Abstract
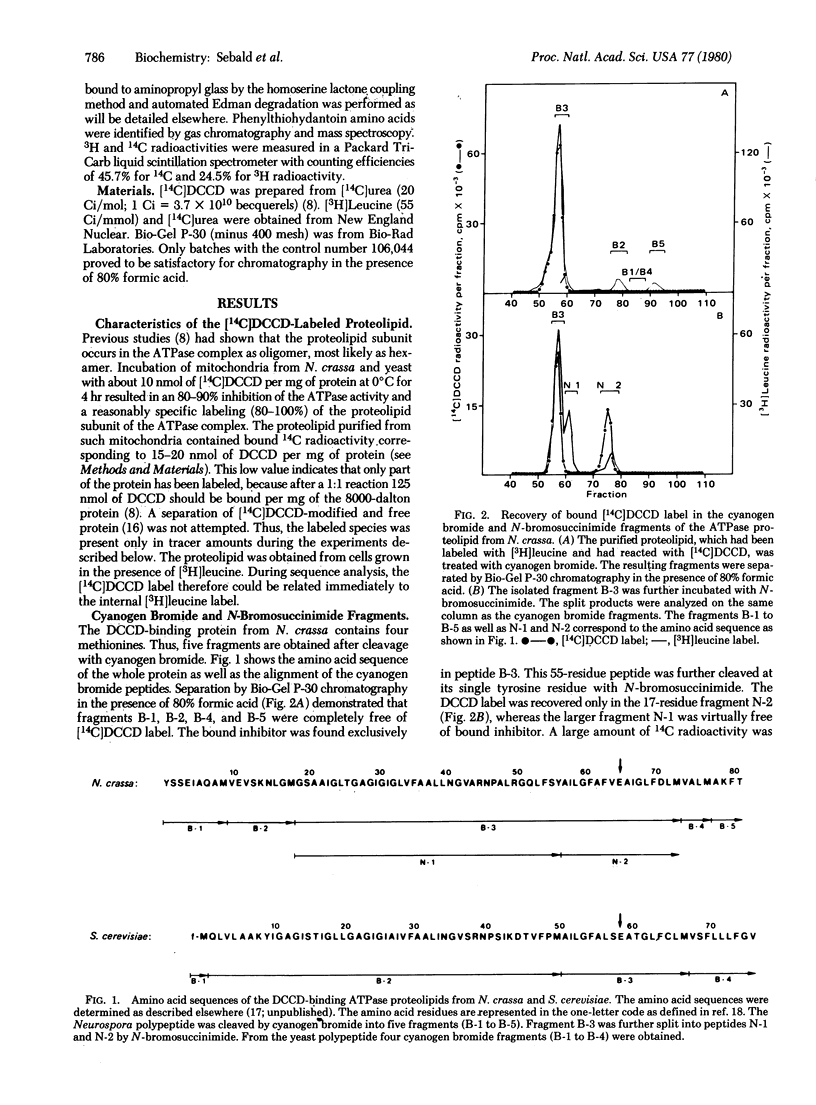
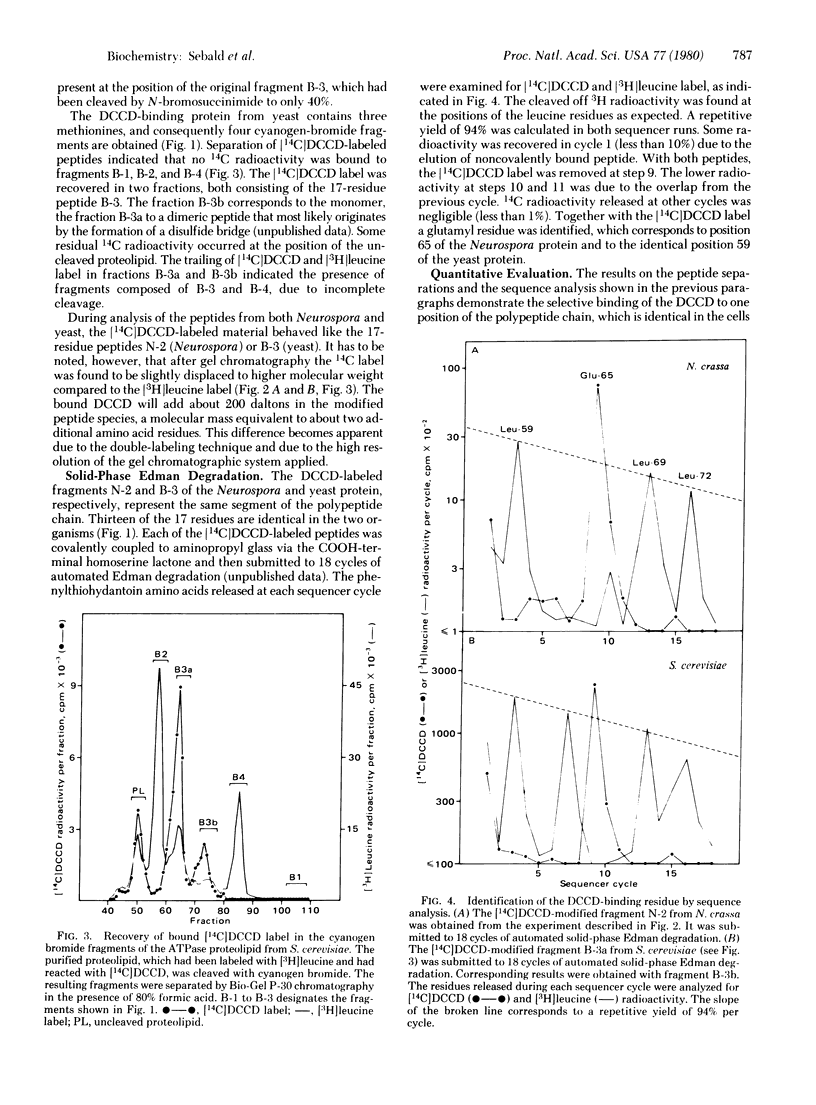
The N,N'-dicyclohexylcarbodiimide-binding proteolipid subunit of the mitochondrial adenosinetriphosphatases (ATP phosphohydrolase, EC 3.6.1.3) of Neurospora crassa and Saccharomyces cerevisiae were purified from mitochondria incubated with the radioactively labeled inhibitor. The specifically labeled subunit was cleaved with cyanogen bromide and N-bromosuccinimide, and the resultant fragments were separated by gel chromatography in the presence of 80% (vol/vol) formic acid. The N,N'-dicyclohexylcarbodiimide label was recovered in each organism exclusively in a 17-residue fragment. Further analysis by automated solid-phase Edman degradation revealed that the bound label was present at only one position, corresponding to a glutamyl residue. The N,N'-dicyclohexylcarbodiimide-modified glutamyl residue is the only identical acidic position in both proteins and occurs in the middle of a hydrophobic sequence of about 25 residues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K., Harold F. M., Simoni R. D. Impairment and restoration of the energized state in membrane vesicles of a mutant of Escherichia coli lacking adenosine triphosphatase. J Biol Chem. 1974 Jul 25;249(14):4587–4593. [PubMed] [Google Scholar]
- Altendorf K. Purification of the DCCD-reactive protein of the energy-transducing adenosine triphosphatase complex from Escherichia coli. FEBS Lett. 1977 Feb 1;73(2):271–275. doi: 10.1016/0014-5793(77)80997-6. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Holloway C. T., Knight I. G., Roberton A. M. Dicyclohexylcarbodiimide--an inhibitor of oxidative phosphorylation. Biochem Biophys Res Commun. 1966 Apr 6;23(1):75–80. doi: 10.1016/0006-291x(66)90271-3. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Roberton A. M., Holloway C. T., Knight I. G. The properties of dicyclohexylcarbodiimide as an inhibitor of oxidative phosphorylation. Biochemistry. 1967 Dec;6(12):3867–3879. doi: 10.1021/bi00864a033. [DOI] [PubMed] [Google Scholar]
- Boyer P. D. A model for conformational coupling of membrane potential and proton translocation to ATP synthesis and to active transport. FEBS Lett. 1975 Oct 15;58(1):1–6. doi: 10.1016/0014-5793(75)80212-2. [DOI] [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle R. S., Packer L., Shieh P. Oligomycin-dependent ionophoric protein subunit of mitochondrial adenosinetriphosphatase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4306–4310. doi: 10.1073/pnas.74.10.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Hoppe J., Schairer H. U., Sebald W. The proteolipid of a mutant ATPase from Escherichia coli defective in H+-conduction contains a glycine instead of the carbodiimide-reactive aspartyl residue. FEBS Lett. 1980 Jan 1;109(1):107–111. doi: 10.1016/0014-5793(80)81321-4. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. Reconstitution of the energy transformer, gate and channel subunit reassembly, crystalline ATPase and ATP synthesis. Biochim Biophys Acta. 1978 Sep 21;505(1):45–93. doi: 10.1016/0304-4173(78)90008-3. [DOI] [PubMed] [Google Scholar]
- Kurzer F., Douraghi-Zadeh K. Advances in the chemistry of carbodiimides. Chem Rev. 1967 Apr;67(2):107–152. doi: 10.1021/cr60246a001. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Morowitz H. J. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Eytan E., Notsani B. E., Sigrist H., Sigrist-Nelson K., Gitler C. Isolation of a chloroplast N,N'-dicyclohexylcarbodiimide-binding proteolipid, active in proton translocation. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2375–2378. doi: 10.1073/pnas.74.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. Structure and function of chloroplast ATPase. Biochim Biophys Acta. 1976 Nov 30;456(3-4):314–338. doi: 10.1016/0304-4173(76)90003-3. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Sone N., Hirata H., Yoshida M., Kagawa Y. Purified proton conductor in proton translocating adenosine triphosphatase of a thermophilic bacterium. J Biol Chem. 1977 Sep 10;252(17):6125–6131. [PubMed] [Google Scholar]
- Pick U., Racker E. Purification and reconstitution of the N,N'-dicyclohexylcarbodiimide-sensitive ATPase complex from spinach chloroplasts. J Biol Chem. 1979 Apr 25;254(8):2793–2799. [PubMed] [Google Scholar]
- Sebald W. Biogenesis of mitochondrial ATPase. Biochim Biophys Acta. 1977 Jun 21;463(1):1–27. doi: 10.1016/0304-4173(77)90002-7. [DOI] [PubMed] [Google Scholar]
- Sebald W., Graf T., Lukins H. B. The dicyclohexylcarbodiimide-binding protein of the mitochondrial ATPase complex from Neurospora crassa and Saccharomyces cerevisiae. Identification and isolation. Eur J Biochem. 1979 Feb 1;93(3):587–599. doi: 10.1111/j.1432-1033.1979.tb12859.x. [DOI] [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Carbodiimide-binding protein of H+-translocating ATPase and inhibition of H+ conduction by dicyclohexylcarbodiimide. J Biochem. 1979 Feb;85(2):503–509. doi: 10.1093/oxfordjournals.jbchem.a132357. [DOI] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Resolution of the membrane moiety of the H+-ATPase complex into two kinds of subunits. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4219–4223. doi: 10.1073/pnas.75.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assesmbly of the mitochondrial membrane system. VI. Mitochondrial synthesis of subunit proteins of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1972 Jan 25;247(2):594–603. [PubMed] [Google Scholar]
- Wikstrom M. K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977 Mar 17;266(5599):271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- Williams R. J. The history and the hypotheses concerning ATP-formation by energised protons. FEBS Lett. 1978 Jan 1;85(1):9–19. doi: 10.1016/0014-5793(78)81238-1. [DOI] [PubMed] [Google Scholar]



