Abstract
Our lab has previously demonstrated that cytoplasmic trafficking and subsequent nuclear entry of non-viral plasmid DNA can be significantly enhanced through the application of cyclic stretch following transfection in vitro 1,2. Here, we demonstrate that cyclic stretching of the murine lung using ventilation immediately following the endotracheal administration and transthoracic electroporation of plasmid DNA increases exogenous gene expression up to 4-fold over Our mice that were not ventilated after plasmid administration and transfection via electroporation in vivo. This increase is time and sequence specific (i.e. the ventilation must occur immediately after the transfection event). The ventilation-enhanced gene transfer is also amplitude-dependent, confirming similar studies completed in vitro, and is mediated, at least in part, through the cytoplasmic tubulin deacetylase, HDAC6. Using immunohistochemistry, we demonstrate that this increase in expression is due to an increase in the number of cells expressing the exogenous protein rather than an increase in the amount of protein produced per cell. These studies demonstrate the potential mechanical stimulation has in vivo in significantly increasing non-viral DNA gene expression, and may ultimately pave the way for more successful clinical trials using this type of therapy in the future.
Introduction
Currently, non-viral gene therapy in the lung is limited by two factors: side effects of certain vectors, and inefficiency of others for gene transfer. Over the past decade, numerous viral and non-viral approaches have been proposed and developed for transferring genes to the lung but all have serious limitations. Inefficiency of gene transfer, immunological responses and non-specificity of cell targeting are just a few of the problems. One promising method that has been used effectively in the living lung is electroporation 3-9. This approach results in high level expression of genes in multiple cell types, including type I and type II alveolar epithelial cells, airway epithelial cells, endothelial cells, and airway and vascular SMCs. We have shown that this approach does not result in inflammation because electroporation bypasses the TLR9 innate immunity signaling pathway to deliver DNA directly into the cell without activating TLR9 10. However, despite achieving transfer and expression of therapeutic genes at levels sufficient to prevent or treat existing lung disease 8,11, increased gene expression is needed if the approach is to move toward the clinical arena.
To date, most studies designed to optimize transfection and characterize the process of gene trafficking and delivery have been done in vitro using cultured cells grown in a static environment. This growth environment may not offer researchers an accurate representation of how cells in the body behave, as in vivo, cells mostly grow under dynamic conditions. When cells are perturbed via a variety of forces, including normal (tensile/compressive) and shear forces, it has been shown that gene expression, cytoskeletal structures, signal transduction pathways, and cell physiology can be altered 12-15. Any or all of these alterations can significantly impact the transport barriers in a cell. Therefore, it is imperative to understand how these forces influence gene transfer in order to improve gene delivery methodologies.
In the lung, normal stresses resulting from constant cyclical expansion and contraction of the basement membrane due to respiration may play an important role in a lung cell's in vivo morphology. Furthermore, an injured lung may undergo larger perturbations, resulting in increased stresses, due to mechanical ventilation or decreased lung capacity resulting from edema or damaged lung tissue 16. Previous studies from our lab have shown that physiological levels of equibiaxial cyclic stretch (10% change in basement surface area 17) applied to cultured human pulmonary epithelial cells (A549 cells or primary rat type II pneumocytes) after transfection, either by lipoplexes or electroporation, significantly increased gene transfer and expression 1,2. We suspect that this enhancement is due to altered cytoplasmic trafficking of plasmid DNA as a result of cytoskeletal reorganization and post-translational modification of microtubules 1,18. With these findings, we were interested in determining if cyclic stretch-mediated enhancement of gene transfer and expression would work in vivo. In this study we instilled plasmid DNA into the lungs of mice and after electroporation across the chest, animals were placed on a mechanical ventilator to affect the strain profiles of the cells within the lung. Reporter gene expression was measured to determine the efficiency of gene transfer.
Materials and Methods
Plasmids
pCMV-Lux-DTS and pCMV-LacZ-DTS contain an SV40 DNA nuclear targeting sequence (DTS) and express firefly luciferase and ß-galactosidase, respectively, from the CMV immediate early promoter/enhancer 4. Both plasmids were purified with Qiagen's Giga-prep columns (Valencia, CA) for use in cell studies and animals.
Cell Culture and Cyclic Stretch
A549 cells (ATCC, Manassas, VA), a human lung adenocarcinoma cell line, were grown in high glucose DMEM supplemented with 10% fetal bovine serum, kanamyacin, and antibiotic/antimycotic solution (Invitrogen, Carlsbad, CA). Cells were passaged every three to five days and maintained at 37°C with 5% CO2. For cyclic stretch, cells were plated on Pronectin treated BioFlex culture plates (Flexcell International Corporation, Hillsborough, NC). After allowing for attachment for 48-72 hours, the cells were stretched using 25 mm BioFlex loading stations according to the prescribed surface area change at 30 cycles per minute and a 50% duty cycle. The 25 mm loading stations ensure an equibiaxial stretch in the radial and circumferential directions over the width of the loading post. For transfection experiments, cells were electroporated at 80-90% confluency using a BTX ECM 830 Square Wave Electroporator coupled with a Petri-Pulser PP35-2P electrode (Harvard Apparatus, Holliston, MA) using a single 10 ms 160V square wave to introduce plasmid DNA into the adherent cells. Cells were lysed using Promega Lysis Buffer and luciferase expression was measured using the Promega Luciferase Assay System (Promega, Madison, WI) as previously described19.
Mouse lung gene transfer
Female BALB/c mice (18-22 g) were anesthetized with an intraperitoneal injection of sodium pentobarbital (70 mg/kg body weight) and placed in the supine position. An endotracheal tube (20G catheter cut at an angle 2 cm from the end) was placed through the vocal cords into the trachea using a guide wire. A solution of 50 μg plasmid DNA in 50 μl of buffer (10 mM Tris, pH 8.0, 1 mM EDTA, 140 mM NaCl) was instilled into the lungs through the catheter with a Hamilton syringe. Immediately afterwards, an electric field was applied transthoracically to the animal's chest with a BTX ECM 830 using a field strength of 200 V/cm, 8 square wave pulses, 10 msec each with pre-gelled pediatric pacemaker electrodes (Medtronic; Minneapolis, MN). After electroporation, mice were mechanically ventilated through the catheter with a Harvard small animal ventilator at tidal volumes between 10-80% TLC (5-40 ml/kg BW), 0 cm PEEP, rate 150 BPM, for either 5 or 20 minutes. In experiments using NCT-10b, the drug was diluted in DMSO at a concentration of 200 μM and 100 ul were delivered via tail vein injection four hours prior to DNA delivery and electroporation. The animals were allowed to recover from anesthesia and returned to the vivarium. All experiments were conducted in accordance with institutional guidelines in compliance with the recommendations of the Guide for Care and Use of Laboratory Animals.
Lung sample retrieval and preparation
Forty-eight hours after gene transfer, mice were euthanized by sodium pentobarbital overdose and lungs were removed and snap frozen in liquid nitrogen or processed for immunohistochemistry. Lung lysates were prepared using a Bio-Pulverizer (Biospec Products; Bartlesville, OK) and the powder suspended in 750 ul of lysis buffer (Promega; Madison, WI), thawed and vortexed with three freeze/thaw cycles 4. The supernatant was separated from debris by centrifugation. Luciferase activity was measured in duplicate with a Turner luminometer (Sunnyvale, CA) using purified recombinant luciferase to produce a standard curve. Total protein content of the lung lysates was measure with the Bradford assay.
Immunohistochemistry
Lungs were inflated to total capacity with 1:1 OCT:PBS, embedded in OCT and frozen. Seven micron thick sections were cut on a cryostat, dried on Superfrost slides (Fischer Scientific; Pittsburgh, PA) two hours to overnight at room temperature, and stored at −80ºC Slides were equilibrated for 30 minutes at room temperature, fixed in ice cold absolute ethanol for 10 minutes, air dried for 30 minutes at room temperature, and rinsed in 1× PBS for 5 minutes. ß-galactosidase was detected with a 1:1200 dilution of rabbit anti-ß-galactosidase antibody (AB1211-5MG; Chemicon; Temecula, CA) using the Vector Labs (Burlingame, CA) ABC-AP kit. Endogenous biotin was blocked with Vector Labs Avidin/Biotin Blocking Kit. Signal was detected with Vector Blue with added levamisole to block endogenous alkaline phosphatase activity. Additional sections were stained with hematoxylin and eosin.
Statistical Analysis
All statistical analysis was performed with Prism software (GraphPad Software, San Diego, CA), using the Mann-Whitey U-test for non-parametric samples. Statistically significant results were denoted at a p value of less than or equal to 0.05.
Results
Moderate changes in basement membrane surface area increases gene transfer in vitro
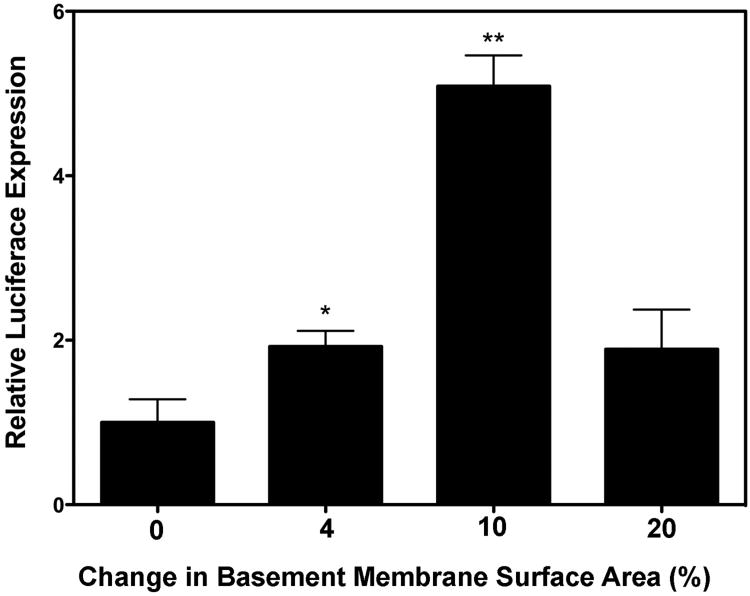
Although we had previously shown that a 10% change in basement membrane surface area (∆SA) applied in a cyclic fashion immediately following transfection caused a significant increase in exogenous gene expression 1,2, we wanted to determine if larger or smaller changes in basement membrane surface area also caused appreciable increases in gene transfer. Cells were stretched for 30 minutes following the electroporation of 10 μg of pCMV-Luc-DTS at either 0%, 4%, 10% or 20% ∆SA, followed by a rest for 23.5 hours. According to the data from Tschumperlin et al., these surface area changes correspond to 0%, 35%, 60% and 80% total lung capacity (TLC) 17,20. Since the TLC of a mouse is approximately 1.05 ml 21, this corresponds to ventilation at 0, 14, 24, and 32 ml/kg body weight, respectively. As seen in Figure 1, a 10% change in basement membrane surface area resulted in the largest significant increase in gene expression compared to unstretched cells, with an approximate 5-fold increase in relative gene expression (n=6, p<0.01). Stretching at either a 4% or 20% change in basement membrane surface area (n=6) resulted in an approximate 2-fold increase in relative gene expression versus unstretched cells, with only the 4% change demonstrating statistical significance (p<0.05).
Figure 1. Stretch-enhanced gene transfer is amplitude dependent.
Cells were grown on Pronectin treated BioFlex culture plates (Flexcell International Corporation, Hillsborough, NC) and electroporated with 10 μg of pCMV-Lux-DTS. The cells were then stretched for 30 minutes at either 0%, 4%, 10% or 20% change in basement membrane area (0.5 Hz, 50% duty cycle) followed by static growth for 23.5 hours. After 24 hours, luciferase expression was determined (n = 6 for each condition). *, p < 0.05, **, p < 0.01 between stretched and unstretched pairs.
Moderate tidal volume ventilation increases gene transfer in vivo
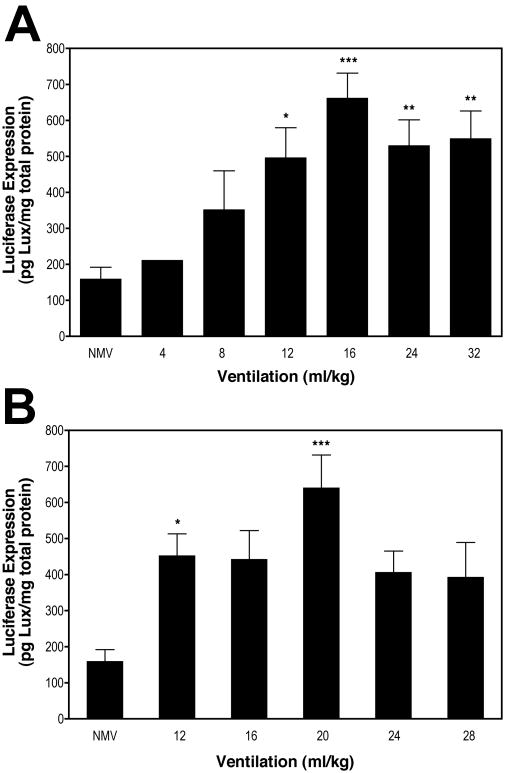
Based on our results with cultured cells 1,2,18, we wanted to determine if stretch in the living murine lung also enhanced gene transfer and expression. Using our in vitro experiments as a guideline, we chose a range of tidal volumes that complemented this data. As such, mice were briefly mechanically ventilated at 4 to 32 ml/kg (10-80% TLC) for 5 minutes, or 12 to 28 ml/kg (30-70% TLC) for 20 minutes, immediately after delivery of pCMV-Luc-DTS. As shown in Figure 2A, luciferase expression in electroporated mice that were not mechanically ventilated (“NMV”) was 160 ± 33 pg/mg total protein (n=13). With mechanical ventilation for 5 minutes, luciferase expression significantly increased 4-fold (p<0.001) to a maximum of 662 ± 69 pg/mg total protein (n=10) at 16 ml/kg. This tidal volume corresponds to roughly a 5% ∆SA 17. Luciferase expression was lower at tidal volumes above and below 16 ml/kg (40% TLC), but values significantly higher than electroporation alone were observed between 12 and 32 ml/kg (30-80% TLC). Mechanical ventilation for 20 minutes following electroporation resulted in a similar luciferase expression profile as for 5 minutes of ventilation (Fig. 2B), except that maximal luciferase expression occurred at 20 ml/kg (641 ± 91 pg/mg total protein; n=9). This is also a significant increase of four-fold over electroporation alone (p<0.001), and is roughly equivalent to 8% ∆SA 17.
Figure 2. Mechanical ventilation increases gene transfer and transgene expression.
Fifty μg of pCMV-Lux-DTS were instilled into the lungs of anesthetized Balb/c mice via an endotracheal tube and electroporated within one minute of DNA delivery. Immediately following electroporation, animals were subjected to no mechanical ventilation (“NMV”) or ventilation at 150 breaths per minute at the indicated volumes for (A) 5 min or (B) 20 min, and then allowed to recover. Gene expression was measured in the lungs 2 days later (n = 5 for each condition). *, p< 0.5 vs. not ventilated; **, p<0.01 vs. not ventilated; ***, p<0.001 vs. not ventilated.
Only ventilation immediately post-electroporation results in increased gene transfer
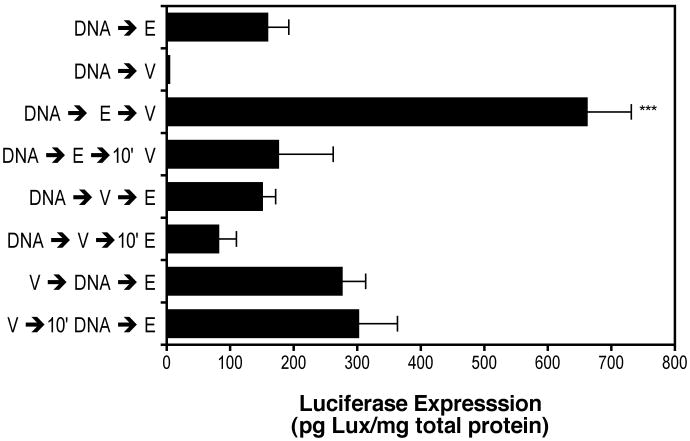
To test whether mechanical stretch must follow transgene delivery, mice were treated as described above, but the order of DNA instillation, electroporation and ventilation was varied (Fig. 3). All ventilations were done at 16 ml/kg for 5 minutes, and the elapsed time between steps was one minute unless otherwise noted. In the absence of electroporation but with mechanical ventilation after DNA instillation, there was essentially no luciferase expression detected compared to electroporation following DNA instillation (DNA→V vs. DNA→E). Thus, mechanical ventilation by itself does not promote gene transfer. As shown in Figure 2, mechanical ventilation at 16 ml/kg for 5 minutes immediately post-electroporation resulted in a four-fold increase in luciferase expression compared to electroporation alone (DNA→E→V vs. DNA→E). When 10 minutes was allowed to elapse between electroporation and mechanical ventilation (DNA→E→10′V), luciferase expression was the same as with electroporation alone. If mechanical ventilation was performed after DNA instillation but before electroporation, either immediately or 10 minutes post-instillation (DNA→V→E or DNA→V→10′E) then luciferase expression was equal to or less than electroporation alone, respectively. When mechanical ventilation was done before DNA instillation and electroporation, either immediately before or 10 minutes prior (V→DNA→E or V→10′DNA→E) luciferase expression was slightly, but not significantly increased compared to electroporation alone. Thus, only when DNA instillation, electroporation and mechanical ventilation in mouse lung were All statistical analysis was performed with Prism software (GraphPad Software, San Diego, CA), using the Mann-Whitey U-test for non-parametric samples. Statistically significant results were denoted at a p value of less than or equal to 0.05.performed in that order, and one immediately following the other, was expression of the luciferase transgene significantly increased over electroporation alone.
Figure 3. Enhanced gene transfer requires ventilation following electroporation.
Fifty μg of pCMV-Lux-DTS were instilled into the lungs of anesthetized Balb/c mice via an endotracheal tube (“DNA”), electroporated (“E”), and/or ventilated at 150 breaths per minute at 16 ml/kg for 5 min (“V”) in the order indicated (n = 5 per condition). If not indicated, the time between treatments was less than 60 seconds; where indicated, 10′ represents a 10 minute lag period between treatments. Following treatment, animals were allowed to recover and two days later gene expression was measured in the lungs of the animals. ***, p<0.001 vs. DNA followed immediately by electroporation in the absence of ventilation.
Ventilation increases distribution of expressing cells
To determine if the increased transgene expression was due to a higher level of expression per cell or due to more cells expressing the transgene, the pCMV-LacZ-DTS plasmid was transferred to the mouse lung as detailed in the Methods section. Mice were either electroporated only (NMV) or electroporated and ventilated at 40% TLC for 5 minutes. Thin frozen lung sections were assayed by immunohistochemistry against ß-galactosidase using a rabbit anti-ß-galactosidase antibody. As shown in Figure 4, mouse lungs ventilated post-electroporation showed an increased distribution of LacZ expressing cells compared to mouse lungs that were electroporated only. To detect any morphological changes due to mechanical ventilation, hematoxylin and eosin staining was performed on adjacent frozen lung sections. Compared to non-ventilated lungs, lungs that were ventilated at 40% or 60% TLC showed no physical damage or cellular infiltration (Fig. 5). Thus, mechanical ventilation post-electroporation at inflation levels that significantly increase gene transfer and expression increased the distribution of exogenous gene expression while resulting in no injury.
Figure 4. Mechanical ventilation increases the distribution of gene expression in the electroporated lung.
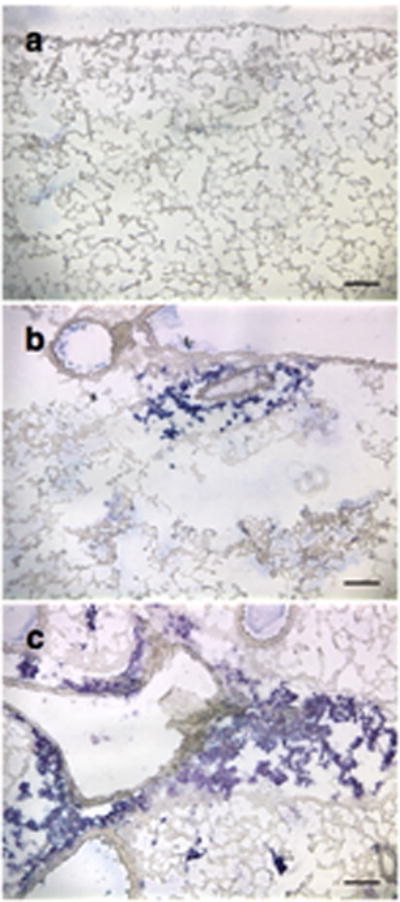
Fifty μg of pCMV-lacZ-DTS were instilled into the lungs of anesthetized Balb/c mice via an endotracheal tube, electroporated within 60 seconds of DNA delivery, and then either not ventilated (NMV) or ventilated for 5 min at 150 breaths per minute at 16 ml/kg. Gene expression (blue) was evaluated by IHC using a rabbit anti-ß-galactosidase antibody at 2 days post-transfer. (a) Naïve (no DNA transferred), (b) NMV, (c) 16 ml/kg.
Figure 5. Moderate mechanical ventilation causes no apparent lung injury.
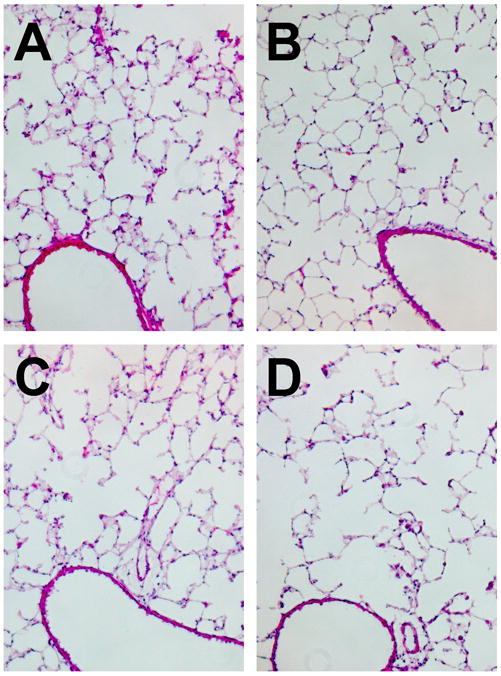
Thin sections of lungs from (A) naïve mice or those receiving DNA by electroporation and (B) no mechanical ventilation, or ventilation for 5 min at 150 breaths per minute at (C) 16 ml/kg or (D) 24 ml/kg were prepared two days after gene delivery as in Figure 4. Sections were stained with hematoxylin and eosin to evaluate lung injury and the presence or absence of infiltrating cells.
Pharmacological inhibition of HDAC6 increases gene transfer without post-electroporation ventilation
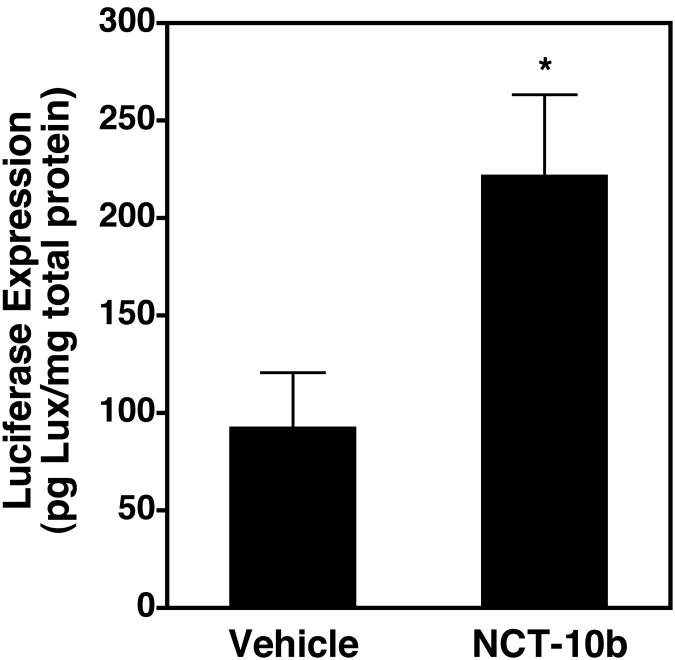
We have shown that the increased gene delivery seen as a result of cyclic stretch in cultured cells is due to inhibition of HDAC6 activity and the resulting increase in levels of acetylated microtubules 18. We have also shown that as mouse lungs are ventilated, there is an increase in the levels of acetylated microtubules in the parenchyma that then returns toward baseline levels at higher tidal volumes 22. Since this biphasic response mimics that seen for gene transfer in similarly ventilated lungs, we wanted to ask whether HDAC6 also played a role in this in vivo gene transfer. To test this, the HDAC6-specific small molecule inhibitor NCT-10b23 or a vehicle control was administered to mice via tail vein injection and four hours later, plasmids were administered to the lungs by electroporation without subsequent ventilation (Fig. 6). Even in the absence of ventilation following electropoation-mediated gene delivery, inhibition of HDAC6 by NCT-10b resulted in an almost 3-fold increase in gene transfer and expression at 2 days, similar to the increases seen with ventilation (Fig. 2), suggesting that HDAC6 plays a role in the enhanced gene delivery seen with ventilation.
Figure 6. Systemic injection of an HDAC6 inhibitor increases lung gene transfer without ventilation.
NCT-10b (100 μl of 200 μM) or DMSO vehicle (100 μl) was injected via the tail vein of mice. Four hours later, 50 μg of pCMV-lacZ-DTS were instilled into the lungs via an endotracheal tube and electroporated within 60 seconds of DNA delivery without any subsequent mechanical ventilation. Gene expression was measured 2 days later (n = 5).
Discussion
In this report we show that, like in cultured cells, cyclic stretch by mechanical ventilation can significantly improve gene transfer and expression in the murine lung. As little as five minutes of ventilation at 16 ml/kg (40% TLC) resulted in a significant four-fold increase in expression. This method also resulted in more cells within the lung expressing the transferred gene than in lungs in the absence of ventilation, and was completely safe for the animals. Further, as in cell culture studies 18, inhibition of HDAC6 by NCT-10b resulted in similar levels of enhanced gene transfer as seen with ventilation (i.e., cyclic stretch). Taken together with our published results that ventilation inhibits HDAC6 activity and causes enhanced microtubule acetylation 22, this strongly suggests that cyclic stretch increases gene transfer in cultured cells and in vivo through HDAC6.
We have previously shown that in cultured cells, mild cyclic stretch (10% ∆SA) results in a significant reorganization of the cytoskeletal network 1. Specifically, the microtubule network was remodeled and depolymerized, whereas the actin network was reorganized, such that fibers were shorter and peripherally localized, but were not depolymerized. This restructuring of the networks appears to be at least partly responsible for improved gene transfer as stabilizing either network diminished the influence of stretch. While destabilization did not prevent stretch-mediated enhancement, it also did not result in increased gene expression in static cells transfected by electroporation 1. Therefore, stretch-mediated reorganization of the cytoskeleton is required for enhancement of expression, but is not sufficient. We have shown that cyclic stretch also inhibits the enzymatic activity of the major cytoplasmic tubulin deacetylase, HDAC6, both in cultured cells and in ventilated mice 18,22. The result of this inhibition is an increase in acetylated tubulin in a pool of stabilized microtubules that are responsible for the increased trafficking of protein-DNA complexes through the cytoplasm to the nucleus 18,24. In non-ventilated mice, the majority of the acetylated microtubules are present in ciliated airway epithelial cells, but upon ventilation, acetylated microtubules appear in the parenchyma 22. The levels of acetylated microtubules are dependent on tidal volume in a pattern that is almost identical to that seen for gene transfer and expression, with low levels in non-ventilated mice, a peak at 16 to 20 ml/kg, and a decrease by 24 to 32 ml/kg 22, suggesting a relationship between acetylated microtubule levels and gene transfer. Indeed, inhibition of HDAC6 activity in cells with the drug NCT-10b or by specific siRNAs resulted in greatly enhanced cytoplasmic trafficking of plasmids to the nucleus and a 4-fold increase in gene expression within 3 hours of transfection 18. The results here using NCT-10b by tail vein injection strongly suggest that this is also true in the living lung.
In our previous experiments with cultured cells exposed to cyclic stretch, enhanced gene expression was seen only when cells were exposed to cyclic stretch after uptake (using liposomes or electroporation), but not before 2. Our results here demonstrate a similar dependency on the order in which stretch (ventilation) is applied: increased transfection efficiency and expression is seen only when ventilation is applied after DNA electroporation (Figure 3). In both cases (cultured cells exposed to cyclic stretch and ventilated lungs), the application of stretch/ventilation caused greater gene expression in individual cells and in the numbers of cells expressing gene product (Figure 4) 2. The fact that transfection enhancement occurs only if animals are ventilated after electroporation suggests that ventilation is not increasing the distribution of DNA within the lung prior to cellular uptake, as would be the obvious possibility. Rather, it is likely that plasmids are delivered to a large number of cells in the lung by electroporation, but that efficient cytoplasmic trafficking, nuclear import, and transcription only occur in a subset of these cells. When the lungs are subsequently ventilated, the resulting cyclic stretch increases the transport and/or transcription of the plasmids within these cells, causing more gene expression in a greater distribution of cells. Our results demonstrating that cyclic stretch inhibits HDAC6 in cultured cells, causing greater trafficking of plasmids along microtubules to the nucleus and enhanced gene expression, supports this hypothesis 18. Taken together with the fact that ventilation also inhibits HDAC6 in mice and that the HDAC6 inhibitor NCT-10b causes enhanced gene expression in mice in the absence of ventilation (Figure 6), strengthens this model for ventilator-enhanced gene delivery 22.
Depending on the duration of ventilation, peak gene transfer and expression levels were attained at ventilation volumes of 40% and 50% TLC at 5 minutes and 20 minutes respectively. These volumes correspond to approximately 5% and 8% ∆SA 17, which corresponds quite well with the 10% ∆SA used in cultured cells to achieve maximal expression. These values are all less than the 12% ∆SA value shown by Tschumperlin and Margulies to cause injury and death of alveolar epithelial cells after one hour of cyclic stretch 20. Our lack of morphological changes in H&E stained lung sections (Figure 5) demonstrate that at these levels of stretch, lung injury was not apparent. Furthermore, mortality levels were reduced in animals that were ventilated post-electroporation, with no deaths at 40% or 50% TLC (n=15 and 17, respectively; data not shown). Trials using a Minivent mechanical ventilator at 6 ml/kg BW (12% TLC) for as little as one minute reduced mortality on average to 12% (n=17). This volume and duration of ventilation was not enough to cause a significant difference in expression compared to electroporation alone (data not shown). Most likely the decreased mortality from ventilation is not due to stretch, but from clearance of the airway after electroporation. It has been observed that electroporation produced foam in the trachea, most likely from the instilled DNA solution. So mechanical ventilation, immediately post-electroporation, could help clear the airway and restore normal breathing, even when applied at low volumes and short duration.
Mechanical-ventilation mediated stretch of the lung can conceivably be coupled with other plasmid DNA delivery methods besides intratracheal instillation. Since stretch enhances cytoplasmic trafficking of plasmids once they have entered a cell, it is independent of the method by which DNA is introduced into the cell. Such prospective delivery methods include aerosolized DNA and nanoparticles. Aerosol delivery of DNA may be a more tenable route for human therapy than intratracheal instillation. Evidence suggests aerosolized PEI-DNA complexes are more efficient at lung cell transfection than intratracheal instillation, requiring only nanogram rather than microgram amounts of DNA to give significantly higher levels of expression 25-27. This is perhaps due to wider dispersal of plasmid DNA throughout the lung compared to instillation. Compacted DNA, such as stabilized poly-lysine PEGylated DNA nanoparticles, are more efficient at transfecting non-dividing cells than noncompacted DNA. The diameter of the minor axis of these rod-shaped compacted particles is 7-14 nm. This size is permissive for passage through nuclear pores with a diameter of 25 nm 28. These nanoparticles were shown to transfect airway epithelial cells from the apical surface via either intratracheal or intranasal delivery routes 29.
Overall, our findings here demonstrate that the stretch-mediated enhancement of exogenous gene expression shown in vitro correlates very well with ventilator-mediated enhancement of exogenous gene expression in vivo in a manner that is consistent with the literature correlating %TLC to %∆SA 17. These results demonstrate that simple ventilation strategies following non-viral plasmid DNA transfections can significantly improve the exogenous expression of an appropriate transgene, and could significantly impact the use of such therapies in a clinical setting.
Acknowledgments
We would like to thank Patricia Chess, James DeGiulio, and Erin Vaughan for insightful discussions and technical advice. This work was supported in part by grants HL081148, HL091960, and EB009903.
References
- 1.Geiger RC, Taylor W, Glucksberg MR, Dean DA. Cyclic stretch-induced reorganization of the cytoskeleton and its role in enhanced gene transfer. Gene Ther. 2006;13:725–731. doi: 10.1038/sj.gt.3302693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor W, Gokay KE, Capaccio C, Davis E, Glucksberg MR, Dean DA. Effects of cyclic stretch on gene transfer in alveolar epithelial cells. Mol Ther. 2003;7:542–549. doi: 10.1016/s1525-0016(03)00041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean DA. Electroporation of the vasculature and the lung. DNA Cell Biol. 2003;22:797–806. doi: 10.1089/104454903322625000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level non-viral gene transfer to the lung. Gene Ther. 2003;10:1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GRS, Yeldandi A, et al. Gene transfer of the Na+,K+-ATPase b1 subunit using electroporation increases lung liquid clearance in rats. Am J Respir Crit Care Med. 2005;171:204–211. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 2005;175:7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazdhar A, Bilici M, Pierog J, Ayuni EL, Gugger M, Wetterwald A, et al. In vivo electroporation and ubiquitin promoter--a protocol for sustained gene expression in the lung. J Gene Med. 2006;8:910–918. doi: 10.1002/jgm.911. [DOI] [PubMed] [Google Scholar]
- 8.Mutlu GM, Machado-Aranda D, Norton JE, Bellmeyer A, Urich D, Zhou R, et al. Electroporation-mediated gene transfer of the Na+,K+-ATPase rescues endotoxin-induced lung injury. Am J Respir Crit Care Med. 2007;176:582–590. doi: 10.1164/rccm.200608-1246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pringle IA, McLachlan G, Collie DD, Sumner-Jones SG, Lawton AE, Tennant P, et al. Electroporation enhances reporter gene expression following delivery of naked plasmid DNA to the lung. J Gene Med. 2007;9:369–380. doi: 10.1002/jgm.1026. [DOI] [PubMed] [Google Scholar]
- 10.Zhou R, Norton JE, Zhang N, Dean DA. Electroporation-mediated transfer of plasmids to the lung results in reduced TLR9 signaling and inflammation. Gene Ther. 2007;14:775–780. doi: 10.1038/sj.gt.3302936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto K, Nagayasu T, Hishikawa Y, Tagawa T, Yamayoshi T, Abo T, et al. Keratinocyte growth factor accelerates compensatory growth in the remaining lung after trilobectomy in rats. J Thorac Cardiovasc Surg. 2009;137:1499–1507. doi: 10.1016/j.jtcvs.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa K, Sato N, Obinata T. Dynamic reorientation of cultured cells and stress fibers under mechanical stress from periodic stretching. Exp Cell Res. 2001;268:104–114. doi: 10.1006/excr.2001.5270. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Xu Q. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal. 2000;12:435–445. doi: 10.1016/s0898-6568(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Post M. Mechanochemical signal transduction in the fetal lung. J Appl Physiol. 2000;89:2078–2084. doi: 10.1152/jappl.2000.89.5.2078. [DOI] [PubMed] [Google Scholar]
- 15.Putnam AJ, Cunningham JJ, Dennis RG, Linderman JJ, Mooney DJ. Microtubule assembly is regulated by externally applied strain in cultured smooth muscle cells. J Cell Sci. 1998;111:3379–3387. doi: 10.1242/jcs.111.22.3379. [DOI] [PubMed] [Google Scholar]
- 16.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cellsEffect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162:357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 17.Tschumperlin DJ, Margulies AS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol. 1999;86:2026–2033. doi: 10.1152/jappl.1999.86.6.2026. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan EE, Geiger RC, Miller AM, Loh-Marley PL, Suzuki T, Miyata N, et al. Microtubule Acetylation Through HDAC6 Inhibition Results in Increased Transfection Efficiency. Mol Ther. 2008;16:1841–1847. doi: 10.1038/mt.2008.190. [DOI] [PubMed] [Google Scholar]
- 19.Vacik J, Dean BS, Zimmer WE, Dean DA. Cell-specific nuclear import of plasmid DNA. Gene Therapy. 1999;6:1006–1014. doi: 10.1038/sj.gt.3300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschumperlin DJ, Margulies AS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 1998;275:L1173–L1183. doi: 10.1152/ajplung.1998.275.6.L1173. [DOI] [PubMed] [Google Scholar]
- 21.Lai YL, Chou HC. Respiratory mechanics and maximal expiratory flow in the anesthetized mouse. J Appl Physiol. 2000;88:939–943. doi: 10.1152/jappl.2000.88.3.939. [DOI] [PubMed] [Google Scholar]
- 22.Geiger RC, Kaufman CD, Lam AP, Budinger GR, Dean DA. Tubulin acetylation and histone deacetylase 6 activity in the lung under cyclic load. Am J Respir Cell Mol Biol. 2009;40:76–82. doi: 10.1165/rcmb.2007-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki T, Kouketsu A, Itoh Y, Hisakawa S, Maeda S, Yoshida M, et al. Highly potent and selective histone deacetylase 6 inhibitors designed based on small-molecular substrate. J Med Chem. 2006;49:4809–4812. doi: 10.1021/jm060554y. [DOI] [PubMed] [Google Scholar]
- 24.Vaughan EE, Dean DA. Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol Ther. 2006;13:422–428. doi: 10.1016/j.ymthe.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Densmore CL, Orson FM, Xu B, Kinsey BM, Waldrep JC, Hua P, et al. Aerosol delivery of robust polyethyleneimine-DNA complexes for gene therapy and genetic immunization. Mol Ther. 2000;1:180–188. doi: 10.1006/mthe.1999.0021. [DOI] [PubMed] [Google Scholar]
- 26.Gautam, Densmore CL, Xu B, Waldrep JC. Enhanced gene expression in mouse lung after PEI–DNA aerosol delivery. Mol Ther. 2000;2:63–70. doi: 10.1006/mthe.2000.0087. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph C, Schillinger U, Ortiz A, Plank C, Golas MM, Sander B, et al. Aerosolized nanogram quantities of plasmid DNA mediate highly efficient gene delivery to mouse airway epithelium. Mol Ther. 2005;12:493–501. doi: 10.1016/j.ymthe.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Li D, Pasumarthy MK, Kowalczyk TH, Gedeon CR, Hyatt SL, et al. Nanoparticles of compacted DNA transfect postmitotic cells. J Biol Chem. 2003;278:32578–32586. doi: 10.1074/jbc.M305776200. [DOI] [PubMed] [Google Scholar]
- 29.Ziady AG, Gedeon CR, Miller T, Quan W, Payne JM, Hyatt SL, et al. Transfection of airway epithelium by stable PEGylated poly-L-lysine DNA nanoparticles in vivo. Mol Ther. 2003;8:936–947. doi: 10.1016/j.ymthe.2003.07.007. [DOI] [PubMed] [Google Scholar]