two metals in bridging juxtaposition at a known distance and variation of the atom or bridging group between them. The dianion of 2 in Figure 1 is a first approach to the problem.7 The upper NNN site, obviously related to the pincer ligand 1 with two deprotonated amide groups, binds NiII while the lower triamine portion binds FeII. Various bridges including hydroxo, formate, and cyano may be interposed between the two metal sites. A further advantage of the system is that the terminal hydroxo pincer complex [Ni(pyN2Me2)(OH)]1−, 8 a virtual replica of the binuclear NiII site, may be prepared and its reactivity examined separately.9,10 This complex reacts quantitatively and reversibly with CO2 to afford an η1-HCO3 product in an exceptionally fast second-order reaction in DMF (k2298 ≈ 106 M−1s−1).
Figure 1.
Depictions of NNN-pincer ligand 1 and binucleating macrocycles 2-6 with ligand abbreviations. The subscript to the symbol ⊂ refers to the number of atoms in the macrocyclic ring.
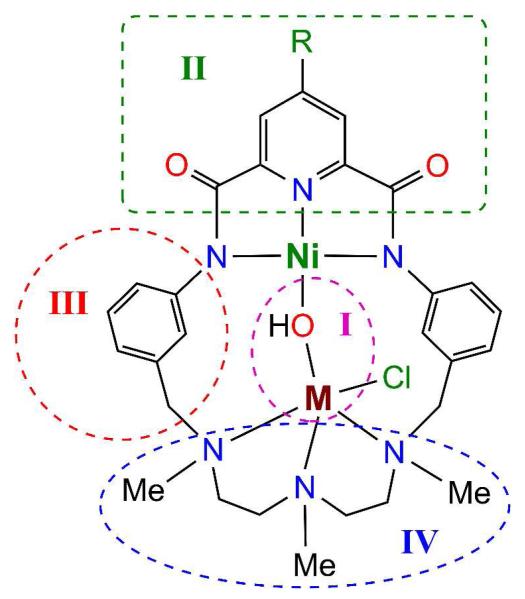
We are engaged in producing a new set of macrocycles and their complexes that sustain proximal Ni⋯M binding with bridge formation and retain the pincer binding site but with otherwise variable and/or improved properties. With attention to the reference macrocyclic complex in Figure 2 which includes hydroxo as a representative bridging group, we note four regions of potential modification. Region I is the site of atom M and associated ligands. Various atoms M and ligands, bridging and terminal (if present), in this region are subject to incorporation dependent on several factors. That of primary interest in ligand design is the binding functionality present in region IV. Introduction of a suitable substituent R in region II would enhance the solubility of neutral complexes such as [Ni(μ2-OH)FeCl(⊂22-pyN2dienMe3)] (see below), allowing solution studies. Adjustment of the spacer group between binding sites in region III could control the Ni⋯M separation. Variation of the ligand framework in region IV (M site) might lead to tighter binding of atom M, a problem we have encountered on occasion in this work. Lastly, given the huge number of known binucleating (or “compartmental”) ligands,11-13 it need be recognized that the binuclear complex in Figure 2 and any alterations of it constitute a small subset of possibilities for examination of the chemistry of proximally bound metals. In this report, we demonstrate certain modifications in regions I-IV by synthesis and structure determinations. As will be seen, all modifications are illustrative of further possibilities and all but that in region II allow bridge formation.
Figure 2.
The binuclear complex [Ni(OH)ML(⊂22-pyN2dienMe3)] with regions I-IV designated for possible structural alteration.
EXPERIMENTAL SECTION
Preparation of Compounds
Unless otherwise stated, all reactions and manipulations were performed under a dinitrogen atmosphere. Volume reduction and drying steps were performed in vacuo; filtrations were through Celite. Commercial grade chemicals were used without further purification. Acetonitrile, THF, and diethyl ether were purified by an Innovative Technology or MBraun solvent purification system. DMF was freshly distilled from CaH2 and dried over molecular sieves for 24 h. Compounds were identified by combinations of elemental analyses, spectroscopic measurements (mainly 1H NMR where possible), and X-ray structure determinations. Representative compounds were analyzed (Midwest Microlab, Inc.). The structures of the NNN-pincer ligand 1 and binucleating macrocycles 2-6 are provided in Figure 1.
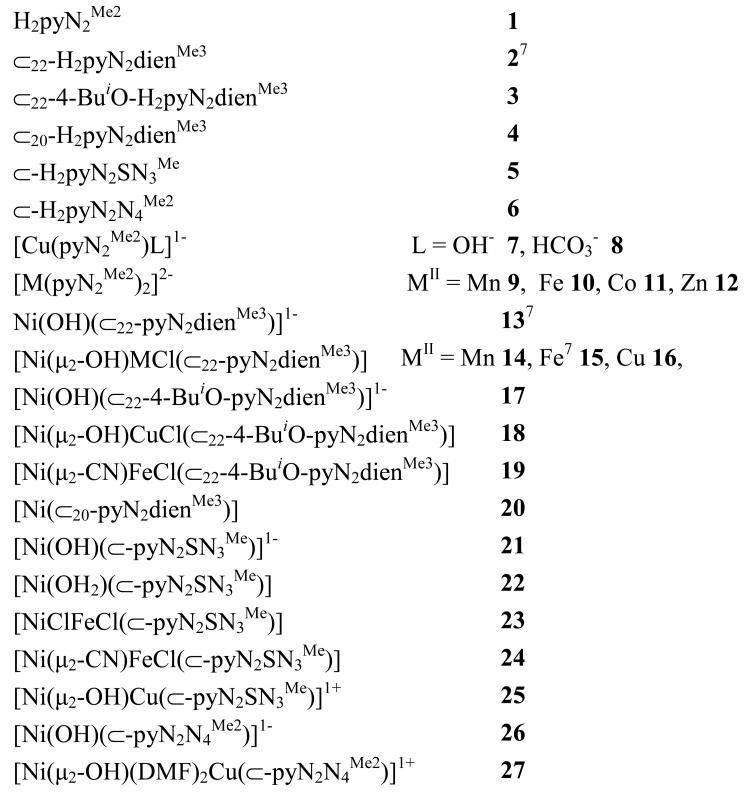
In the sections that follow, ligands and complexes are numerically designated according to Chart 1 and Figures 1, 3-7, 9, 11, 12, and 14.
Chart 1. Designation of Ligands and Complexes*.
Figure 3.
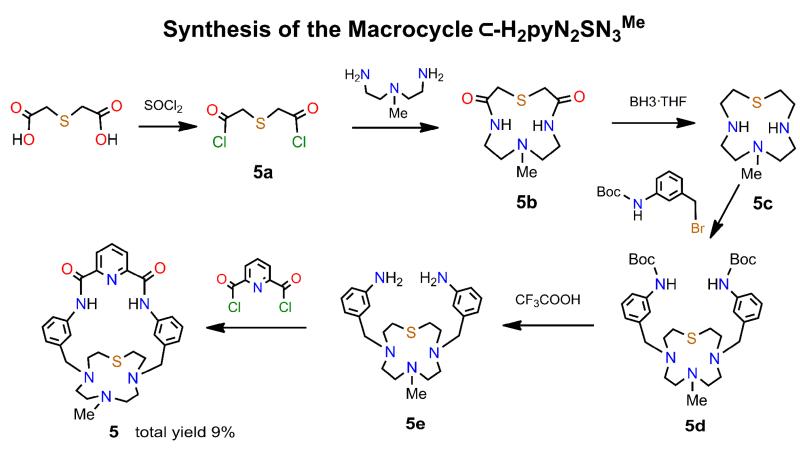
Synthetic scheme for macrocycle 5 via intermediates 5a-5e.
Figure 7.
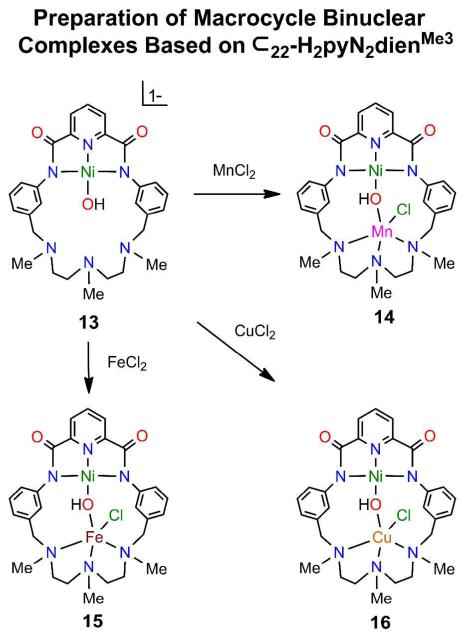
Formation of binuclear hydroxo-bridged complexes 14-16 from NiII-OH complex 13 based on macrocycle 2.
Figure 9.
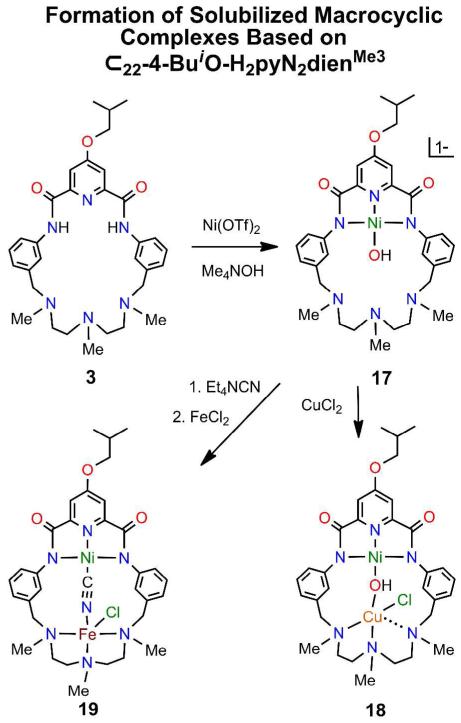
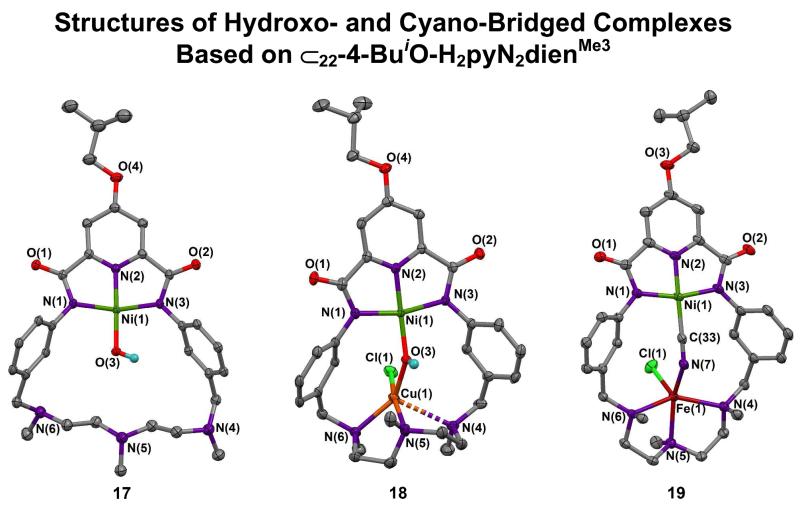
Formation of solubilized binuclear bridged complexes 18 and 19 from NiII-OH complex 17 based on macrocycle 3.
Figure 11.
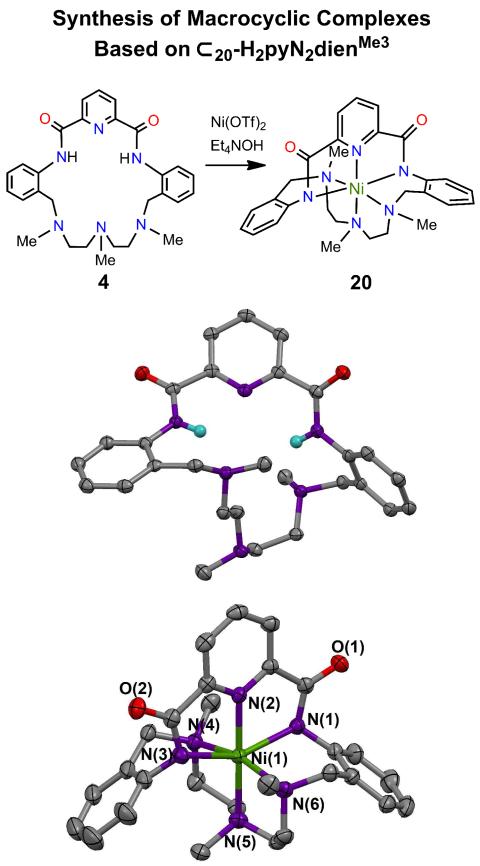
Formation of the NiII complex 20 derived from macrocycle 4 and the structures of the macrocycle and the complex.
Figure 12.
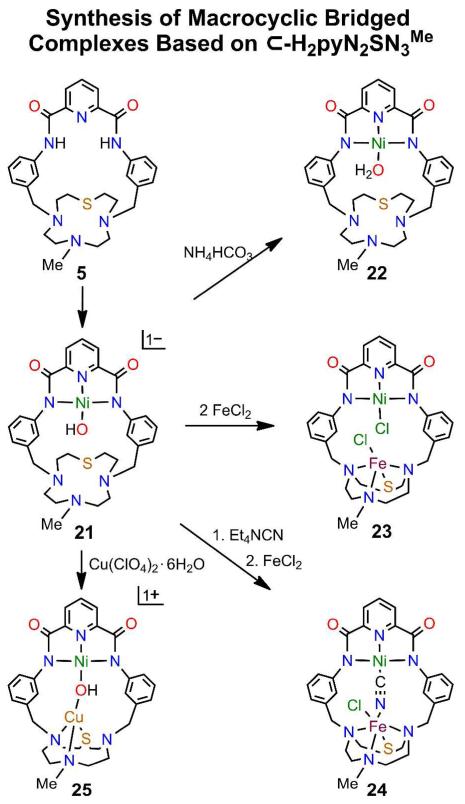
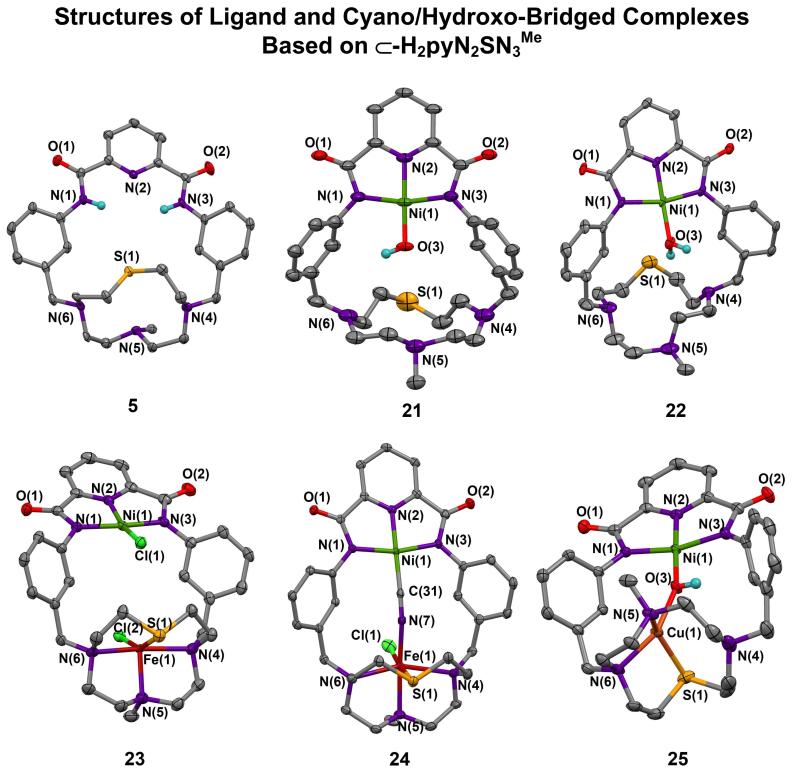
Formation of mononuclear NiII-OH complex 21, aquo complex 22, unbridged binuclear Ni-Fe complex 23, and bridged binuclear complexes 24 and 25 based on macrocycle 5.
Figure 14.
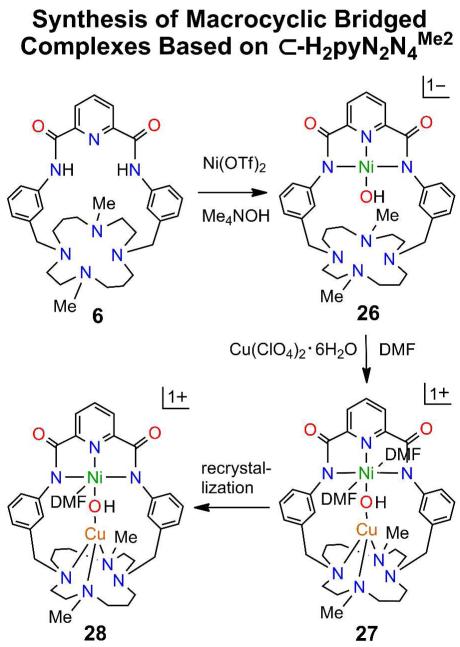
Formation of mononuclear NiII-hydroxo complex 26 and binuclear hydroxo-bridged complexes 27 and 28 based on macrocycle 6.
(1) Complexes Derived from the Pincer Ligand H2pyN2Me2·(Et4N)[Cu(pyN2Mw2)(OH)]
H2pyN2ME2 (75 mg, 0.20 mmol)7 and Cu(OTf)2 (72 mg, 0.20 mmol) were stirred in DMF (3 mL) for 20 min. The light green solution was treated slowly with Et4NOH (25% in methanol, 353 mg, 0.60 mmol) and the mixture was stirred for 12 h to form a purple-blue solution and a small amount of sticky precipitate which was removed by filtration. Ether (18 mL) was added to the filtrate resulting in a cloudy suspension from which crystals separated over 3 h. The product was collected as a purple-blue crystalline solid (85 mg, 73%). Absorption spectrum (DMF): λmax (εM) 594 (br, 270) nm. IR (KBr): νOH 3608 cm!1. Anal. Calcd. for C31H42CuN4O3: C, 63.95; H, 7.27; N, 9.62. Found: C, 63.85; H, 7.23; N, 9.52.
(Et4N)[Cu(pyN2Me2)(HCO3)]
A solution of (Et4N)[Cu(pyN2)(OH)] (29 mg, 0.050 mmol) in DMF (3 mL) was bubbled with carbon dioxide for 15 min. Diffusion of ether into the blue solution afforded the product as blue crystals (25 mg, 80%). Absorption spectrum (DMF): λmax (εM) 610 (br, 320) nm.
(Et4N)2[Fe(pyN2Me2)2]
A solution of H2pyN2 (112 mg, 0.30 mmol) in DMF (3 mL) was treated with NaH (15 mg, 0.060 mmol) slowly to give a light yellow solution, to which was added Fe(OTf)2 (106 mg, 0.30 mmol). The dark purple reaction mixture was stirred for 2 h and filtered. Ether (20 mL) was added to the filtrate causing separation of a purple oil, which was treated with ether to form a purple solid. The solid was dissolved in THF (5 mL) and solution was filtered through Celite to give a deep purple solution. A solution of Et4NOH (25% in methanol, 176 mg, 0.30 mmol) was slowly added, and the mixture was stirred for 2 h and filtered to remove a sticky white precipitate. Ether (15 mL) was added to the filtrate to form a blue solid, which was dissolved in DMF (2 mL). Addition of ether by diffusion caused separation of the product as blue-red crystals (71 mg, 45%). Anal. Calcd. for C62H82FeN8O4: C, 70.30; H, 7.80; N, 10.58. Found: C, 70.19; H, 7.83; N, 10.66.
(Et4N)2[Co(pyN2Me2)2]
A mixture of H2pyN2 (75 mg, 0.20 mmol) and Co(OTf)2 (71 mg, 0.20 mmol) in DMF was stirred for 30 min. The pink-red solution was slowly treated with a solution of Et4NOH (25% in methanol, 353 mg, 0.60 mmol) and the reaction mixture was stirred for 6 h to form a red solution and a sticky precipitate, which as removed by filtration through Celite. Ether (15 mL) was added to the filtrate causing formation of a red oil. Addition of ether to the oil formed a red solid, which was washed with THF (5 mL) and dissolved in DMF (2 mL). Diffusion of ether into the solution afforded the product as violet block-like crystals (53 mg, 50%). Anal. Calcd. for C62H82CoN8O4: C, 70.10; H, 7.78; N, 10.55. Found: C, 69.92; H, 7.92; N, 10.66.
(Et4N)2[Mn(pyN2Me2)2]
The preceding method was followed with use of Mn(OTf)2 (71 mg, 0.20 mmol) and gave the product as a yellow crystalline solid (70 mg, 66%). Anal. Calcd. for C62H82MnN8O4: C, 70.36; H, 7.81; N, 10.59. Found: C, 70.04; H, 7.87; N, 10.46.
(Et4N)2[Zn(pyN2Me2)2]
The preceding method was followed with use of Zn(OTf)2 (73 mg, 0.20 mmol) and gave the product as a yellow crystalline solid (77 mg, 72%). Anal. Calcd. for C62H82N8O4Zn: C, 69.68; H, 7.73; N, 10.48. Found: C, 69.51; H, 7.91; N, 10.52
(2) Binuclear Complexes Derived from Macrocycle ⊂22-H2pyN2dienMe3
Macrocycle 2 and complexes 13 and 15 were prepared as described.7
[Ni(μ2-OH)MnCl(⊂22-pyN2Me3)]
To a solution of (Et4N)[Ni(OH)(⊂22-pyN2dienMe3)] (28 mg, 0.040 mmol) in DMF (2 mL) was added a solution of MnCl2 (5.0 mg, 0.040 mmol) in DMF (1 mL). The reaction mixture was stirred for 1 h, filtered, and ether was diffused into the deep red filtrate for 2 d. The solid was washed with acetonitrile (3 mL) and dried to afford the product as red crystals (11 mg, 42%). Anal. Calcd. for C28H33ClMnN6NiO3: C, 51.68; H, 5.11; N, 12.92. Found: C, 51.62; H, 5.18; N, 13.01.
[Ni(μ2-OH)CuCl(⊂22-pyN2dienMe3)]
To a solution of (Et4N)[Ni(OH)(⊂22-pyN2dienMe3)] (21 mg, 0.030 mmol) in DMF (2 mL) was added a solution of CuCl2 (4.1 mg, 0.030 mmol) in DMF (1 mL). The reaction mixture was stirred for 30 min, filtered, and ether was diffused into the brown-red filtrate to form a solid. This material was washed with acetonitrile (3 mL) and dried in vacuo to give the product as orange-yellow crystals (8.0 mg, 41%).
(3) Complexes Derived from Macrocycle ⊂22-4-BuiO-H2pyN2dienMe3
The five-step synthesis of macrocycle 3, obtained in 16% overall yield based on chelidamic acid, is given elsewhere.14
(Me4N)[Ni(OH)((⊂22-4-BuiO-pyN2dienMe3)]
Macrocycle 3 (56 mg, 0.10 mmol) and Ni(OTf)2 (37 mg, 0.10 mmol) were mixed and stirred in DMF (2 mL) for 15 min to give a light yellow suspension. Me4NOH (25% in methanol, 55 mg, 0.015 mmol) was added and the mixture was stirred for 1 h, forming a red suspension. A second equal portion of Me4NOH was added and the mixture was stirred for 4 h to give a deep red solution with some sticky precipitate which was removed by filtration. Addition of ether (40 mL) to the filtrate deposited an orange-red material, which was dissolved in DMF/THF (6 mL, 1/5 v/v) to give a red solution. Ether (5 mL) was added and a white solid was filtered off. A second portion of ether (10 mL) was added and the solution was stored overnight, resulting in the separation of a red crystalline solid (31 mg, 44%). Absorption spectrum (DMF): λmax (εM) 400 (5460) nm. IR (KBr): νOH 3608 cm!1. 1H NMR (DMF-d7): δ −4.71 (s, 1), 1.03 (d, 6), 2.05 (s, 3), 2.10 (m, 1), 2.18 (m, 4), 2.26 (m, 4), 2.28 (s, 6), 3.35 (s, 4), 3.99 (d, 2), 6.79 (d, 2), 7.04 (m, 4), 7.18 (s, 2), 7.25 (d, 2). Anal. Calcd. for C36H53N7NiO4: C, 61.20; H, 7.56; N, 13.88. Found: C, 60.29, H, 7.70; N, 14.02.
[Ni(μ2-OH)CuCl(⊂22-4-BuiO-pyN2dienMe3)]·DMF
CuCl2 (4.0 mg, 0.030 mmol) in DMF (0.5 mL) was added dropwise to a solution of (Me4N)[Ni(OH)(⊂22-4-BuiO-pyN2-dienMe3)] (21 mg, 0.030 mmol) in DMF (2 mL). The reaction mixture was stirred for 1 h and filtered. Diffusion of ether into the filtrate deposited the product as tea-brown block-like crystals (15 mg, 62%). IR (KBr): νOH 3557 cm!1. Anal. Calcd. for C35H48ClCuN7NiO5: C, 52.25; H, 6.01; N, 12.19. Found: C, 51.91; H, 5.90; N, 11.97.
[Ni(μ2-CN)FeCl(⊂22-4-BuiO-pyN2dienMe3)]·½Et2O
A mixture of (Me4N)-[Ni(OH)(⊂22-4-BuiO-pyN2dienMe3)] (35 mg, 0.050 mmol) and Et4NCN (9.4 mg, 0.060 mmol) was stirred in DMF (3 mL) for 3 h and filtered. FeCl2 (6.3 mg, 0.050 mmol) in DMF (0.5 mL) was added to the orange-red filtrate. The mixture was stirred for 1 hr, filtered, and ether (18 mL) was added slowly to the filtrate to deposit a white precipitate, which was filtered off. Ether was diffused into the filtrate, causing separation of a red crystalline solid. This material was dissolved in 4 mL of DMF/propionitrile (1:1 v/v) and diffused with ether to deposit the product as orange-red crystals (17 mg, 44%). IR: νCN 2117 cm!1. Anal. Calcd. for C35H45ClFeN7NiO3.5: C, 54.61; H, 5.89; N, 12.74. Found: C, 54.59; H, 5.85; N, 12.80.
(4) NiII Complex Derived from Macrocycle ⊂20-H2pyN2dienMe3
The preparation of macrocycle 4 (25% overall yield based on o/nitrobenzaldehyde) is described elswhere.14
[Ni(⊂20-pyN2dienMe3)]
Macrocycle 4 (97 mg, 0.20 mmol) and Ni(OTf)2 (71 mg, 0.20 mmol) were reacted in DMF/THF (5 mL, 3:2 v/v) for 1 h to give a light yellow suspension. Et4NOH (25% in methanol, 118 mg, 0.20 mmol) was added and the reaction mixture was stirred for 1 h forming a light green suspension. A second equal portion of Et4NOH was added and the mixture was stirred for 10 h to give a green solution and a white precipitate, which was removed by filtration. Ether (20 mL) was added to the filtrate, forming a green oil. The mixture was allowed to stand overnight, yielding the product as a green crystalline solid (43 mg, 40%).
(5) Macrocylic Ligand ⊂-H2pyN2SN3Me and Complexes
The synthesis of macrocycle 5 (11% overall yield based on 5a) is given in Figure 3.
(a) Intermediates 5a-5e
To a mixture of thioglycollic acid (21.0 g, 0.140 mol) in thionyl chloride (45 mL) was added 0.15 mL of dry DMF as a catalyst. The mixture was stirred for 4 h at 45EC to give a clear orange solution. Excess thionyl chloride was partially removed to leave a red-black solution from which pure 5a15 was obtained by distillation (130E/3.0 mm Hg). Solutions of 5a (2.80 g, 15.0 mmol) and 3-N-methylaminopentane-1,5-diamine (3.51 g, 30.0 mmol), each in dry toluene (40 mL), were added simultaneously to chloroform (500 mL) by syringe pump over 16 h. After removal of a white precipitate and evaporation of the filtrate, the yellow-orange solid was purified on a silica column eluted with CH2Cl2/MeOH/NH4OH (300/80/1 v/v) to yield 5b as a white solid (1.80 g, 52%). 1H NMR (CDCl3): δ 2.30 (s, 3), 2.48 (t, 4), 3.24 (s, 4), 3.30 (q, 4), 7.26 (s, 2). ESMS: m/z 232 (M+H)+.
To a suspension of 5b (1.80 g, 7.8 mmol) in dry THF (20 mL) was added a solution of Me2SBH3 (16 mL, 32 mmol). The mixture was refluxed at 75EC for 5 h. Methanol was added to the white slurry, the mixture was stirred for 1 h, and the solvent was removed to leave a foam-like residue. This material was refluxed in 6 M aqueous HCl (35 mL) for 3 h and solvent was removed to give a white solid, which was treated with 2 M aqueous NaOH until pH ~14 was reached. The mixture was extracted with chloroform (5 × 70 mL), the combined organic layers were dried (MgSO4), and solvent was removed to leave 5c as a colorless crystalline solid (0.95 g, 60%). 1H NMR (CDCl3): δ 2.17 (s, 3), 2.37 (t, 4), 2.55 (q, 8), 2.63 (t, 4), 2.68 (br, s, 2).
A mixture of N-(tert-butoxycarbonyl)-3-bromomethylaniline16 (1.35 g, 4.72 mmol), 5c (0.48 g, 2.36 mmol), K2CO3 (0.65 g, 4.72 mmol), and KI (0.39 g, 2.36 mmol) was refluxed in acetonitrile (200 mL) for 36 h, the mixture was filtered, and the solvent evaporated to leave a light yellow solid residue. This material was dissolved in dichloromethane (100 mL), the solution was filtered, and the solvent removed. The residue was purified on a silica column eluted with CH2Cl2/MeOH/NH4OH (70/8/5 v/v) to yield 5d as a white foam-like solid (1.04 g, 72%). 1H NMR (CDCl3): δ 1.53 (s, 18), 2.11 (s, 3), 2.45 (s, 4), 2.54 (s, 4), 2.76 (t, 4), 2.98 (t, 4), 3.59 (s, 4), 6.67 (s, 2), 7.05 (d, 2), 7.23 (t, 2), 7.30 (d, 2), 7.35 (s, 2). ESMS: m/z 614 (M+H)+.
To a solution of 5d (1.04 g, 1.70 mmol) in dichloromethane (10 mL) was added trifluoroacetic acid (3 mL); the mixture was stirred for 2 h. The orange-red mixture was added slowly to a 2 M aqueous NaOH solution (50 mL), stirred for 20 min, and diluted with water (20 mL). The organic layer was collected and the aqueous layer was extracted with dichloromethane. The combined organic layers were washed with brine (2 × 70 mL) and dried (MgSO4). Solvent was removed to afford 5e as a pale yellow oil (0.674 g, 96%). 1H NMR (CDCl3): δ 2.12 (s, 3), 2.48 (s, 4), 2.55 (s, 4), 2.76 (t, 4), 2.96 (t, 4), 3.53 (s, 4), 3.66 (s, 4), 6.56 (d, 2), 6.71-6.74 (m, 4), 7.08 (t, 2).
(b) Macrocycle ⊂-H2pyN2SN3Me)]
To a solution of Et3N (6.0 mL) in dry THF (350 mL) was added simultaneously solutions of pyridine-2,6-dicarbonylchloride (0.365 g, 1.79 mmol) in THF (50 mL) and 5e (0.674 g, 1.63 mmol) in THF/acetonitrile (50 mL, 4/1 v/v) by syringe pump over 20 h. The mixture was filtered and solvent was evaporated to give a yellow-brown solid, which was dissolved in dichloromethane (7 mL). Upon standing for several h, the solution deposited a colorless crystalline solid which was collected and washed with dichloromethane (2 × 3 mL). The solid was dissolved in dichloromethane/methanol (8/2 mL) and dried to give the macrocyclic 5 as a white powder (0.47 g, 53%). 1H NMR (Me2SO-d6): δ 2.53 (m, 2), 2.65-2.73 (m, 7), 2.75-2.87 (m, 6), 3.21 (m, 2), 3.64 (s, 4), 3.70 (m, 2), 7.05 (d, 2), 7.37 (t, 2), 7.53 (s, 2), 8.32 (t, 1), 8.39 (m, 4), 11.14 (s, 2). ESMS: m/z 545 (M+H)+.
(c) Complexes. (Me4N)[Ni(OH)(⊂-pyN2SN3Me)]
Macrocycle 5 (82 mg, 0.15 mmol) and Ni(OTf)2 (54 mg, 0.15 mmol) were stirred in DMF (3 mL) for 12 h to give a light yellow suspension. Me4NOH (25% in methanol, 82 mg, 0.15 mmol) was added dropwise and the mixture was stirred for 4 h. An equal portion of Me4NOH was added, the mixture was stirred for 4 h, and was filtered to remove a sticky precipitate. Ether was added to the filtrate to deposit an orange-red solid that was collected and washed with ether (3 mL). The solid was dissolved in DMF (7 mL) and filtered. The red filtrate was diffused with ether to cause separation of a red solid together with some white precipitate. The red product was separated manually and dried (52 mg, 50%). Absorption spectrum (DMF): λmax (εM) 412 (5500), 488 (sh, 1800) nm. IR (KBr): νOH 3601 cm!1. 1H NMR (DMF-d7): δ −4.20 (s, 1), 1.94 (m, 2) 2.12 (s, 3), 2.41 (m, 2), 2.56 (m, 4), 2.80 (m, 2), 2.87 (m, 2), 2.98 (m, 4), 3.48 (d, 2), 3.59 (d, 2), 6.73 (d, 2), 6.98 (t, 2), 7.27 (d, 2), 7.52 (d, 2), 7.67 (s, 2), 8.05 (t, 1). Anal. Calcd. for C34H47N7NiO3S: C, 58.97; H, 6.84; N, 14.16. Found: C, 59.02; H, 6.79; N, 13.97.
[Ni(OH2)(⊂-pyN2SN3Me)]
A solution of (Me4N)[Ni(OH)(⊂-pyN2SN3Me)] (21 mg, 0.03 mmol) was added to solid NH4HCO3 (4.7 mg, 0.06 mmol). The mixture was stirred for 3 h and filtered. The filtrate was diffused with ether to precipitate some colorless crystals which were removed by filtration. The red filtrate was reduced to a red oily solid, which was dissolved in THF (0.5 mL). The solution was diffused with ether to deposit a red microcystalline product (8.0 mg, 43%) which was identified by an X-ray structure determination.
[NiClFeCl(⊂-pyN2SN3Me)]
FeCl2 (3.8 mg, 0.030 mmol) in DMF (0.5 mL) was added dropwise to a red solution of (Me4N)[Ni(OH)(⊂-pyN2SN3Me)] (21 mg, 0.030 mmol) in DMF (3 mL). The reaction mixture was stirred for 2 h and filtered to remove some black precipitate. A second equal portion of FeCl2 in DMF (0.5 mL) was added slowly to the red-brown filtrate. The mixture was stirred for 2 h and filtered. Diffusion of ether into the filtrate afforded the red crystalline product (10 mg, 41%).
[Ni(μ2-CN)FeCl(⊂-pyN2SN3Me)]·Me2SO
A mixture of (Me4N)[Ni(OH)(⊂-pyN2SN3Me3)] (21 mg, 0.030 mmol) and Et4NCN (5.6 mg, 0.036 mmol) was stirred in DMF (2 mL) for 5 h and filtered. Ether was added to deposit a red solid, which was washed with THF/ether (3 mL, 2:1 v/v) and dissolved in DMF (1 mL). FeCl2 (3.8 mg, 0.030 mmol) in DMF (0.5 mL) was added slowly to the red solution, which was stirred for 1 h and filtered. Ether (20 mL) was added to the filtrate to obtain a red solid which was washed with ether (1 mL) and acetonitrile (1 mL). The red solid was dissolved in Me2SO (1 mL) and the solution was layered with ethyl acetate to cause separation of a red crystalline solid and a white precipitate. The red solid was manually separated and dried to yield the product (11 mg, 46%). IR: νCN 2137 cm!1.
[Ni(μ2-OH)Cu(⊂-pyN2SN3Me)](ClO4)
A solution of (Me4N)[Ni(OH)(⊂-pyN2SN3Me3)] (21 mg, 0.030 mmol) in DMF (2.5 mL) was added quickly to a solution of Cu(ClO4)2·6H2O (11 mg, 0.030 mmol) in DMF (0.5 mL). The mixture was stirred for 10 min and filtered. The filtrate was diffused with ether causing the product to separate as a black-green crystalline solid (14 mg, 60%). IR (KBr): νOH 3516 cm!1. Anal. Calcd. for C30H35ClCuN6NiO7S: C, 46.11; H, 4.51; N, 10.76. Found: C, 45.93; H, 4.47; N, 10.54.
(6) Macrocyclic Ligand ⊂-H2pyN2N4Me2 and Complexes
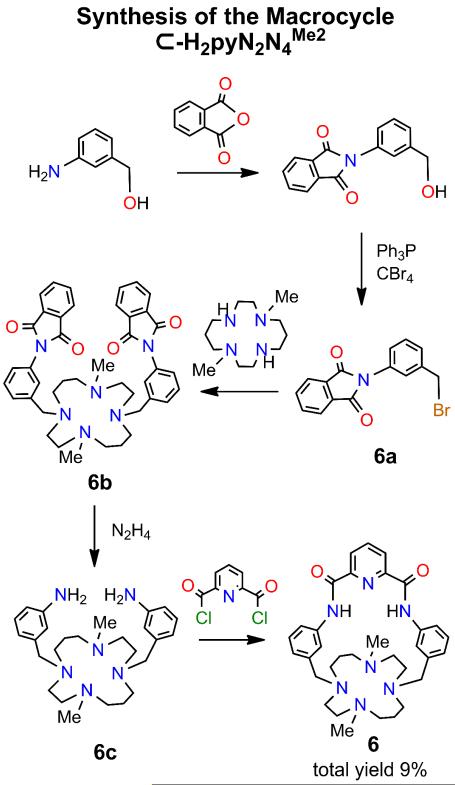
The synthetic route to macrocycle 6 (10% overall yield based on 6a) is provided in Figure 4.
Figure 4.
Synthesic scheme for macrocycle 6 via intermediates 6a-6c.
(a) Intermediates 6a-6c
Compound 6a has been previously used but no preparation was reported.17 PPh3 (4.72 g, 18.0 mmol) was added to a solution of 2-[(3-hydroxymethyl)phenyl]isoindoline-1,3-dione18 (3.04 g, 12.0 mmol) in dichoromethane (30 mL). The mixture was cooled to −5EC and CBr4 (5.97 g, 18.0 mmol) in dichloromethane (30 mL) was added dropwise. The mixture was stirred at −5EC for 2 h and allowed to warm to room temperature. Ethyl acetate (150 mL) was added and the solution was washed with brine (4 × 70 mL). The organic layers were combined and dried over MgSO4, and solvent was removed to leave a light yellow-brown residue. The crude product was purified on a silica column eluted with dichloromethane/hexanes (1:1 v/v) to give 6a as a white solid (3.21 g, 85%). 1H NMR (CDCl3): δ 4.54 (s, 2), 7.42 (m, 2), 7.50 (m, 2), 7.81 (m, 2), 7.96 (m, 2). ESMS: m/z 338 (M + Na)+.
Compound 6a (2.71 g, 8.60 mmol), 1,8-dimethyl-1,4,8,11-tetraazacyclotetradecane (1,8-Me2-[14]aneN4) (0.98 g, 4.3 mmol), K2CO3 (1.19 g, 8.60 mmol), and KI (0.71 g, 4.3 mmol) were refluxed in acetonitrile (300 mL) for 60 h. The mixture was filtered and solvent was removed to leave a light brown solid. This material was purified on a silica column eluted with dichloromethane/methanol/NH4OH to give 6b as a pale yellow solid (0.96 g, 32%). 1H NMR (CDCl3): δ 1.64 (m, 4), 2.11 (s, 6), 2.46 (m, 8), 2.58 (m, 8), 3.61 (s, 4), 7.27 (d, 2), 7.41 (m, 6), 7.78 (m, 4), 7.94 (m, 4). ESMS: m/z 699 (M + H)+, 350 (M + 2H)2+/2.
To a hot solution of 6b (0.96 g, 1.38 mmol) in 30 mL of THF and 10 mL of ethanol was added N2H4·H2O (1.0 g, 20 mmol). The mixture was refluxed at 80EC for 15 h to give a white suspension. Ethanol (50 mL) was added and the mixture was filtered. Solvent removal gave a pale yellow oil. The product was purified on a silica gel column eluted with dichloromethane/methanol/NH4OH (15/3/2 v/v) give 6c as a colorless oily solid (453 mg, 75%). 1H NMR (CDCl3): δ 1.67 (m, 4), 2.14 (s, 6), 2.49 (m, 8), 2.57 (m, 8), 3.46 (s, 4), 3.67 (br s, 4), 6.55 (d, 2), 6.70 (m, 4), 7.06 (t, 2). ESMS: m/z 439 (M + H)+.
(b) Macrocycle ⊂-H2pyN2N4Me2
To a solution of Et3N (5.5 mL) in dry THF (300 mL) was added simultaneously solutions of pyridine-2,6-dicarbonylchloride (0.245 g, 1.20 mmol) in THF (40 mL) and 6c (0.44 g, 1.00 mmol) in THF/acetonitrile (40 mL, 3:1 v/v) by syringe pump over 20 h. The mixture was filtered and the solvent removed to leave a light yellow solid. The crude product was purified on a silica column eluted with dichloromethane/methanol/NH4OH (60/6/1 v/v) to afford the macrocycle 6 as a while powder (285 mg, 50%). 1H NMR (CDCl3): δ 1.70 (m, 2), 1.77 (m, 2), 2.13 (s, 6), 2.21 (m, 6), 2.45 (m, 4), 2.62 (m, 2), 2.92 (m, 2), 3.16 (m, 2), 3.47 (m, 2), 3.74 (m, 2), 6.97 (d, 2), 7.35 (t, 2), 7.44 (s, 2), 8.15 (t, 1) 8.47 (d, 2), 8.56 (d, 2), 9.61 (s, 2). ESMS: m/z 570 (M + H)+, (M + 2H)2+/2.
(c) Complexes. (Me4N)[Ni(OH)(⊂-pyN2N4Me2)]·½Et2O
Ni(OTf)2 (36 mg, 0.10 mmol) in DMF/THF (4 mL, 3:1 v/v) was stirred for 12 h to give a light yellow suspension. Macrocycle 6 (57 mg, 0.10 mmol) was added, followed by dropwise addition of Me4NOH (25% in methanol, 55 mg, 0.15 mmol). The mixture was stirred for 2 h, a second equal portion of Me4NOH was introduced, and stirring was continued for 4 h. The mixture was filtered to remove a sticky precipitate, and ether (20 mL) was added to the filtrate. The orange solid that separated was washed with ether (3 mL), dissolved in DMF (2 mL), and THF (2 mL) was added. The solution was filtered and the filtrate diffused with ether to give a mixture of red and white solids. This was washed with THF (1 mL) and DMF (0.5 mL) to afford the product as red plate-like crystals (35 mg, 46%). Absorption spectrum (DMF): λmax (εM) 412 (5350), 488 (sh, 1800) nm. IR (KBr): νOH 3603 cm!1. 1H NMR (DMF-d7): δ −4.15 (s, 2), 1.45 (m, 2), 1.61 (m, 2), 2.02 (s, 6), 2.07 (m, 2), 2.18 (m, 2), 2.31 (m, 2), 2.42 (t, 4), 2.69 (m, 2), 2.82 (m, 2), 2.99 (m, 2), 3.22 (m, 2), 3.50 (m, 2), 6.72 (d, 2), 6.97 (t, 2), 7.26 (d, 2), 7.52 (m, 4), 8.04 (t, 1). Anal. Calcd. for C39H59N8NiO3.5: C, 62.07; H, 7.88; N, 14.85. Found: C, 62.16; H, 7.91; N, 14.45.
[Ni(μ2-OH)(DMF)2Cu(⊂-pyN2N4Me2)](ClO4)
The preceding compound was recystallized from DMF/THF/C6H5CF3 (1:10:25 v/v) to give the product in essentially quantitative yield as a green-black crystalline solid. The compound was identified by an X-ray structure determination.
X-ray Structure Determinations
Twenty of the compounds in Chart 1 were structurally characterized; for brevity, compounds that are salts are referred to by their cation or anion number. Diffraction-quality crystals were obtained from the following solvents: dichloromethane/ether/hexane, 4; DMF/acetonitrile/ether, 5; methanol/chloroform/ethyl acetate, 6; DMF/ether, 7, 8, 11, 14, 16, 18, 20, 21, 23, 25-27; DMF/toluene/ether, 17, 22; DMF/propionitrile/ether, 19; Me2SO/ethyl acetate, 24; DMF/THF/PhCF3, 28. Diffraction data for 16 (15 K) and 22 (95 K) were obtained using synchrotron radiation at the Advanced Photon Source (APS), Argonne National Laboratory. Intensities of reflections were collected by means of a Bruker APEX II CCD together with the D8 Diffractometer equipped with an Oxford Cryosystems helium/nitrogen open flow apparatus. The collection method involved 0.5E scans in φ at −5E in 2q. Other diffraction data were collected with a Bruker CCD area detector diffractometer equipped with an Oxford 700 low-temperature nitrogen apparatus (100 K). Data integration was carried out using SAINT V7.46A with reflection size optimization, and absorption corrections made with SADABS (Bruker AXS APEX II, Bruker AXS Inc., Madison, WI 53711). Structures were solved by direct methods using the SHELX program package. All non-hydrogen atoms were refined anisotropically and the temperature factors of those disordered atoms were restrained by DELU/SIMU to achieve an anisotropic average. Hydrogen atoms were not added to the cations (7, 11) or solvate molecules (6, 23) owing to highly disordered carbon atoms. The hydrogen atom(s) of −OH groups (7, 17, 18, 28) and the −HCO3 group (8) were located geometrically. Hydrogen atoms of other −OH groups (14, 16, 18, 22, 25-27) were located from difference Fourier maps and refined isotropically. Crystal data and refinement details area given in Tables S1-S4.14 Other refinement details and explanations (wherever necessary) are included in individual CIF files.
The compounds (Et4N)2[M(pyN2Me2)2] with M = Fe, Mn, and Zn yielded the following crystal data. M = Fe (10): triclinic, a = 15.529(3) Å, b = 25.509(5) Å, c = 31.076(6) Å, α = 65.966(4)E, β = 89.778(5)E, γ = 89.945(5)E, V = 11243(4) Å3, Z = 8, P-1; M = Mn (9): triclinic, a = 10.4628(4) Å, b = 16.2333(6) Å, c = 18.9633(7) Å, α = 78.432(1)E, β = 80.522(1)E, γ = 83.378(1)E, V = 3100.9(2), Z = 2, P-1; M = Zn (12): orthorhombic, a = 12.723(5) Å, b = 28.53(1) Å, c = 15,467(6) Å, V = 5614(9), Z = 4, Pbcn. Sufficient data were collected to show that 9 and 10 have the same structure as 11 in its Et4N+ salt. Complex 12 is assigned the structure of 11 based on crystal isomorphism.
The metal contents in selected binuclear complexes were determined at the APS by multiple wavelength anomalous dispersion techniques.14 The following electron densities normalized to one metal atom per molecule were determined and Ni:M atom ratios calculated: 19 – Ni 0.967(3), Fe 0.961(3), 1.01:1; 24 – Ni 0.938(5), Fe 0.851(5), 1.10:1; [25](ClO4) – Ni 1.000(5), Cu 0.964(5), 1.04:l; [28](ClO4) – Ni 0.968(5), Cu 0.953(3), 1.02:1.
RESULTS AND DISCUSSION
Pincer Complexes with Other Metals. (a) CuII
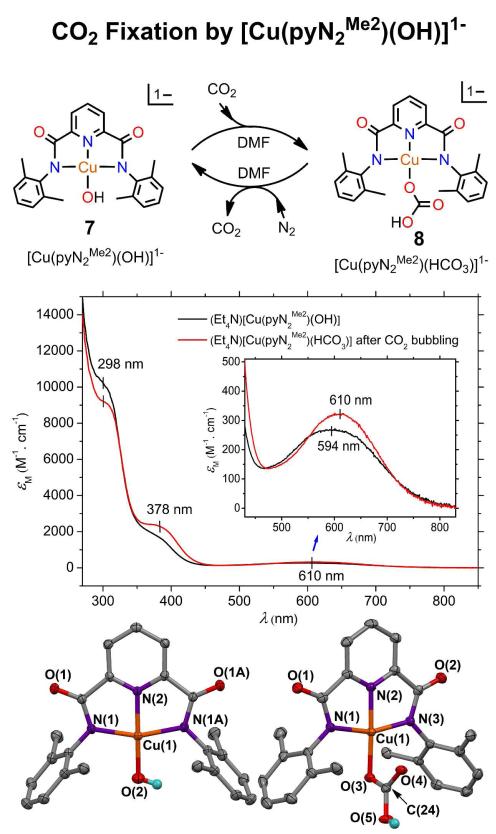
Prior to pursuing ligand alterations in regions I-IV of the complex in Figure 2, we wished to know what other divalent metals might bind in the NNN pincer site. Such metals would be candidates for incorporation into binucleating macrocycles containing that site and also may engage in CO2 fixation. To do so, we utilized for simplicity ligand 1. The planar complexes [M(pyN2Me2)(OH)]1− (M = NiII, PdII) have been previously described. Both react cleanly with CO to afford [M(pyN2Me2)(HCO3)]1− in good yield. The corresponding CuII-OH complex 7 is easily prepared by the reaction of Cu(OTf)2 and Et4NOH in DMF. It reacts immediately with CO2 in DMF to form the η1-HCO3 complex 8 which is isolable in 80% yield. The spectrophotometric conversion 7 → 8 is illustrated with Figure 5 together with the planar structures and selected dimensions of the two complexes which are isostructural with their NiII and PdII counterparts, and nearly isometric with the NiII complex. Mononuclear CuII-OH complexes in different coordination environments have been described.19-21 However, nearly all CuII-mediated CO2 fixation reactions have been carried out with polynuclear bridged oxo and hydroxo reactants and afford carbonate-bridged products.22-25 Only one four-coordinate η1-bicarbonate complex, also a pincer species, has been previously characterized.26,27 It and 8 crystallize as centrosymmetric hydrogen-bonded dimers. The long Cu-O4 distance of 2.542 Å is consistent with a four-coordinate description of 8. Evidently, the system in Figure 5 is the simplest yet devised for CO2 fixation by a CuII reactant.
Figure 5.
UV-visible spectrum of the reaction of carbon dioxide with [Cu(pyN2Me22)(OH)]1− after 15 min bubbling in DMF solution at room temperature and the structures of the reactant and product complexes. Selected bond distances (Å) and angles (deg): [Cu(pyN2Me22)(OH)]1− Cu-O(2) 1.845(3), Cu-N(1) 2.004(2), Cu-N(2) 1.924, N(1)-Cu-N(2) 80.01(6), N(2)-Cu-O(2) 175.5(1); [Cu(pyN2Me2)(HCO3)]1− Cu-O(3) 1.946(2), Cu-N(1) 2.005(3), Cu-N(2) 1.912(3), Cu-N(3) 1.996(2), Cu⋯O(4) 2.542(3), N(2)-Cu-O(3) 178.0(1). In this and succeeding X-ray structures, 50% probability ellipsoids are depicted.
(b) MnII, FeII, CoII, and ZnII
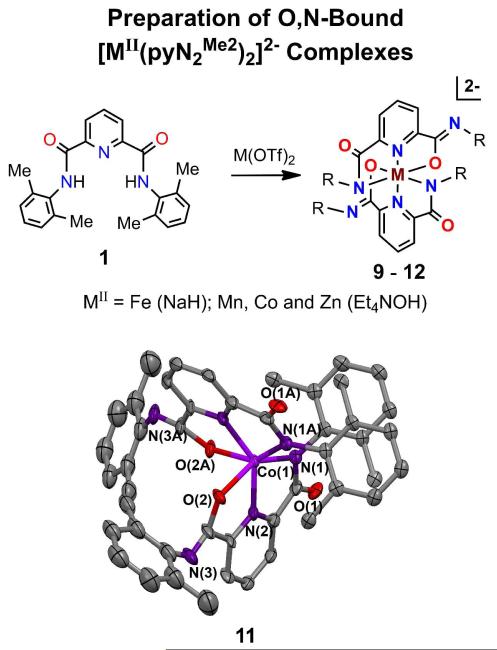
To seek a broader set of reactants, we have examined the reaction of other divalent metals with equimolar H2pyN2Me2. The results with four divalent ions are summarized in Figure 6, from which it is evident that despite the 1:1 reaction stoichiometry six-coordinate bis-ligand complexes 9-12 are formed. Because of ligand steric factors, these isostructural complexes adopt a MN4O2 coordination unit in which one N#substituent of each ligand is rotated in order to place two oxygen atoms mutually cis, thus reducing destabilizing steric interactions between N-substituents. The structure of 11 (Figure 6) reveals a C2-symmetric configuration far removed from octahedral with approximately cis oxygen atoms and trans O/N-Co-N angles much smaller than 180E. This situation arises because, unlike low-spin d8 and d9, the other four metal ions do not have an intrinsic planar stereochemical preference based on d-orbital energetics. Further, the pyN2Me2 ligand dianion does not engender bond angles in the usual tetrahedral range. This type of coordination isomerism has several precedents among 2,6-pyridinedicarboxamido complexes.28-32 These four metal ions are unsuitable for coordination in the pincer site of a macrocycle such as that in Figure 2, but are candidates for binding in the other site.
Figure 6.
Preparation of a set of O,N-bound complexes [MII(pyN2Me22)]2− (M = Mn, Fe, Co, Zn); ligand deprotonation reagents are indicated. The structure of [CoII(pyN2Me22)]2− is shown with the atom labeling scheme. A crystallographically imposed C2 axis relates atoms X(n) and X(nA). Selected bond distances (Å) and angles (deg): Co-N(1) 2.134(3), Co-N(2) 2.028(3), Co-O(2) 2.226(2), N(1)-Co-N(2) 77.1(1), N(2)-Co-O(2) 74.3(1), O(2)-Co-O(2A) 96.4(1), N(2)-Co-N(2A) 145.6(2), O(2)-Co-N(1) 149.4(1), N(2)-Co-N(1A) 127.1(1). Other angles at the Co atom are in the 83-98E range.
Modifications in Complexes of Binucleating Ligands
This phase of our work on complexes of binucleating ligands is focused on synthesis and structure proofs by X-ray methods. Physical properties will be described subsequently as needed in reactivity studies. Displayed in Figure 2 is the reference molecule subject to modification. The changes that follow are illustrative of a much broader set of potential alterations. Yields of isolated compounds are indicated. For example, variation in divalent M is confined to MnII, FeII, and CuII and bridging ligands to hydroxo and cyano, although other M atoms and bridges are surely possible. Cyanide was employed to determine if macrocycle flexibility would accommodate a two-atom bridge NiII-CN-MII approaching linearity. Further, cyanide is an inhibitor of CODH. The X-ray structures of macrocycles 4-6 and complexes 14 and 16-28 were determined; those of macrocycle 2 and complexes 13 and 15 were described earlier.7 Metric features of bridge units are collected in Table 1; other structural information is available in figure legends and elsewhere.14 All complexes except 27 and 28 contain planar NiII coordinated by the NNN pincer ligand. Parameters of the NiN3 unit are nearly identical across the set 13-26; data for 17 and 26 are included in Figures 10 and 15, respectively. Stereochemistry at five-coordinate M sites is approximated in terms of the shape parameter τ = (α − β)/60 where α and β are largest and next-largest bond angles, respectively.33 For idealized square pyramidal (SP) τ = 0 and for trigonal bipyramidal (TBP) τ = 1. Note that for four binuclear complexes, mixed metal contents were determined directly by anomalous dispersion methods.
Table 1.
Ni-X-M Bridge Matric Parameters
| Compounds Ni-X-M |
14 Ni-OH-Mn |
15a Ni-OH-Fe |
16 Ni-OH-Cu |
18 Ni-OH-Cu |
19 Ni-CN-Fe |
|---|---|---|---|---|---|
| Ni-O/C/Cl (Å) | 1.861(3) | 1.927(4) | 1.899(3) | 1.895(2) | 1.878(3) |
| M-O/N/Cl (Å) | 2.089(4) | 2.059(3) | 2.134(4) | 2.026(2) | 2.099(2) |
| Ni-O-M (deg) | 142.5(2) | 140.0(2) | 147.6(2) | 141.1(1) | - |
| Ni-C-N (deg) | - | - | - | - | 170.2(2) |
| Fe-N-C (deg) | - | - | - | - | 138.3(2) |
| Ni···M (Å) | 3.741(2) | 3.747(2) | 3.873(2) | 3.697 (1) | 4.709(1) |
|
| |||||
| Compounds Ni-X-M |
23 Ni-Cl Cl-Fe |
24 Ni-CN-Fe |
25 Ni-OH-Cu |
27 Ni-OH-Cu |
28 Ni-OH-Cu |
|
| |||||
| Ni-O/C/Cl (Å) | 2.178(2) | 1.853(3) | 1.890(3) | 2.056(4) | 2.008(3) |
| M-O/N/Cl (Å) | 2.236(2) | 2.084(3) | 1.936(3) | 1.924(4) | 1.936(3) |
| Ni-O-M (deg) | - | - | 150.4(2) | 153.1(2) | 152.7(2) |
| Ni-C-N (deg) | - | 170.5(3) | - | - | - |
| Fe-N-C (deg) | - | 157.2(2) | - | - | - |
| Ni···M (Å) | 5.315(1) | 4.927(1) | 3.699(1) | 3.871(1) | 3.833(2) |
Ref. 7.
Figure 10.
Structures of NiII-OH complex 17 and binuclear complexes containing the bridge units NiII-OH-CuII (18) and NiII-CN-FeII (19). Ni-N3 site of 17: Ni-N(1) 1.912(2) Å, Ni-N(3) 1.916(2) Å, Ni-N(2) 1.828(2) Å, N(1)-Ni-N(2) 82.5(1)E, N(2)-Ni-N(3) 82.2(1)E, N(1)-Ni-N(3) 163.8(1)E. Cu site of 18: Cu-N(5) 2.033(3) Å, Cu-N(6) 2.130(3) Å, Cu-Cl(1) 2.239(1) Å, Cu⋯N(4) 2.702(3) Å.
Figure 15.
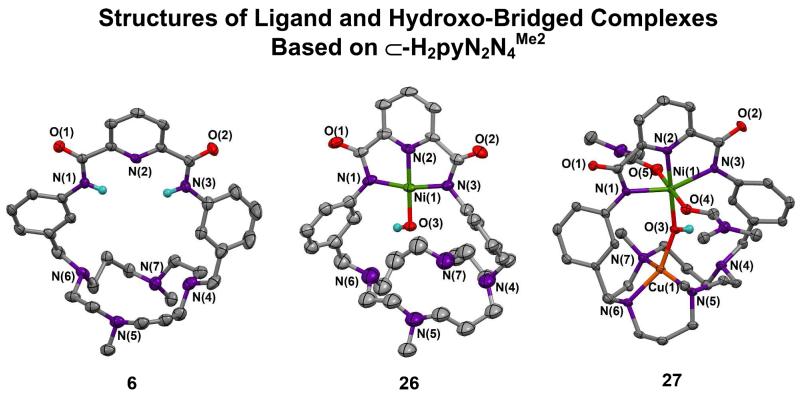
Structures of macrocycle 6, mononuclear NiII-OH complex 26, and binuclear complex 27 containing a NiII-OH-CuII bridge. Ni site in 26 (Å): Ni-N(1) 1.917(9), Ni-N(2) 1.828(3), Ni-N(3) 1.902(8), Ni-O(3) 1.821(2). Ni site in 27 (Å): Ni-N(1) 2.110(4), Ni-N(2) 1.991(4), Ni-N(3) 2.149(4). Cu site in 27 (Å): Cu-N(5) 2.073(4), Cu-N(6) 2.084(4), Cu-N(7) 2.067(5), Cu⋯N(4) 3.070(4).
(a) Region I
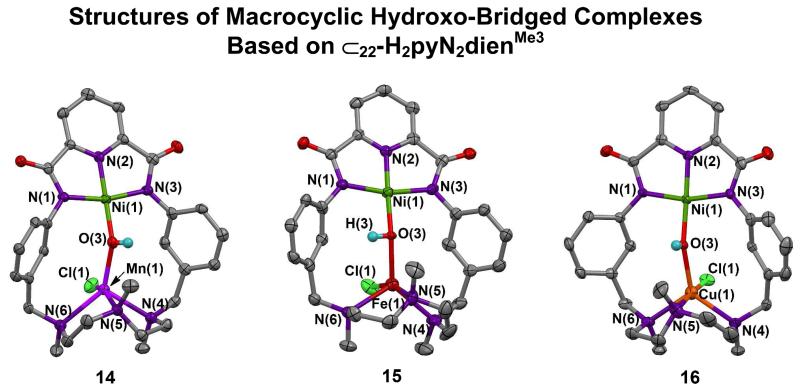
Different metals are incorporated by binding in the vacant site of mononuclear NiII-OH complexes 13, 17, 21, and 26. These precursors are readily prepared and are recognized by upfield −OH resonances at −4.1 to −4.7 ppm (DMF). As shown in Figure 7, different metals M = MnII (14) and CuII (16) can be readily inserted into the vacant triamine site of terminal hydroxo complex 13 in ca. 40% yield by reaction with metal chlorides in DMF. The FeII complex 15, the first binuclear complex of macrocyle 2,7 is included for comparison. The M sites are five-coordinate with intermediate shapes (τ = 0.52 for 14 and 0.50 for 15), and approach TBP with 16 (τ = 0.78). Complexes 19 (44%, Figure 9) and 23 (46%, Figure 12) containing the NiII-CN-MII bridge were obtained by cyanide displacement of hydroxo.
(b) Region II
Neutral complexes 14-16 are insoluble or sparingly soluble in organic solvents such as DMF and acetonitrile; as such they are not amenable to studies of solution properties and reactivity. We find that substitution of an isobutoxy group in the 4-position of the pyridine ring affords complexes that are freely soluble in DMF and soluble (but less so) in acetonitrile. These complexes are derived from macrocycle 3 which is accessible by five steps in 16% overall yield from chelidamic acid.14 Two complexes, hydroxo-bridged 18 (M = CuII, 62%) and cyano-bridged 19 (M = FeII, 44%) were prepared from 17 as indicated in Figure 9. Structures are set out in Figure 10. The bridge structural parameters of 18 are similar to those of the Ni/Cu complex 16 except for the Ni⋯Cu separation which is 0.17 Å shorter. The CuII site does not approach the TBP shape of 16. Instead, the site reveals a highly irregular four-coordinate CuN2OCl unit with a NNCu/CuOCl dihedral angle of 38.6E and a non-bonded Cu-N4 distance of 2.702(3) Å. This arrangement apparently influences the metal-metal distances, but why substitution at the remote 4-position of the pyridyl ring affects structure is unclear. The DMF solvate molecule14 does not interact with this site. Cyanide can be readily interposed in the macrocyclic structure 19, affording nearly linear Ni-C-N (170E) and bent C-N-Fe (138E) bridge segments and an increase of nearly 1.0 Å in metal-metal distance. The FeII site approaches SP coordination (τ = 0.16) with chloride in the apical position. Thus the reference macrocycle can stabilize two-atom bridges.
(c) Region III
Inasmuch as the Ni⋯Fe separation in active and cyanide-inhibited CODH is less than 3.0 Å,1,4,34 we wished to decrease the Ni⋯M distance in the reference macrocycle. This is most readily accomplished synthetically by changing the two connecting phenylene rings from 1,3− to 1,2−substitution. Macrocycle 4 differs from 2 and 3 by having 20 rather than 22 atoms in its inner ring. Relevant information is provided in Figure 11, which includes a structure proof of the macrocycle. Reaction of 4 with Ni(OTf)2 and base leads to the green distorted octahedral complex 20 (40%) instead of a complex with an NiII-OH group and a vacant triamine locus. While the pincer site is retained, the smaller ring and the proclivity of NiII for octahedral coordination favor the observed product. In this context, we note that another NNN pincer macrocycle with a 20-membered ring and a potential pyridyldiamino binding site forms a distorted octahedral complex with FeII.35 A shorter metal-metal distance requires a different macrocycle design.
(d) Region IV
Two changes were made in the M binding site of the reference molecule by replacement of the triamine chain with macrocyclic components. The 1,3-phenylene connectors were retained in both cases. The 7-Me-[12]aneSN3 ring was appended by the procedure of Figure 3 to give macrocycle 5 with a 22-membered internal ring. The unsubstituted [12]aneSN3 ring has been prepared and binds divalent ions including CuII and ZnII with three M-N bonds and a longer M-S interaction.36 The macrocycle readily forms pincer hydroxo complex 21 (50%) from which 22-25 (40-60%) are prepared as indicated in Figure 12. Structures of the macrocycle and these complexes are collected in Figure13. NH4HCO 3acts as a proton donor to 21, yielding aquo complex 22 whose Ni-O bond is 0.10 Å longer than 21; 22 should be reactive to substitution at the NiII site. Two equiv of FeCl2 results in unexpected removal of the hydroxo group for chloride and insertion of FeII in the SN3 site to afford the unbridged complex 23 with a TBP iron site (τ = 0.91). Cyano-bridged Ni/Fe complex 24 results from hydroxo substitution of 21. It features a bridge more closely approaching linearity than 19 and a six-coordinate iron site with a weak Fe-S interaction (2.528(1) Å). Hydroxo-bridged Ni/Cu complex 25 is readily formed by the reaction of CuII with 21 and contains a non-planar CuN2OS site with one nitrogen atom uncoordinated. These results reveal that macrocycle 5 can stabilize binuclear unbridged and hydroxo- and cyano-bridged complexes with variable coordination at the M site. This structural design should be capable of elaboration with other macrocycles as the M site. One closely related example is 1,7-Me2/[12]aneN437 (1,7-Me2-cyclen), which binds divalent metal ions with high affinity.38 Another example is considered next.
Figure 13.
Structures of macrocycle 5, mononuclear NiII complexes 21 (Ni-O 1.824(4) Å) and 22 (Ni-O 1.917(2) Å) derived from 5, unbridged 23, and bridged reaction products 24 and 25 from 21. Fe site in 24 (Å): Fe-Cl(1) 2.342(1), Fe-N 2.084(3)-2.339(2), Fe-S 2.528(1). Cu site in 25 (Å): Cu-N(5) 2.018(4), Cu-N(6) 2.037(3), Cu-S(1) 2.358(1), Cu⋯N(4) 3.011(4).
The procedure in Figure 4, which involves reaction of the benzylic bromide 6a with the substituted cyclam 1,8-Me2-[14]aneN4, deprotection of 6b with hydrazine, and coupling of 6c with pyridine-2,6-dicarbonylchloride, affords macrocycle 6 with the substituted cyclam ring as the M site. Reactions of 6 are summarized in Figure 14 and the structure of the macrocycle and its complexes are presented in Figure 15. Reaction of 6 with Ni(OTf)2 leads to mononuclear 26 (46%) whose coordination unit is isostructural with 13, 17, and 21. Reaction with CuII affords the bis(DMF) hydroxo-bridged complex 27 (80%) which upon recrystallization from a solvent mixture containing a small amount of DMF converts to the mono-DMF adduct 28.14 Bridge dimensions of the two complexes are quite similar (Table 1).
Complex 27 contains two unexpected features. The NiII site is six-coordinate with trans DMF ligands (O-Ni-O = 177.7(2)E) and bond lengths (2.14-2.15 Å) ca. 0.10-0.15 Å longer than in (paramagnetic) [Ni(DMF)6]2+ (Ni-O = 2.04-2.06 Å)39,40 and various cationic pincer complexes (unreported magnetism).41-43 In 28 this distance decreases to 2.052(4) Å in the absence of a trans ligand.43 All NiII-OH complexes in DMF solution show 1H NMR spectra indicative of diamagnetism and weak or nil out-of-plane coordination. We have not encountered additional ligation in any other NiII NNN pincer coordination unit where the strong in-plane ligand field is expected to favor a planar structure. However, compared to diamagnetic 26, the Ni-N bond lengths in 27 and 28 are 0.16-0.25 Å and Ni-OH bond lengths are 0.19-0.24 Å longer (Figure 15, Table 1). These results imply a spin state change at NiII to S = 1 (as in [Ni(DMF)6]2+) upon DMF binding.
The CuII atom in 27 and 28 does not assume CuN4 binding in the cyclam ring but instead assumes an irregular four-coordinate CuN3O structure with N4 uncoordinated at 3.07 Å; bonded Cu-N distances are 2.07-2.08 Å. Similar distorted stereochemistry occurs at the CuII sites in 18, 25, and 28. Given the binding affinity of cyclam-type ligands toward CuII, and the occurrence of the planar CuN4 unit in such complexes,44-46 we conclude that the macrocycle conformation does not favor this conventional coordination mode. A similar factor apparently is operative in 25 as well. Nonetheless, CuII is bound tightly in these structures.
Bridge Structures
Macrocyclic binucleating ligands facilitate entry to and sustain the viability of supported [NiII-X-MII] bridges with X = OH− and CN− and variable M. Whereas homometallic hydroxo and cyano bridges between divalent ions are commonplace, supported or unsupported heterometallic bridges—especially one such bridge per molecule—are not. Such bridges nearly always require one trivalent ion, as exemplified by, e.g., the assemblies [FeIII-(OH)-CuII]47 and [FeIII-(CN)-CuII,I]46,48,49 prepared in this laboratory. We examine briefly the metric features collected in Table 1.
In the mononuclear diamagnetic NiII-OH complexes 13, 17, 21, and 26, Ni-O bond lengths are nearly constant at 1.802(4)-1.825(2) Å. Hydroxo-bridged complexes 14-16, 18, and 25 when compared against the appropriate member of this set show Ni-O bond length increases of 0.06-0.10 Å indicative of diamagnetic NiII. With 27 and 28, increases are larger (0.19, 0.24 Å) because of apparent high-spin NiII in these complexes. Increases in Ni-O bond lengths are to be expected upon bridge formation and are relatively small with diamagnetic NiII. Hydroxo bridges of 14-18 exhibit small variations in Ni-O-M angles (140-147E) and Ni⋯M distances (3.70-3.87 Å). The shortest distance is associated with the near-TBP CuII stereochemistry in 16, and the longest with the unexpected four-coordinate CuII site in 18. The structures in Figures 8 and 10 and the data of Table 1 delineate the NiII-(OH)-MII bridge geometry supported by macrocycles 2 and 3 for metal ionic radii of 0.7-0.9 Å. Figures 13 and 15, and Table 1 convey this information for complexes derived from macrocycles 5 and 6.
Figure 8.
Structures of complexes containing the NiII-OH-MII bridge unit (MII = Mn (14), Fe (15), Cu (16)). Complex 157 is included for comparison.
Cyano-bridged complexes 19 and 24, based on different macrocycles 3 and 5, respectively, reveal Ni-C bonds that differ by 0.04 Å, expected nearly linear Ni-C-N fragments (170E), and bent Fe-N-C segments whose angles (138E, 157E) are presumably dependent on macrocyclic constraints. These results together with the structure of [Ni(CN)FeCl(⊂22-pyN2dienMe3)]7 show that 2, 3, and 5 are capable of supporting two-atom bridges, here at Ni⋯Fe distances of 4.71 and 4.93 Å. Lastly, unbridged 23 with two oppositely placed chloride atoms in the bridging void, exhibits a Ni⋯Fe distance of 5.315(1) Å, the best current measure of the maximum distance available for insertion of a bridging group in macrocycle 5. No such complex has been isolated with the other macrocycles.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by NIH Grant GM 28856. We thank Dr. Shao-Liang Zheng for crystallographic assistance and useful discussions. ChemMetCARS Sector 15 at the APS is principally supported by the National Science Foundation-Department of Energy under grant number NSF-CHE-0822838. Use of the APS was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Footnotes
ASSOCIATED CONTENT
! Supporting Information. Crystallographic data for 20 compounds (Tables S1-S4), structure of complex 28, synthesis of macrocycles 3 and 4, UV-visible spectra of the NiII-OH compounds 17, 21 and 26.
REFERENCES
- 1.Jeoung J/H, Dobbek H. Science. 2007;318:1461–1464. doi: 10.1126/science.1148481. [DOI] [PubMed] [Google Scholar]
- 2.Gong W, Hao B, Wei Z, Ferguson DJ, Tallant T, Krzycki JA, Chan MK. Proc. Natl. Acad. Sci. USA. 2008;105:9558–9563. doi: 10.1073/pnas.0800415105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kung Y, Doukov TI, Seravalli J, Ragsdale SW, Drennan CL. Biochemistry. 2009;48:7432–7440. doi: 10.1021/bi900574h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amara P, Mouesca J/M, Volbeda A, Fontecilla/Camps JC. Inorg. Chem. 2011;50:1868–1878. doi: 10.1021/ic102304m. [DOI] [PubMed] [Google Scholar]
- 5.Panda R, Berlinguette CP, Zhang Y, Holm RH. J. Am. Chem. Soc. 2005;127:11092–11101. doi: 10.1021/ja052381s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Tessier C, Holm RH. Inorg. Chem. 2007;46:2691–2699. doi: 10.1021/ic062362z. [DOI] [PubMed] [Google Scholar]
- 7.Huang D, Holm RH. J. Am. Chem. Soc. 2010;132:4693–4701. doi: 10.1021/ja1003125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbreviations are given in Chart 1.
- 9.Huang D, Makhlynets OV, Tan LL, Lee SC, Rybak/Akimova EV, Holm RH. Proc. Natl. Acad. Sci. USA. 2011;108:1222–1227. doi: 10.1073/pnas.1017430108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang D, Makhlynets OV, Tan LL, Lee SC, Rybak-Akimova EV, Holm RH. Inorg. Chem. 2011;50:10070–10081. doi: 10.1021/ic200942u. [DOI] [PubMed] [Google Scholar]
- 11.Vigato PA, Tamburini S, Bertolo L. Coord. Chem. Rev. 2007;251:1311–1492. [Google Scholar]
- 12.Vigato PA, Tamburini S. Coord. Chem. Rev. 2008;252:1871–1995. [Google Scholar]
- 13.Vigato PA, Peruzzo V, Tamburini S. Coord. Chem. Rev. 2012;256:953–1114. [Google Scholar]
- 14.See paragraph at the end of this article for Supporting Information available.
- 15.Coulter KR, McAuley A, Rettig S. Can. J. Chem. 2001;79:930–937. [Google Scholar]
- 16.Brown FJ, Bernstein PR, Cronk LA, Dosset DL, Hebbel KC, Maduskuie TP, Jr., Shapiro HS, Vacek EP, Yee YK, Willard AK, Krell RD, Snyder DW. J. Med. Chem. 1989;32:807–826. doi: 10.1021/jm00124a014. [DOI] [PubMed] [Google Scholar]
- 17.Rensing S, Schrader T. Org. Lett. 2002;2:2161–2164. doi: 10.1021/ol025976q. [DOI] [PubMed] [Google Scholar]
- 18.dos Santos JL, Lanaro C, Lima LM, Gambero S, Franco-Penteado CF, Alexandre-Moreira S, Wade M, Yerigenhally S, Kutlar A, Meiler SE, Costa FF, Chung M. J. Med. Chem. 2011;54:5811–5819. doi: 10.1021/jm200531f. [DOI] [PubMed] [Google Scholar]
- 19.(a) Harata M, Hasegawa K, Jitsukawa K, Masuda H, Einaga H. Bull. Chem. Soc. Jpn. 1998;71:1031–1038. [Google Scholar]; (b) Fujisawa K, Kobayashi T, Fujita K, Kitajima N, Moro/oka Y, Miyshita Y, Yamada Y, Okamoto K. Bull. Chem. Soc. Jpn. 2000;73:1797–1804. [Google Scholar]; (c) Berreau LM, Mahapatra S, Halfen JA, Young VG, Jr., Tolman WB. Inorg. Chem. 1996;35:6398–6341. [Google Scholar]; (d) Jitsukawa K, Harata M, Arii H, Sakurai H, Masuda H. Inorg. Chim. Acta. 2001;324:108–116. [Google Scholar]; (e) Tubbs KJ, Fuller AL, Bennett B, Arif H, Berreau LM. Inorg. Chem. 2003;42:4790–4791. doi: 10.1021/ic034661j. [DOI] [PubMed] [Google Scholar]; (f) Price JR, Fainerman-Melnikova M, Fenton RR, Gloe K, Lindoy LF, Rambusch T, Skelton BW, Turner P, White AH, Wichmann K. Dalton Trans. 2004:3715–3726. doi: 10.1039/B412437E. [DOI] [PubMed] [Google Scholar]; (g) Cheruzel LE, Cecil MR, Edison SE, Mashuta MS, Baldwin MJ, Buchanan RM. Inorg. Chem. 2006;45:3191–3202. doi: 10.1021/ic051280s. [DOI] [PubMed] [Google Scholar]; (h) Yao L, Li W-H. Acta Crystallogr. 2009;E65:m1532. doi: 10.1107/S1600536809043189. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Huang M-L. Acta Crystallogr. 2010;E66:m1312. doi: 10.1107/S1600536810037529. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Yoshida Y, Miyamoto R, Nakato A, Santo R, Kuwamura N, Gobo K, Nishioka T, Hirotsu M, Ichimura A, Hashimoto H, Kinoshita I. Bull. Chem. Soc. Jpn. 2011;64:600–611. [Google Scholar]
- 20.Lee SC, Holm RH. J. Am. Chem. Soc. 1993;115:11789–11798. [Google Scholar]
- 21.Donoghue PJ, Tehranchi J, Cramer CJ, Sarangi R, Solomon EI, Tolman WB. J. Am. Chem. Soc. 2011;133:17602–17605. doi: 10.1021/ja207882h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitajima N, Hikuchi S, Tanaka M, Moro-oka Y. J. Am. Chem. Soc. 1993;115:5496–5508. [Google Scholar]
- 23.Bazzicalupi C, Bencini A, Bencini A, Bianchi A, Corana F, Fusi V, Giorgi C, Paoli P, Paoletti P, Valtancoli B, Zanchini C. Inorg. Chem. 1996;35:5540–5548. doi: 10.1021/ic9603262. [DOI] [PubMed] [Google Scholar]
- 24.Nishida Y, Yatani A, Nakao Y, Taka J, Kashino S, Mori W, Suzuki S. Chem. Lett. 1999:135–136. [Google Scholar]
- 25.Fenandes C, Neves A, Bortoluzzi AJ, Szpoganicz B, Schwingel E. Inorg. Chem. Commun. 2001;4:354–357. [Google Scholar]
- 26.Jones PG, Hrib CG, Petrovic D, Tamm M. Acta Crystallogr. 2007;E63:m2768. [Google Scholar]
- 27.Petrovic D, Bannenberg T, Randoll S, Jones PG, Tamm M. Dalton Trans. 2007:2812–2822. doi: 10.1039/b703183a. [DOI] [PubMed] [Google Scholar]
- 28.Wasilke J-C, Wu G, Bu X, Kehr G, Erker G. Organometallics. 2005;24:4289–4297. [Google Scholar]
- 29.Dwyer AN, Grossel MC, Horton PN. Supramol. Chem. 2004;16:405–410. [Google Scholar]
- 30.Wallenhorst C, Axenov KV, Kehr G, Samec JSM, Fröhlich R, Erker GZ. Naturforsch. 2007;62b:783–790. [Google Scholar]
- 31.Tyler LA, Olmstead MM, Mascharak PK. Inorg. Chim. Acta. 2001;321:135–141. [Google Scholar]
- 32.Harrop TC, Tyler LA, Olmstead MM, Mascharak PK. Eur. J. Inorg. Chem. 2003:475–481. [Google Scholar]
- 33.Addison AW, Nageswara Rao T, Reedijk J, van Rijn J, Verschoor GC. J. Chem. Soc., Dalton Trans. 1984:1349–1356. [Google Scholar]
- 34.Jeoung J-H, Dobbek H. J. Am. Chem. Soc. 2009;131:9922–9923. doi: 10.1021/ja9046476. [DOI] [PubMed] [Google Scholar]
- 35.Brooker S, Simpson TJ. J. Chem. Soc., Dalton Trans. 1998:1151–1154. [Google Scholar]
- 36.Marcus ST, Bernhardt PV, Grondahl L, Gahan LR. Polyhedron. 1999;18:3451–3460. [Google Scholar]
- 37.Piersanti G, Varrese MA, Fusi V, Giorgi L, Zappia G. Tetr. Lett. 2010;51:3436–3438. [Google Scholar]
- 38.Ciampolini M, Micheloni M, Nardi N, Paoletti P, Dapporto P, Zanobini F. J. Chem. Soc., Dalton Trans. 1984:1357–1362. [Google Scholar]
- 39.Li W-S, Blake AJ, Champness NR, Schröder M, Bruce DW. Acta Crystallogr. 2012;C54:349–351. [Google Scholar]
- 40.McKee V, Metcalfe T, Wikaira J. Acta Crystallogr. 1996;C52:1139–1141. [Google Scholar]
- 41.Behrens H, Fröhlich R, Würthwein E-U. Eur. J. Org. Chem. 2005:3891–3899. [Google Scholar]
- 42.Chen X, Zhang L, Yu J, Hao X, Liu H, Sun W-H. Inorg. Chim. Acta. 2011;370:156–163. [Google Scholar]
- 43.Huang D, Deng L, Sun J, Holm RH. Inorg. Chem. 2009;48:6159–6166. doi: 10.1021/ic900494u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tasker PA, Sklar LJ. Cryst. Mol. Struct. 1975;5:329–344. [Google Scholar]
- 45.Elias H. Coord. Chem. Rev. 1999;187:37–73. [Google Scholar]
- 46.Scott MJ, Holm RH. J. Am. Chem. Soc. 1994;116:11357–11367. [Google Scholar]
- 47.Scott MJ, Zhang HH, Lee SC, Hedman B, Hodgson KO, Holm RH. J. Am. Chem. Soc. 1995;117:568–569. [Google Scholar]
- 48.Scott MJ, Lee SC, Holm RH. Inorg. Chem. 1994;33:4651–4662. [Google Scholar]
- 49.Lim BS, Holm RH. Inorg. Chem. 1998;37:4898–4908. doi: 10.1021/ic9801793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.