Abstract
Transcription factors (TFs) can regulate different sets of genes to determine specific cell types by means of combinatorial codes. We previously identified closely-spaced TF binding motifs located 8.2-8.5 kb 5′ to the ATG of the murine Pou4f3 gene, a gene required for late hair cell (HC) differentiation and survival. These motifs, 100% conserved among four mammalian species, include a cluster of E-boxes preferred by TCF3/ATOH1 heterodimers as well as motifs for GATA factors and SP1. We hypothesized that these factors might interact to regulate the Pou4f3 gene and possibly induce a HC phenotype in non-sensory cells of the cochlea. Cochlear sensory epithelium explants were prepared from postnatal day 1.5 transgenic mice in which expression of GFP is driven by 8.5 kb of Pou4f3 5′ genomic DNA (Pou4f3/GFP). Electroporation was used to transfect cells of the greater epithelial ridge with multiple plasmids encoding human ATOH1 (hATOH1), hTCF3 (also known as E2A or TEF2), hGATA3, and hSP1. hATOH1 or hTCF3 alone induced Pou4f3/GFP cells but hGATA3 and hSP1 did not. hATOH1 but not hTCF3 induced conversion of greater epithelial ridge cells into Pou4f3/GFP and myosin VIIa double-positive cells. Transfection of hATOH1 in combination with hTCF3 or hGATA3 induced 2-3X more Pou4f3/GFP cells, and similarly enhanced Pou4f3/GFP and myosin VIIa double-positive cells, when compared to hATOH1 alone. Triple or quadruple TF combinations were generally not more effective than double TF combinations except in the middle turn, where co-transfection of hATOH1, hE2A, and hGATA3 was more effective than hATOH1 plus either hTCF3 or hGATA3. The results demonstrate that TFs can cooperate in regulation of the Pou4f3 gene and in the induction of at least one other element of a HC phenotype. Our data further indicate that combinations of TFs can be more effective than individual TFs in the inner ear.
Keywords: ATOH1, combinatorial code, TFE2, GATA3, hair cell, POU4F3
Introduction
The hair cell (HC) is a critical cell type of the inner ear, required for normal auditory and vestibular function, and exquisitely specialized for mechanotransduction and communication with the nervous system. HCs do not regenerate in the mammalian inner ear and loss of these cells in the cochlea or in the vestibule results in permanent hearing loss or vestibular deficit. Gene therapy has been proposed as a means of stimulating HC regeneration or of preventing HC loss due to genetic or environmental factors (e.g. Kawamoto et al., 2003; Ono et al., 2009; Zheng and Gao, 2000).
A promising approach to HC regeneration has been to introduce regulators of HC development into other types of inner ear cells. In particular, the Class II basic helix-loop-helix (bHLH) transcription factor (TF) ATOH1 (also known as Math1) is required for HC fate determination, survival and differentiation. Atoh1 deletion leads to perinatal lethality, with some poorly differentiated cells in the organ of Corti expressing the early HC marker myosin 7A (Myo7A) (Bermingham et al., 1999). Conditional deletion of Atoh1 in the inner ear leads to the death of most cells in the organ of Corti prior to birth, but a few surviving cells in the organ express Myo7A at later stages (Pan et al., 2011). Premature termination of inner ear Atoh1 expression that has begun normally leads to the death of most partially differentiated HCs (Pan et al., 2012). However, forced Atoh expression can induce ectopic HC formation, in which nonsensory cochlear epithelial cell types adopt a HC-like phenotype (Gubbels et al., 2008; Izumikawa et al., 2005, 2008; Kawamoto et al., 2003; Woods et al., 2004; Zheng and Gao, 2000). However, despite advances in identifying TFs critical for inner ear development and applying them to HC replacement, there has been less progress in understanding how these factors exert their specific effects in HCs. For example, ATOH1 is also expressed in several other cell types with very different phenotypes, playing a significant role in the development of, for example, Merkel cell cutaneous touch receptors (Leonard et al., 2002), spiral ganglion and cochlear nucleus cells (Maricich et al., 2009), cerebellar granule cells (Gazit et al., 2004), and secretory epithelial cells in the colon (Yang et al., 2001), as well as regulating mucin gene expression in various mucosal epithelia (Sekine et al., 2006). Why then does ATOH1 expression induce a HC phenotype in inner ear epithelial cells, but not in these other cells? A potential explanation is that ATOH1 interacts with other transcriptional regulators to generate cellular specificity. There is extensive evidence that TFs can combine with distinct TF partners in different cell types, in a combinatorial code, which regulates different sets of genes and contributes to the determination of cell phenotype. This concept was originally developed to explain conserved, modular elements in an Arabadopsis gene (Curie et al., 1993) and then applied to mammalian Hox gene regulation (Shashikant et al., 1995). Recently, such combinatorial coding was found to regulate expression of ATOH1 itself (Ahmed et al., 2012). TF interactions are highly likely to occur physically, on the regulatory DNA of genes. If so, in the case of HC genes regulated by ATOH1, this may be apparent in conserved binding motifs for TFs in the regulatory DNA of ATOH1 target genes expressed in this cell type. One potential target is the gene encoding the POU domain TF POU4F3 (also known as Brn-3.1, Brn-3c)..
POU4F3 is required for late HC differentiation, including their transduction and synaptic specializations, and for HC survival. Deletion of POU4F3 leads to failure of HC differentiation and the death of most HCs (Erkman et al., 1996; Xiang et al., 1997, 2003), although some undifferentiated HCs are retained into adulthood (Xiang et al., 1997, 2003; Pauley et al., 2008). Cochlear POU4F3 expression is initiated around embryonic day 13 (E13), just after that of ATOH1 and preceding myosin VIIa expression, another HC specific marker, which begins on E15 (Bermingham et al., 1999; Chen et al., 2002; Pan et al., 2012; Sage et al., 2006; Woods et al., 2004; Xiang et al., 1997, 2003). It is associated with the regulation of the motor protein prestin in outer HCs in newborn rodents (Gross et al., 2011), and continues to be expressed into adulthood. It is the gene mutated in DFNA 15, a form of dominant, progressive hereditary hearing loss (Vahava et al., 1998, Collin et al., 2008). Like ATOH1, POU4F3 is also expressed in other developing and adult sensory or neural populations. For example, it is expressed in Merkel cells (Leonard et al., 2002), trigeminal neurons (Artinger et al., 1998) and a subset of retinal ganglion cells (Erkman et al., 1996), which have quite different specializations and do not express HC-specific genes. There is also evidence that different members of the Pou4f family (1-3) are combinatorially expressed and cross-regulate in retinal and somatosensory neurons (Badea et al., 2012).
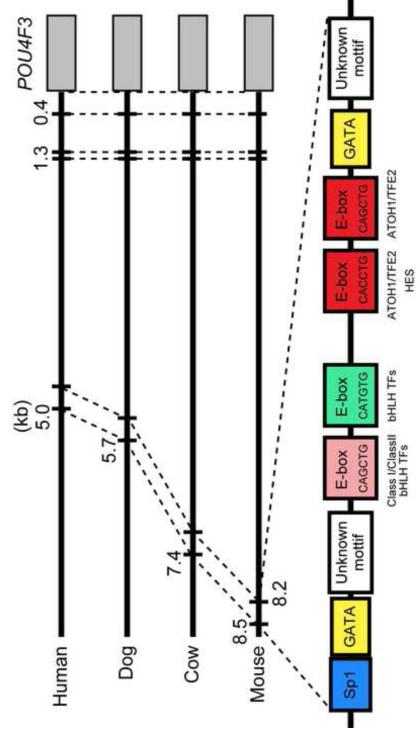
The upstream regulation of the Pou4f3 gene is not well characterized. However, using bioinformatic analysis, we previously identified several closely-spaced motifs for TF binding located 8.2-8.5 kb 5′ to the ATG of the murine Pou4f3 gene (Masuda et al., 2011). These motifs are 100% conserved among four mammalian species: mouse, human, dog, and cow (Fig. 1). They include three E-boxes (CAGCTG x 2, CACCTG) to which Class I/Class II bHLH heterodimers such as TCF3/ATOH1 can bind with high affinity (Akazawa et al., 1995; Ledent et al., 2002; Massari and Murre, 2000; Scheffer et al., 2007b). Two of these are consistent with the motifs known to be activated by ATOH1 (Klisch et al., 2011). We also demonstrated that ATOH1 directly binds to this conserved region using chromatin immunoprecipitation (Masuda et al., 2011). The remaining E-box (CATGTG) is typically preferred by Class III bHLH and Class IV bHLH factors (Fisher et al., 1992; Hamid and Kakar, 2004). In addition, preferred binding sites for SP1 and GATA3 are present (Ko and Engel, 1993; Masuda et al., 2011; Merika and Orkin, 1993; Wierstra, 2008). Moreover, co-transfection of a reporter construct in which 8.5 kb of 5′ Pou4f3 DNA drives eGFP with an expression construct encoding ATOH1 enhanced eGFP expression in HEK293 and VOT-E36 cells, when compared to transfection with the transgene alone (Masuda et al., 2011).
Fig. 1. Conserved 5′ TF biinding sites in the Pou4f3 gene.
A region that is conserved across four mammalian species is located 8.2-8.5 kb 5′ to the ATG of Pou4f3 in the mouse, where it supports ATOH1 binding (Masuda et al., 2011). Clustered E-boxes (CANNTG) are conserved at this site, including two of a type that is activated by ATOH1 (red boxes) (Klisch et al., 2011). The E-boxes are located in close proximity to conserved binding motifs for GATA and SP1.
We speculated that TCF3, GATA3, and/or SP1 might act cooperatively at this conserved cluster of TF binding sites to control Pou4f3 gene expression. We further speculated that these factors might interact to induce a HC-like phenotype in non-sensory cells of the cochlea. To test these hypotheses, we evaluated whether these TFs, alone or in combination, enhance the ability of ATOH1 to induce ectopic inner ear Pou4f3 and/or Myo7a gene expression. Electroporation was used to transfect cells of the greater epithelial ridge (GER) of postnatal day 1.5 (P1.5) cochlear epithelial explants from transgenic mice (Pou4f3/GFP mice) in which expression of GFP is driven by 8.5 kb of 5′ Pou4f3 genomic DNA (Masuda et al., 2011). We demonstrate that ATOH1 can act in a combinatorial fashion with TCF3 or GATA3 to enhance both Pou4f3-promoter-induced eGFP and myosin VIIa expression in nonsensory cells of the GER.
Materials and Methods
Animals
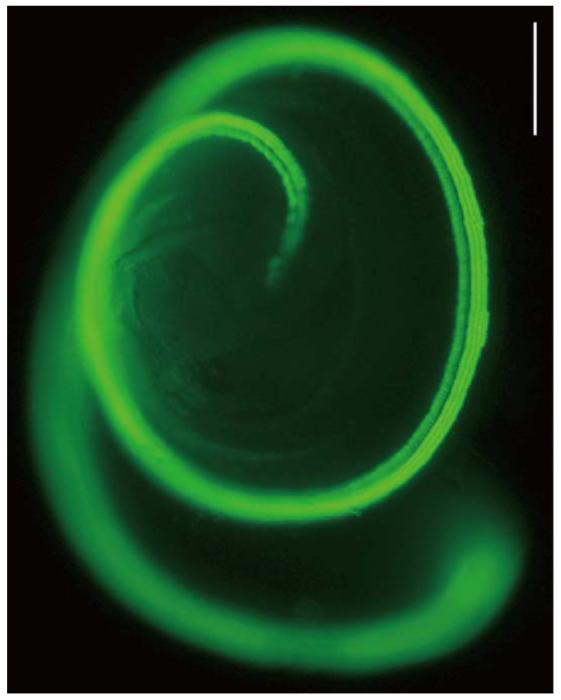
P1.5 wildtype CBA/J or Pou4f3/GFP mice on a CBA/J background were used. In the transgenic mice, robust GFP (Pou4f3/GFP) expression is noted in neonatal cochlear and vestibular HCs, and not in other cochlear cells (Fig. 2). Preliminary studies showed that transfection of P1.5 sensory epithelia was less robust than at P0.5. P1.5 was chosen so that any combinatorial enhancement of ATOH1 effects might be detected more readily.
Fig. 2. A Pou4f3/GFP transgenic.
Neonatal cochlea of a transgenic mouse (Pou4f3/GFP mouse) in which GFP driven by 8.5 kb of 5′ Pou4f3 DNA is expressed only in hair cells (HCs). The scale bar = 200 μm.
The local animal subjects committee of the VA San Diego Healthcare System approved all procedures in accordance with the guidelines laid down by the National Institutes of Health regarding the care and use of animals for experimental procedures.
Plasmid DNA preparation
All plasmids were prepared with the EndoFree Plasmid Maxi Kit (Qiagen, Valencia, CA) following the manufacture’s protocol. Plasmids were resuspended to 3 μg/μl in HBSS (without calcium, magnesium and phenol red), aliquotted and stored at −20°C. The stock was diluted in HBSS just before initiating dissection of the sensory epithelium.
Dissection of the sensory epithelium, electroporation and sensory epithelium explant culture
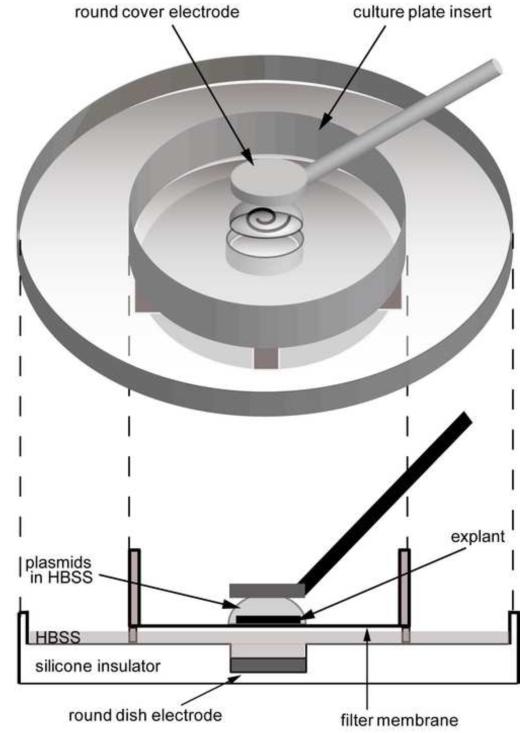
All media were from Invitrogen (Carlsbad, CA). The temporal bones were dissected from P1.5 mice and placed in cold DMEM/F12. The cochlear sensory epithelia were isolated in cold DMEM/F12 with 30 U/ml penicillin (Sigma-Aldrich, St. Louis, MO) using the methods of Sobkowicz et al. (1993). In particular, the lateral wall was separated from the sensory epithelium at its outer edge, which is a natural division point. The tissue was then carefully separated from the spiral ganglion by teasing, so that only the sensory epithelium was used for transfection studies. The hook portion of the epithelium was also removed to render the sensory epithelium flat between electrodes for electroporation. The epithelia were electroporated according to Kawabata et al. (2004) with substantial modification (Fig. 3). A Millipore filter membrane (30 mm diameter culture plate insert; Millipore, Billerica, MA) was placed in a sterile 60 mm diameter petri dish, and the whole surface of the filter membrane was moistened by adding 40 μl HBSS to its center. The dissected sensory epithelium was washed with HBSS and transferred to the filter membrane. Five hundred l HBSS was placed at the center of a dish electrode (2 mm diameter flat round electrode; CUY700P2E, NEPA GENE, Chiba, Japan). Approximately 20 μl HBSS around the epithelium was removed by pipette, the tissue was relocated to the center of the membrane, and the HBSS around the tissue was removed completely by suction with a pipette and natural evaporation to enhance attachment to the membrane. Immediately after the excess HBSS around the explant had evaporated, 5 πl of expression plasmid in HBSS was applied to the sensory epithelium while confirming that the tissue was well attached to the filter membrane. The epithelium on the filter membrane was placed at the center of the dish electrode with HBSS. A cover electrode (2 mm diameter flat round electrode; CUY700P2L, NEPA GENE) was placed above the tissue. The plate electrode and the cover electrode were set as the anode and the cathode, respectively. An electroporator (CUY21EDIT, NEPA GENE) was used to apply five rectangular pulses (12 V, 30 ms duration with an interval of 970 ms). The tissue was left undisturbed for 1 min after completion of electroporation, and then 1 ml pre-warmed Opti-MEM was added in the membrane. The tissue was removed from the filter membrane with a needle and transferred into a petri dish filled with 3 ml pre-warmed Opti-MEM, where it was divided into two pieces. The two pieces were transferred into one well of a 24-well plate filled with 250 μl of pre-warmed culture medium (high glucose DMEM, 10% fetal bovine serum, 25 mM HEPES, 10 μl/ml N-2 supplement, 20 ng/ml epidermal growth factor). About 85 πl of culture medium was removed to enhance the attachment of the explants with the bottom of the well. The explants were cultured in an incubator at 37[g864][g864]with 5% CO2 and 95% humidity for 2-3 hours, and then 500 μl fresh culture medium was added into the well. Thereafter, the culture medium was replaced with 500 μl of fresh culture medium every 24 hours.
Fig. 3. Electroporation paradigm.
A postnatal day 1.5 (P1.5) cochlear sensory epithelial explant is placed on a filter membrane and covered with 5 μl of HBSS containing a desired plasmid(s). The gap between the filter membrane and the dish electrode is filled with 500 μl HBSS. Five rectangular electrical pulses (12 V, 30 ms duration, 970 ms interval) are applied through the 2 mm diameter round cover (anode) and dish (cathode) electrodes.
The explants were imaged daily and native HCs were followed to differentiate them from transdifferentiated Pou4f3/GFP+ cells by transfection with plasmids. These images also allowed differentiation between inner and outer HCs, and determination of which HCs were lost due to electroporation damage.
Transfection of sensory epithelia with dsRed and/or eGFP
To determine the location of cells in the P1.5 sensory epithelia of transgenic mice that were transfected using our electroporation protocol, the samples were transfected with 1 μg/μl Discosoma sp. red (dsRed)-expression vector in which dsRed was driven by a cytomegalovirus (CMV) promoter (Clontech, Mountain View, CA). To confirm that multiple plasmids could enter into the same cell during electroporation, as previously reported (LoTurco et al., 2009; Ono et al., 2009; Tabata and Nakajima, 2008), the sensory epithelia of wildtype mice were co-transfected with 0.5 μg/μl of an eGFP-expression vector in which eGFP was driven by a CAG promoter (CMV/beta-actin promoter) plus 0.5 μg/μl of the dsRed-expression vector. All explants were fixed with 4% paraformaldehyde (PFA) for 15 min, 2 days after transfection, and then stained with DAPI to label nuclei. The area of maximal transfection for each explant was identified and imaged on a fluorescent microscope at 200x. Six microscopic fields of the GER from five explants were imaged, and the number of cells positive for eGFP, dsRed, or both reporters was counted. The ratio of cells expressing one or both reporters was then calculated to estimate the degree of co-transfection.
Transfection of sensory epithelia with TFs and myosin VIIa staining
To determine the effect of various TFs on cochlear cells, plasmids encoding human TFs and driven by a CMV promoter were used singly or in combination at 0.5 μg/μl or 1.0 μg/μl. The plasmids included human ATOH1 (hATOH1), hTCF3, hGATA3, or hSP1. An empty vector was used as a transfection procedure control. All plasmids were purchased from OriGene (Rockville, MD). The cochlear sensory epithelial explants of Pou4f3/GFP mice were transfected with the combinations of plasmids shown in Table I. Five days after transfection, immunolabelling was carried out to detect myosin VIIa, which in the inner ear is a specific marker for the HC phenotype (Hasson et al., 1997). For immunolabelling, the explants were fixed with 4% PFA for 15 min, permeabilized with 0.5% Triton X-100 (Sigma, St. Louis, MO, USA) for 8 min and blocked with 10% fetal bovine serum for 30 min. They were then incubated overnight at 4°C with anti-myosin VIIa antibody (1:500; Proteus Biosciences; Ramona, CA). Texas-Red-conjugated anti-rabbit antibody (1:100; Jackson ImmunoResearch, West Grove, PA) was used as the secondary antibody. All explants prepared as above were mounted using mounting medium (Vectashield Hard Set, Vector Laboratories, Burlingame, CA) and covered with round cover glasses. The fluorescent signal was visualized using an inverted fluorescent microscope (IX 70, Olympus, Tokyo, Japan). The entire length of each explant was imaged from the two segments. Each cochlear epithelium was then divided into three equal sections by length, which were defined as the apical, middle, and basal turns. The numbers of Pou4f3/GFP and myosin VIIa positive cells were then counted for each turn.
| Plasmid Concentration |
Transfection Factor Combination | Sample Number |
|||
|---|---|---|---|---|---|
| hATOH1 | 11 | ||||
| hE2A | 10 | ||||
| hGata3 | 6 | ||||
| hATOH1 | + hSP1 | 10 | |||
| hATOH1 | + hGATA3 |
10 | |||
| hATOH1 | + hE2A | 10 | |||
| 0.5 μgμL | hATOH1 | + hSP1 | + hE2A | 10 | |
| hATOH1 | + hGATA3 |
+ hE2A | 11 | ||
| hATOH1 | + hGATA3 |
+ hE2A | + Empty vector | 10 | |
| hATOH1 | + hSP1 | + hGATA3 |
+ hE2A | 10 | |
| Empty vector | 5 | ||||
|
| |||||
| 1.0 μgμL | hATOH1 | 10 | |||
GFP quantification
To determine whether the expression of Pou4f3/GFP by cells that also express myosin VIIa was different from that of cells that did not express myosin VIIa, the average brightness of individual cells was measured by image analysis using Photoshop (version 10.0.1, Adobe Systems Incorporated). Images of the apical, middle or basal turn (200x) that contained native HCs, Pou4f3/GFP+/myosin VIIa+, and Pou4f3/GFP+/myosin VIIa-GER cells were chosen from each sample transfected with hATOH1 alone (n=11), hATOH1 plus hTCF3 (n=10), or hATOH1 plus hTCF3 plus hGATA3 (n=11) at 0.5 μg/μl. The background of each slide was determined by measuring fluorescence intensity in the GFP channel of widely spaced areas in which no cells were present. This background was subtracted from all cell measurements. For each HC, Pou4f3/GFP+/myosin VIIa+ GER cell, and Pou4f3/GFP+/myosin VIIa-GER cell in the image, the cell body was outlined and captured, and the average intensity of GFP fluorescence of the cell was measured. Untransfected cell background was determined by measuring fluorescence intensity in cells that showed no visible evidence of GFP expression. For the determination of cell numbers, only GFP+ cells that displayed fluorescence intensity more than 2X greater than that of untransfected cells were included in cell counts.
For measures of GFP intensity, background-subtracted GFP brightness of transfected GER cells in each sample was then standardized against the average background-subtracted GFP brightness of HCs in the same image, to correct for any differences in image exposure.
Data analysis
The numbers of Pou4f3/GFP+, myosin VIIa+ and double-positive cells in the GER of each cochlear turn were counted for each transfected explant. One-way ANOVA with Dunnett’s multiple comparisons test was used to evaluate differences between individual conditions using Prism 5 (GraphPad Software Inc., La Jolla, CA). Samples transfected with 0.5 or 1.0 μg/μl hATOH1 alone were used as the reference group for the Dunnett’s test. One-way ANOVA with Tukey’s multiple comparison test was used to evaluate differences between cochlear turns (i.e., apical, middle or basal turn) in a condition. Differences with a corrected p value less than 0.05 were considered significant. The data were presented as means ± standard deviations.
To compare normalized GFP intensity between any two groups of HCs, p value was calculated for pairs of groups with the nonparametric Mann-Whitney’s U test using Bonferroni correction for multiple comparison. Differences with a corrected p value less than 0.05 were considered significant.
Results
GER cells were preferentially transfected by electroporation
The location of transfected cells was evaluated by transfecting sensory epithelial explants with plasmid encoding dsRed driven by a constitutive (CMV) promoter. With the electroporation method used, the majority of transfected cells observed in P1.5 cochlear epithelial samples were located in the GER, medial to the sensory cell region, as has been reported previously (Zheng and Gao, 2000; Jones et al., 2006; Driver and Kelley, 2010; Zhao et al., 2011). Cells lateral to the HCs or HCs themselves were occasionally transfected, but this was rare.
Multiple plasmids were transferred into the same cells using electroporation
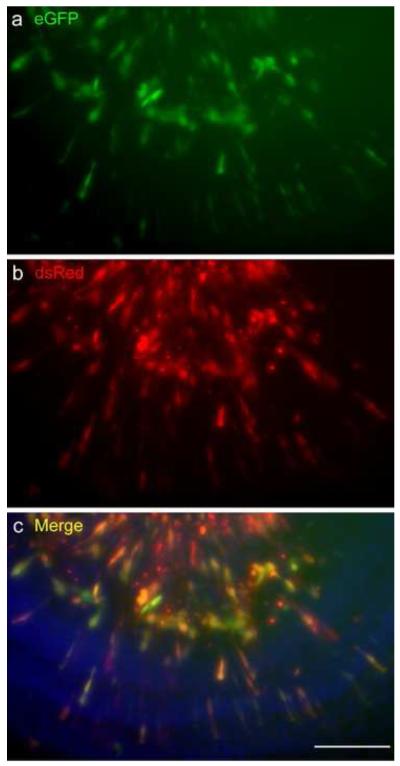
It has been reported that almost all cells in brain or inner ear tissue that are transfected with two plasmids will express both (Ono et al., 2009; Tabata and Nakajima, 2008). To confirm this with our methods, we introduced two different reporter plasmids in which either eGFP or dsRed are driven by a constitutive promoter, into P1.5 cochlear explants. We used wildtype mice for this experiment to avoid confusing extrinsic eGFP with intrinsic Pou4f3/GFP signal. dsRed expression was similar to that seen after dsRed transfection alone. Almost all dsRed+ cells also showed eGFP expression (Fig. 5). A total of 475 dsRed+ cells and 468 eGFP+ cells were counted. Of 475 dsRed+ cells, 458 were also positive for eGFP (96%). Of 468 eGFP+ cells, 458 were also positive for dsRed (98%). These results indicate that our co-transfection method transferred two different plasmids into virtually the same population of cells. They also suggest that combining two plasmids does not lead to significant differences in the number or population of cells that are transfected.
Fig. 5. Verification of single and double transfections.
A P1.5 wildtype cochlear sensory epithelial explant (in which HCs do not express GFP) co-transfected with eGFP- and dsRed-expression plasmids at 0.5 μg/μl for each plasmid (a, b). Almost all transfected cells (94%) expressed both eGFP and dsRed. The scale bar = 100 μm.
Transfection of hATOH1 or hTCF3 induces Pou4f3/GFP+ cells in the GER of Pou4f3/GFP mice
Zheng and Gao (2000) previously found that electroporation of postnatal rat cochlear explants with an expression of plasmid encoding ATOH1 induced the formation of ectopic HCs in the GER (Woods et al., 2004; Zheng and Gao, 2000). In our P1.5 transgenic mouse epithelia, we found that transfection of either 0.5 μg/μl or 1.0 μg/μl of hATOH1 alone induced similar numbers of Pou4f3/GFP+ cells in the GER (Fig. 6 a-d). The explants were imaged daily and the positions of all HCs were followed. No migration of HCs into the GER was ever observed. It also should be noted that GER cells showed increasing expression of Pou4f3/GFP from Day 1 to 5, while Pou4f3/GFP+ HCs spread outward and many outer HCs were lost (see Fig. 7 a-f; Time course of Pou4f3/GFP expression in the GER in the explant transfected with hAtoh1 plus hTCF3 plus hGATA3). We noted a greater number of eGFP+ cells in the GER of the apical turn than in the basal turn (p<0.05; Fig. 8 b-d). The fact that similar numbers of GFP+ cells were noted in the GER after transfection with 0.5 versus 1.0 μg/μl of plasmid (Fig. 8) suggests that plasmid dose alone does not influence the number or location of transfected cells.
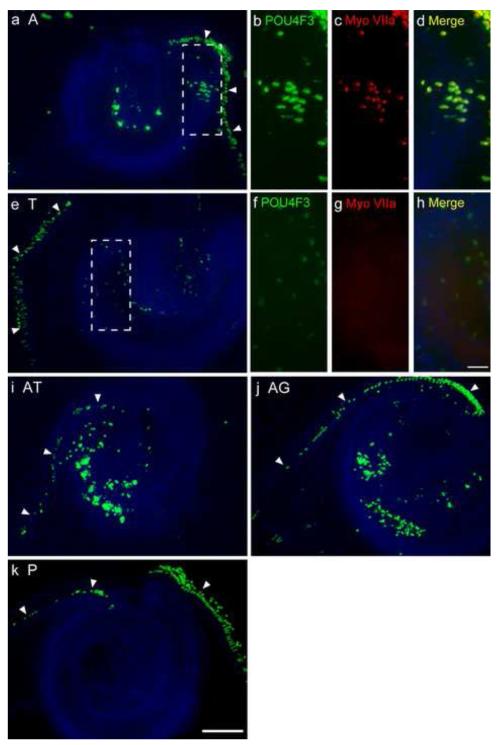
Fig. 6. Co-transfection enhances Pou4f3/GFP induction in the GER by ATOH1.
The apical turn of P1.5 explants, 5 days after transfection with transcription factors (TFs). Transfection with 0.5 μg/μl of hATOH1 alone (a) or hTCF3 alone (e) induced Pou4f3/GFP+ cells in the GER, well inside the native HCs (arrow heads) of Pou4f3/GFP mouse sensory epithelium. Transfection of hATOH1 in combination with either hTCF3 (i) or hGATA3 (j) induced more Pou4f3/GFP+ GER cells than hATOH1 alone. Transfection of an empty vector (k) did not induce Pou4f3/GFP+ GER cells. The figure also illustrates the loss of HCs (arrowheads) induced by electroporation. It should be noted that the explants were imaged daily and the position of all HCs were traced, and that no migration of HCs into the GER was ever observed (see Fig. 7) Panels b-d and f-h show a higher magnification of the sample transfected with hATOH1 alone (a) and hTCF3 alone (e) respectively. hATOH1 alone induced myosin VIIa (Myo VIIa) expression, but hTCF3 did not. The scale bar in e = 200 μm. The scale bar in h = 50 μm. A, 0.5 μg/μl of hATOH1 expression plasmid; T, 0.5 μg/μl of hTCF3 expression plasmid; G, 0.5 μg/μl of hGATA3 expression plasmid; P, 0.5 μg/μl of empty plasmid.
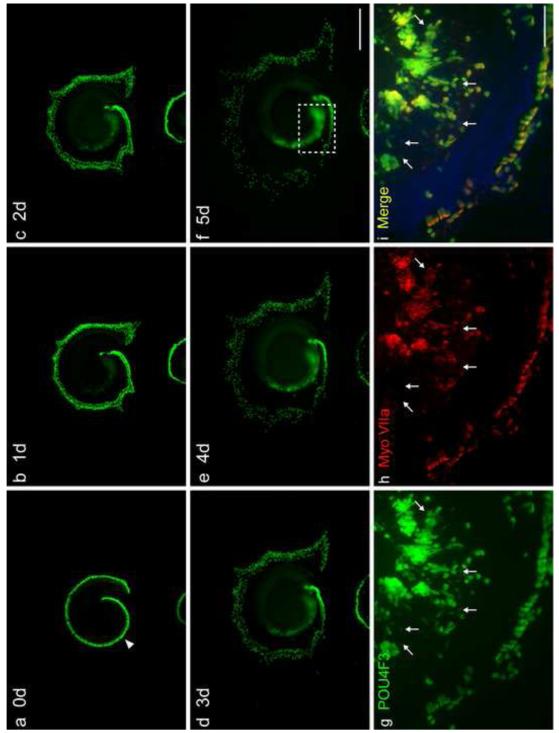
Fig. 7. TF co-transfection enhances Myo7A induction in the GER by ATOH1.
Time course of Pou4f3/GFP expression in the GER of a middle and basal turn explant transfected with hATOH1 plus hTCF3 plus hGATA3, and myosin VIIa expression in the same sample. GER cells showed increasing expression of Pou4f3/GFP from Day 1 to 5, while Pou4f3/GFP+ HCs spread outward and many outer HCs were lost (a-f). Panels g-i show a higher magnification of Pou4f3/GFP, and myosin VIIa expression, for the region indicated in panel f on Day 5. Many Pou4f3/GFP+ GER cells (g) were also positive for myosin VIIa (h, i). While no myosin VIIa+ cells were negative for Pou4f3/GFP, not all Pou4f3/GFP+ GER cells expressed myosin VIIa. Pou4f3/GFP+ cells negative for myosin VIIa tended to show weaker GFP intensity (arrows in g-i indicate some such cells). The scale bar in f = 500 μm. The scale bar in i = 100 μm.
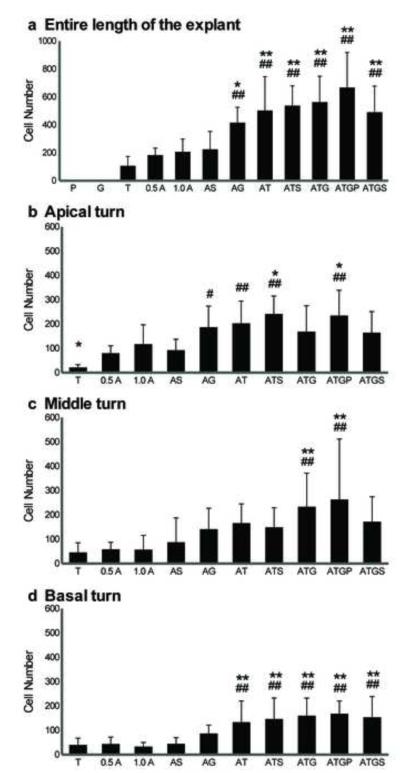
Fig. 8. Quantitative analysis of Pou4f3/GFP induction by TF co-transfection.
The numbers of Pou4f3/GFP+ cells observed in the GER following transfection with plasmids encoding TFs, alone or in combination. The numbers of Pou4f3/GFP+ GER cells observed along the entire length of the sensory epithelium (a), in the apical turn (b), in the middle turn (c), or in the basal turn (d) are shown separately. Transfection of hATOH1 alone and hTCF3 alone induced Pou4f3/GFP+ GER cells, but hGATA3 alone did not. Co-transfection of hATOH1 with either hTCF3 or hGATA3 induced more Pou4f3/GFP+ GER cells, and the increase was greater than that induced by hATOH1 alone or hTCF3 alone in the entire cochlea (a) and apical turn (b), although the effect of adding hGATA3 to hATOH1 was not significant statistically in the basal turn, while the triple combination of hATOH1, hTCF3 and hGATA3 was required to increase Pou4f3/GFP+ GER cells significantly over that observed with hATOH1 alone in the middle turn. These indicate that transfection of hATOH1 in combination with either hTCF3 or hGATA3 enhances the hATOH1 effect on 8.5 kb of 5′ Pou4f3 regulatory DNA synergistically.
0.5 A, 0.5 μg/μl of hATOH1 expression plasmid; 1.0 A, 1.0 μg/μl of hATOH1 expression plasmid; T, 0.5 μg/μl of hTCF3 expression plasmid; G, 0.5 μg/μl of hGATA3 expression plasmid; S, 1.0 μg/μl of hSP1 expression plasmid; P, 0.5 μg/μl of empty vector.
# or ## indicates a significant difference at p<0.05 or p<0.01 from 0.5 μg/μl of hATOH1 alone by one-way ANOVA with Dunnett’s multiple comparison test. *or ** indicates a significant difference at p<0.05 or p<0.01 from 1.0 μg/μl of hATOH1 alone with the same test.
Interestingly, transfection of plasmid encoding hTCF3 by itself also induced Pou4f3/GFP+ cells in the GER (Fig. 6e, Fig. 8a). Moreover, with hTCF3, there was no statistical difference in the number of Pou4f3/GFP+ cells among the apical, middle, and basal turns. In contrast, transfection of hGATA3 alone (Fig. 8a) or hSP1 alone (not shown) did not induce any Pou4f3/GFP+ cells in the GER. Similarly, in control cultures transfected with 0.5 μg/μl of empty vector, no Pou4f3/GFP+ GER cells were observed (Fig. 6k).
Co-transfection of hATOH1 with either hTCF3 or hGATA3 produced significantly more Pou4f3/GFP+ cells in the GER than hATOH1 alone
Explants were transfected with hATOH1 in combination with either hTCF3 or hGATA3. Along the entire length of the sensory epithelium, co-transfection of hATOH1 with either hTCF3 or hGATA3 at 0.5 μg/μl induced 2-3 times more Pou4f3/GFP+ cells in GER than 0.5 or 1.0 μg/μl hATOH1 alone (Fig. 6, Fig. 8). This difference was statistically significant (p<0.01) for both 0.5 and 1.0 μg/μl transfection dosages. In contrast, co-transfection with hSP1 did not alter the effects of hATOH1 (Fig. 8).
Co-transfection of hATOH1 plus hGATA3 plus hTCF3 was not statistically more effective than either the double combinations of hATOH1 plus hTCF3 or hATOH1 plus hGATA3 in the cochlea as a whole (Fig. 8a) or in the apical turn. However, the triple combination was more effective in the middle turn (Fig. 8b). Similarly, co-transfection of hATOH1 plus hSP1 plus hGATA3 plus hTCF3 was no more effective than the double combinations. Adding 0.5 μg/μl empty vector to the triple combination also did not alter the outcome significantly (Fig. 8).
Co-transfection of hATOH1 with either hTCF3 or hGATA3 produced more myosin VIIa+ cells in the GER than hATOH1 alone
After birth, the expression of POU4F3 is restricted to HCs, and thus the expression of Pou4f3/GFP in P1.5 GER cells provides evidence of conversion to a HC phenotype. However, since we have hypothesized that the TFs used interact directly with the Pou4f3 promoter and enhancers, it is possible that regulation of the regulatory DNA of the transgene occurred in isolation. We therefore evaluated the expression of myosin VIIa, a different HC specific marker that appears later in normal development than POU4F3 (Bermingham et al., 1999; Woods et al., 2004; Xiang et al., 1997, 2003).
hATOH1 alone induced myosin VIIa+ GER cells (Fig. 6 a-d), in accordance with the previous report of Zheng and Gao (2000). Moreover, the cells overlapped with cells expressing Pou4f3/GFP. Along the entire length of the sensory epithelium, about 60% of Pou4f3/GFP+ GER cells induced by 0.5 μg/μl hATOH1 alone were also positive for myosin VIIa. No cells were myosin VIIa positive and Pou4f3/GFP negative (Fig. 6 b-d, Fig. 7 g-i). In contrast, neither hTCF3 nor hGATA3 alone induced myosin VII a+ GER cells (Fig. 6 e-h, Fig. 9), although as mentioned above hTCF3 induced GER cells positive for Pou4f3/GFP.
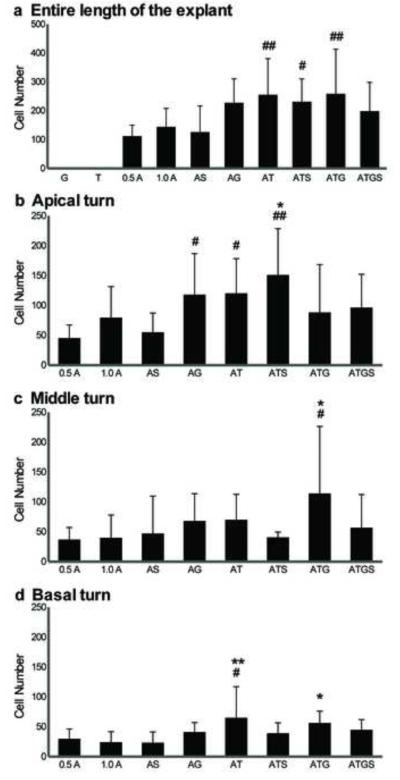
Fig. 9. Quantitative analysis of Myo7A induction by TF co-transfection.
Comparison of the number of Pou4f3/GFP+ GER cells that also expressed myosin VIIa+, observed after transfection with hATOH1 alone or TF combinations. The numbers of Pou4f3/GFP+/myosin VIIa+ GER cells in the entire length of the sensory epithelium (a), in the apical turns (b), in the middle turn (c), and in the basal turn (d) are shown separately. While hATOH1 alone induced Pou4f3/GFP+/myosin VIIa+ GER cells, hTCF3 alone or hGATA3 alone did not. However, co-transfection of each of these TFs with hATOH1 induced more Pou4f3/GFP+/myosin VIIa+ GER cells than hATOH1 alone in the apical turn (a). In the basal turn only hTCF3 enhanced the efeects of hATOH1, while in the middle turn both hTCF3 and hGATA3 were required. The result suggests a synergistic effect among hATOH1, hTCF3, and hGATA3 in the regulation of myosin VIIa expression.
Indicators and abbreviations are the same as those in Fig. 8.
Co-transfection of hATOH1 with either hTCF3 or hGATA3 produced significantly more (p<0.05) myosin VIIa+ cells, by approximately 2-3 times, than 0.5 πg/πl hATOH1 alone in the apical turn (Fig. 9b). As observed for hATOH1 alone, with double combinations more than 50% of Pou4f3/GFP+ GER cells were also positive for myosin VIIa. Again, no cells were myosin VIIa positive but Pou4f3/GFP negative. The number of Pou4f3/GFP+/myosin VIIa+ GER cells decreased along the cochlear spiral from the apex to the base (Fig. 9b-d), although TF combinations were still more effective than hATOH1 alone, even in the basal turn (p<0.01 for hATOH1 plus hTCF3 and hATOH1 plus hTCF3 plus hGATA3 compared to either 0.5 or 1.0 μg/μl hATOH1 alone; Fig. 9d). Again, only the triple combination of hATOH1 plus hTCF3 plus hGATA3 was more effective than hATOH1 alone in the middle turn.
Double-positive Pou4f3/GFP+/myosin VIIa+ GER cells expressed more GFP than Pou4f3/GFP+/myosin VIIa-GER cells
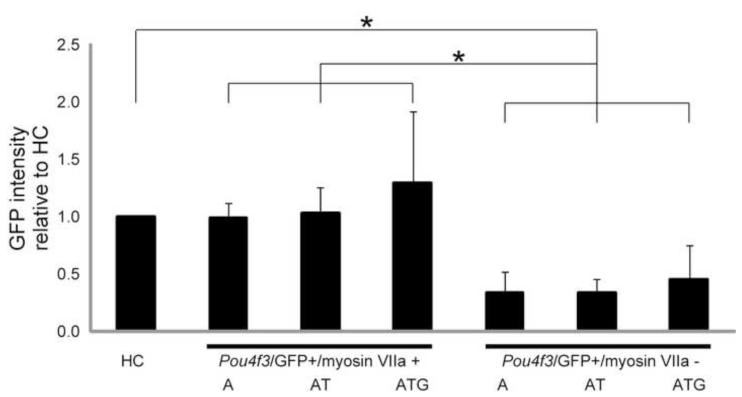
To determine whether the amount of Pou4f3/GFP expressed by GER cells was related to myosin VIIa expression, we compared the level of GFP fluorescence in Pou4f3/GFP+/myosin VIIa+ GER cells with that observed in Pou4f3/GFP+/myosin VIIa-cells. A total of 712, 474, and 637 HCs, 284, 321, and 572 Pou4f3/GFP+/myosin VIIa+ cells, and 163, 395, and 516 Pou4f3/GFP+/myosin VIIa-GER cells were measured in samples transfected with 1) hATOH1 alone, 2) hATOH1 plus hTCF3, or 3) hATOH1 plus hTCF3 plus hGATA3, respectively. As can be seen in Figure 7, Pou4f3/GFP+/myosin VIIa+ GER cells exhibited significantly greater GFP intensity when compared to Pou4f3/GFP+/myosin VIIa-cells (p<0.0001; Fig. 7 g-i, Fig. 10). The Pou4f3/GFP+/myosin VIIa+ GER cells were similar in GFP intensity to endogenous Pou4f3/GFP+ native HCs. In contrast, Pou4f3/GFP+/myosin VIIa-cells exhibited less than 50% of the fluorescent intensity of HCs. When GFP intensity of Pou4f3/GFP+/myosin VIIa+ GER cells was compared across the three transfection groups, no differences were observed. Similarly, GER cells that were Pou4f3/GFP+ but myosin VIIa-showed comparable GFP intensity across all three groups.
Fig. 10. High levels of Pou4f3/GFP induction are required for Myo7A induction.
Comparison of normalized GFP intensity between native HCs, and transfected GER cells that expressed Pou4f3/GFP alone or Pou4f3/GFP and myosin VIIa. GFP intensities were measured in the samples transfected with 0.5 μg/μl hATOH1 alone (A), hATOH1 plus hTCF3 (AT), and hATOH1 plus hTCF3 plus hGATA3 (ATG). GER cells that expressed both Pou4f3/GFP and myosin VIIa exhibited significantly greater GFP intensity than cells that expressed only Pou4f3/GFP. While the intensity of Pou4f3/GFP+/myosin VIIa+ GER cells was similar to that of native HCs, there were no differences of GFP intensity of GER cells among the three transfection groups.
* indicates p<0.001 with the Mann-Whitney’s U test using Bonferroni correction for multiple comparison. Abbreviations are the same as those of Fig. 8.
Discussion
As mentioned above, ATOH1 plays a key role in early HC differentiation (Ahmed et al., 2012; Fritzsch et al., 2010; Kawamoto et al., 2003; Pan et al., 2011, 2012; Zheng and Gao, 2000). However, it is also expressed in several other cell types, where it is instrumental in determining quite different cell phenotypes. Unlike in some non-mammalian organisms, where duplication of the Atoh1 gene has allowed distinct functions for different gene isoforms, in mammals the same TF must play multiple roles (see Fritzsch et al., 2010 for a review of the evolution of Atoh1 and other bHLH genes important for inner ear cell type specification). Therefore, we speculated that cell-specific cues must exist to specify the role of ATOH1 in HC formation. Because it is known that combinatorial TF codes can contribute to define specific cell phenotype determination (e.g. Shashikant et al., 1995; Ma, 2006), we hypothesized that this might be true of HC specification by ATOH1 as well. To approach this issue, we determined whether combinatorial coding can influence expression of a reporter gene driven by 5′ Pou4f3 genomic DNA, as well as expression of an independent HC marker, myosin VIIa. In the current study, we present evidence that TCF3 proteins and GATA3 can function in combination with ATOH1, to regulate expression of the Pou4f3 gene in cells of the GER. Enhancement of myosin VIIa expression in these cells provides evidence that these combinations more broadly affect HC phenotype.
TCF3 factors and Class II bHLH TFs
Our results with TCF3 (E2A) are well supported by current understanding of bHLH TFs. The two TCF3 proteins, E12 and E47 produced by differential splicing of the Tcf3 gene, are ubiquitously expressed Class I bHLH TFs (Massari and Murre, 2000; Sun and Baltimore, 1991), although the TCF3 expression pattern has not been well studied in the cochlea. In general, TCF3 proteins are capable of forming either homo-or heterodimers, and binding to E-boxes in the regulatory DNA of genes (Akazawa et al., 1995; Ellenberger et al., 1994; Massari and Murre, 2000). The binding affinity of TCF3 is enhanced by heterodimerization with Class II TFs like ATOH1 (Akazawa et al., 1995; Krizhanovsky et al., 2006; Massari and Murre, 2000). The TCF3 preferred E-box sequences are CAGCTG and CACCTG (Atchley and Fitch, 1997; Ledent et al., 2002), and two copies of CAGCTG and one CACCTG are clustered in a highly conserved sequence region within Pou4f3 regulatory DNA, as mentioned previously (Fig.1). Moreover, Akazawa et al. (1995) has demonstrated that the binding affinity of TCF3 to the motifs is strongly enhanced by heterodimerization with ATOH1, although ATOH1 alone does not bind to this E-box. On the other hand, Klisch et al. (2011) has demonstrated in medulloblastoma cells that ATOH1 binds to a motif consisting of a subset of E-boxes plus flanking DNA with high affinity and activates a downstream reporter. Two of the E-boxes in the highly-conserved Pou4f3 sequence region fit the Klisch et al. motif (see Figure 1).
While we did not directly test negative regulation of TCF3 protein function in this report, it is also well documented. For example, inhibitors of differentiation and DNA binding (Ids) form transcriptionally inactive heterodimers with the TCF3 proteins, and thereby prevent the TCF3 proteins from forming functional heterodimers with tissue-specific bHLH proteins such as ATOH1 (Norton, 2000). HES proteins also can achieve repression in two ways (Norton, 2000; Palaparti et al., 1997). In the first, HES factors bind N-boxes on regulatory DNA and recruit the co-repressor transducin-like enhancer of split. Second, in a non-DNA-binding manner, HES protein can interfere with complex formation between TCF3 proteins and Class II bHLH TFs. As noted above, these proteins are known to be involved in HC development. Fritzsch et al. (2006) provides a comprehensive review of the role of Id and HES factors in HC development (see Figure 3 of their paper). We identified a potentially HES-binding N-box that overlaps one of the highly conserved E-boxes within Pou4f3 regulatory DNA that can bind TCF3/Class II bHLH TFs (Fig. 1), suggesting that HES may negatively regulate the Pou4f3 gene via direct DNA binding.
TCF3 alone induces Pou4f3/GFP expression in the GER
We observed that transfection of hTCF3 alone induced Pou4f3/GFP expression in the neonatal GER (Fig. 6 e-h). Overexpression of hTCF3 would be expected to generate an excess of TCF3 homodimers. These could bind to E-boxes preferred by TCF3 TFs in the transgene, and induce Pou4f3/GFP expression directly. Another possibility is interaction of hTCF3 with an endogenously expressed Class II bHLH TF, to increase binding to CAGCTG and CACCTG E-boxes in the transgene. This could occur if there were an excess of a Class II bHLH TF such as ATOH1 expressed within GER cells. ATOH1 seems unlikely, since RT-PCR results suggest that there is little ATOH1 in the GER (Zhang et al., 2007), and since addition of ATOH1 alone to GER cells is enough to induce Pou4f3/GFP expression.
Alternatively, the overexpressed hTCF3 could inhibit endogenous Ids and/or HES factors that prevent the binding of bHLH factors to the transgene. In the cochlea, it has been suggested that Ids or HES1 in the GER of postnatal rodent cochlea are one reason why GER cells normally do not give rise to HCs (Jones et al., 2006; Murata et al., 2006; Zhang et al., 2007), although they have the capacity to differentiate into HCs in response to exogenous ATOH1 (Zheng and Gao, 2000). Excess hTCF3 could bind with Ids and/or HES factors, decreasing the level of Ids and HES available to prevent activation of the transgene by endogenous bHLH factors. Alternatively, high levels of hTCF3/Class II heterodimers could more successfully compete for binding to the E-boxes, displacing Ids or HES factors. At least in the case of ATOH1, this is unlikely for the reasons stated above.
Finally, it should be noted that indirect regulation of the transgene is also a possibility. Transfection with hTCF3 could induce other TFs, and these other factors could bind to the regulatory sequences in the transgene and control Pou4f3/GFP expression. For example, TFE2 could combine with other bHLH factors to bind E-boxes in the promoter of Atoh1 itself (Helms et al., 2000; Pan et al., 2012).
ATOH1 induces Pou4f3/GFP expression in the GER, and this effect is enhanced by TCF3
Transfection with hATOH1 induced GER cells to express GFP under the control of Pou4f3 regulatory DNA and this effect was enhanced by co-transfection with hTCF3 (Fig. 6 a, i, Fig. 8). Class II bHLH TFs like ATOH1 are incapable of forming homodimers, and require heterodimerization with E proteins to bind E-boxes with high affinity (Akazawa et al., 1995; Krizhanovsky et al., 2006; Massari and Murre, 2000). Therefore, it is highly likely that transfection of hATOH1 alone generated heterodimers with endogenous TCF3 products such as E47 or E12, and in some transfected cells, the amount of the heterodimers was sufficient to activate Pou4f3 regulatory DNA in the transgene and induce Pou4f3/GFP expression. Our previous demonstration of direct binding of hATOH1 to the region of clustered E-boxes in the transgene supports this possibility (Masuda et al., 2011). If the level of endogenous TCF3 factors was low, co-transfection of hATOH1 and hTCF3 would be expected to generate more hATOH1/hTCF3 heterodimers, activating the transgene in more transfected GER cells, than either hATOH1 or hTCF3 alone. This seems the most likely explanation for our results.
Of course, the hTCF3 may inhibit endogenous Ids and/or HES factors as mentioned above, allowing more hTCF3/hATOH1 heterodimers to bind to E-boxes, which could also account for increased Pou4f3/GFP expression.
It should be noted that the increase of Pou4f3/GFP+ GER cells by the TF combination of hATOH1 plus hTCF3 was greater than that induced by hTCF3 alone plus hATOH1 alone (Fig. 8). This synergistic enhancement seems likely to result from interactions among TFs, as mentioned above, rather than an additive effect.
The possibility that increasing the amount of plasmid or number of plasmid species might, by itself, increase transfection efficiency is not supported by our data, since combining reporter plasmids, increasing hATOH1 plasmid dose, or combinating plasmids beyond three did not enhance GFP expression (Fig. 8). The possibility that some unique structural feature of the hTCF3 plasmid enhanced transfection efficiency seems remote.
GATA3 also enhances the effect of ATOH1 on Pou4f3/GFP expression in the GER
GATA3 is necessary for neurosensory development of the vertebrate ear (Karis et al., 2001; Lawoko-Kerali et al., 2002), and its haploinsufficiency causes hearing loss (Van Esch et al., 2000). Prolonged and high level expression of GATA3 in virtually all parts of the auditory system suggests that it may help to define and integrate the different, spatially diverse components of the system during development (Lawoko-Kerali et al., 2002). Interactions between GATA factors and Class II bHLH TFs have also been reported. In other tissues, GATA3 enhances the effect of bHLH TFs synergistically (Iwahori et al., 2004; Yang et al., 2009), reinforces feedback interactions with bHLH TFs (Hendershot et al., 2008; Moriguchi et al., 2006), or inhibits Ids or Notch signals like HES1 (Batts et al., 2009; Dydensborg et al., 2009; Wang et al., 2009). For example, in lymphocyte development, GATA3 reinforces an autoregulatory loop of a bHLH TF expression, acts as a co-factor of other bHLH TFs for target gene expression, and also inhibits Notch signaling (Iwahori et al., 2004; Wang et al., 2009; Yang et al., 2009). In breast cancer, GATA3 inhibits Ids (Dydensborg et al., 2009).
The mechanisms for these interactions have not been explored in the cochlea. Considering that clustered Class I/Class II preferred E-boxes are located between GATA binding motifs in the highly conserved region (Fig. 1), interaction between ATOH1 and GATA3 on Pou4f3 regulatory DNA seems a reasonable possibility. Another possibility is interaction with LIM homeodomain proteins, which have been shown to interact with GATA3 and bHLH proteins in cell fate specification (Matthews and Visvader, 2003). Several LIM-domain proteins are involved in cochlear development (e.g. Nichols et al., 2008; Hertzano et al., 2007).
SP1 does not enhance the effect of ATOH1 on Pou4f3/GFP expression in the GER
hSP1 did not enhance the effect of hATOH1 in the present study. Polymorphisms affecting SP1 binding sites have recently been observed in the human Pou4f3 gene (Nolan et al., 2007), although the binding sites are not at the highly conserved locus included in our transgene. In any event, either this factor is not involved in Pou4f3/GFP transgene expression in P1.5 GER cells, or there is sufficient endogenous SP1 that induced expression has no additonal effect.
ATOH1 alone induces myosin VIIa+ GER cells, but TCF3 or GATA3 do not
Transfection with hATOH1 induced myosin VIIa+ GER cells, as has been reported previously (Fig. 6 a-d; Zheng and Gao, 2000). We observed less robust induction than reported by Zheng and Gao in P0 rat epithelia, which may be related to the age of the samples used, and/or to differences in the relative developmental ages of the mouse and rat (Dazert et al., 2007; Freeman et al., 1996).
However, in contrast to the induction of Pou4f3/GFP+ cells, hTCF3 alone did not induce myosin VIIa+ GER cells (Fig. 6 e-h). Jones et al. (2006) similarly found that overexpression of E47 alone via electroporation did not induce myosin VIIa+ cells in the sensory epithelia of the E13 mouse inner ear. The myosin VIIa gene also has an E-box (CAGGTG) in its regulatory DNA which is essential but not sufficient to control the HC expression of myosin VIIa (Boeda et al., 2001). However, interaction with other TFs or the epigenetic state of myosin VIIa regulatory DNA may be different than that of the transgene. Similarly, transfection with GATA3 did not induce myosin VIIa expression in the GER.
TCF3 or GATA3 enhances the effect of ATOH1 for myosin VIIa induction in the GER
Our results demonstrate that TF combinations of hTCF3 or hGATA3 with hATOH1 were more effective in inducing expression of Pou4f3/GFP than hATOH1 alone (Fig. 8). These results provide support for our hypothesis that TFs with conserved, closely-spaced binding sites in its 5′ regulatory DNA can influence the expression of the Pou4f3 gene. However, we also noted that hTCF3 or hGATA3 enhanced myosin VIIa induction in the GER (Fig. 9). This seems unlikely to occur as a consequence of POU4F3 expression, since transfection with POU4F3 itself has not been shown to induce myosin VIIa+ cells in GER or other non-sensory inner ear cells (Zheng and Gao, 2000). However, ATOH1 target genes in general are thought to contain evolutionarily conserved clustered E-boxes (Krizhanovsky et al., 2006), as we observed in the Pou4f3 gene (Masuda et al., 2011). It is possible that other ATOH1 target genes in HCs contain binding sites for TCF3/ATOH1 and nearby sites for GATA3. Consistent with this hypothesis, co-transfection of hATOH1 with either hTCF3 or hGATA3 induced about 2 times more myosin VIIa+ cells than hATOH1 alone. Our data certainly suggest similar co-operative mechanisms among ATOH1, TCF3, and GATA3 in the regulation of myosin VIIa, either directly or indirectly.
Overall, these results demonstrate that TF combinations can enhance the effect of ATOH1 for myosin VIIa expression as well as for POU4F3 expression and suggest that they may also cooperate more generally in specification of HC fate and differentiation. Supporting this conclusion is the observation that induction of retinal ganglion cells by another Atonal homolog, Atoh7, can also be modified by the co-expression of other TFs (Kiyama et al., 2011).
The effects of exogenous TFs on cochlear cells must be considered in the context of endogenous expression. At the age used for our studies, TCF3 as a ubiquitously expressed factor is presumably expressed in all cochlear cells. GATA3 is expressed at high levels in the GER, and much less so in HCs at this age (Rivolta and Holley, 1998). The presence of endogenous GATA3 may partially explain the ability of Atoh1 alone to preferentially induce HC-like cells in the GER. Other TFs known to be important to HCs could also have an influence, such as FGF10 and Sox 2, both of which are highly expressed in the rodent GER around P0 (Pan et al., 2011).
Not all Pou4f3/GFP+ cells express myosin VIIa, although all myosinVIIa+ cells expressed GFP
While hATOH1 alone or in combination with other TFs induced Pou4f3/GFP+/myosin VIIa+ GER cells, not all Pou4f3/GFP+ cells showed myosin VIIa positivity (Fig. 7). In the developing mouse inner ear, ATOH1 and POU4F3 expression precedes that of myosin VIIa, as mentioned previously (Bermingham et al., 1999; Woods et al., 2004; Xiang et al., 1997, 2003). Considering the expression patterns of these genes, Pou4f3/GFP+/myosin VIIa-cells could represent a more immature state than Pou4f3/GFP+/myosin VIIa+ cells. It is possible that weaker Pou4f3/GFP+ cells might express myosin VIIa given more time than was used in the present study. However, Hu et al. (2010) found that low levels of ATOH1 do not drive cochlear neural progenitors into mature HCs. Moreover, a recent study by Pan et al. (2012), using a conditional expression transgenic model, found that the level and duration of ATOH1 expression played a significant role not only in the survival of HCs, but also in their degree of differentation or even HC type. Consistent with these reports, Pou4f3/GFP+/myosin VIIa-GER cells showed weaker Pou4f3/GFP intensity than the intensity of Pou4f3/GFP +/myosin VIIa+ cells (Fig. 7, Fig. 10). The hATOH1 expression of some GER cells may not have reached the threshold for induction of myosin VIIa or a HC fate. This would imply that the threshold of POU4F3 expression, or more specifically our Pou4f3/GFP transgene, is lower than that of myosin VIIa.
The effects of TCF3 and GATA3 on ATOH1 did not depend upon cochlear location
The present study also suggests that the difference in maturation between the apex and the base may influence the effect of ATOH1 in GER cells. Differentiation of the cochlear sensory epithelium begins in a gradient that extends from the base of the cochlear spiral to the apex, and it is still actively developing at around P1 (Chen et al., 2002; Scheffer et al., 2007a; Zuo et al., 1999). At the time of electroporation, cells located in the middle and basal turns were substantially more mature than those of the apex (Scheffer et al., 2007a). We found that the number of Pou4f3/GFP+/myosin VIIa+ GER cells that were induced by ATOH1 decreased along the cochlear spiral from the apical to the basal turn (Fig. 9), suggesting that maturation decreases the ability of GER cells to express the transgene and/or myosin VIIa, although we did not evaluate age as a variable and a positional effect cannot be ruled out by our results. In contrast, the ability of TCF3 and GATA3 combinations to enhance the effects of ATOH1 were proportionally equivalent in all turns, indicating that cooperative enhancement is a relatively stable property of GER responses.
Triple or quadruple TF combinations are generally not more effective than double TF combinations
In general, triple or quadruple TF combinations did not enhance the effect of hATOH1 over that seen with the double TF combinations of hATOH1 plus hTCF3 or hATOH1 plus GATA3. We confirmed that this ineffectiveness did not result from the toxicity of higher plasmid concentrations. Transfection with 2 μg/μl plasmids, including hATOH1, hGATA3, hTCF3 and an empty vector, did not have adverse effect on the expression of Pou4f3/GFP, compared with 1.5 μg/μl plasmid, including hATOH1, hTCF3, and hGATA3. These data also provide an additional control demonstrating that increasing the amount of plasmid, by itself, does not alter the effects of a fixed amount of hATOH1 plasmid.
However, hATOH1 plus hTCF3 plus hGATA3 was required to generate significantly more GFP+ and myosin VIIa+ GER cells than hATOH1 alone, in the middle turn (Fig. 8c, Fig. 9c). Both hATOH1 plus hTCF3 and hATOH1 plus GATA3 transfection enhanced GFP and myosin VIIa in GER cells in the middle turn, but these increases were not statistically significant. It is thus possible that multiple combinations are more effective than paired combinations under some conditions.
TF transfection did not induce GFP or Myosin VIIa in all GER cells
Transfection of P1.5 sensory epithelium with dsRed resulted in widespread expression of the reporter in the GER, indicating that electroporation was highly effective in achieving transfection. It is clear from a comparison of dsRed and eGFP plasmid transfection efficiency in the GER (Fig. 4, Fig. 5), versus the number of GER cells that responded to TF transfection with Pou4f3/GFP or myosinVIIa expression (Fig. 6), that either transfection efficiency of TF plasmids was lower than that of the dsRed or eGFP plasmids, or that TF transfection induced a response in a minority of GER cells. The limited cellular conversion observed after ATOH1 transfection of the GER cells observed by other laboratories may favor the latter explanation (Zhang et al., 2007). If so, a potential contributing factor to lack of response to TF transfections may be lack of still other TFs that are necessary for conversion of GER cells. Our observation of additional, highly-conserved TF binding sites in the transgene used for this study may be relevant to this issue (Masuda et al., 2011).
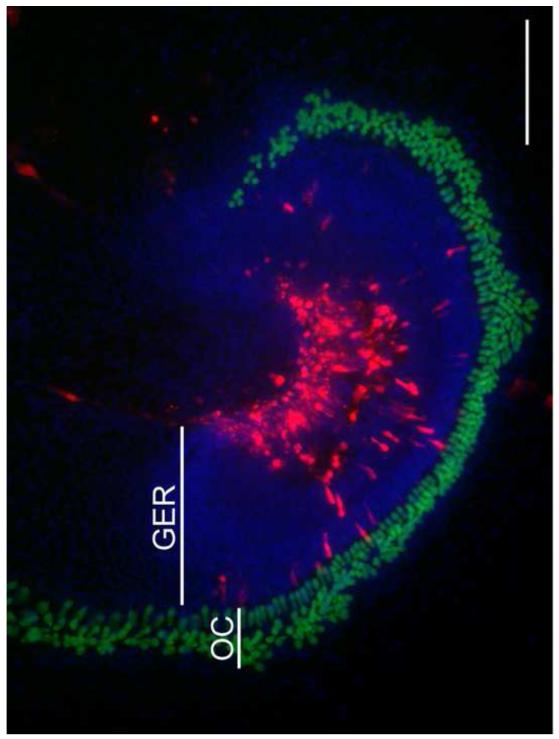
Fig. 4. Typical location of transfected cells in the P1.5 sensory epithelium of the Pou4f3-GFP transgenic mouse.
The explant shown was electroporated with 1 πg/πl dsRed-expression vector, and fixed 2 days after transfection. The majority of the transfected cells are located in the greater epithelial ridge (GER) which was medial to the organ of Corti (OC). As in this example, HCs (expressing GFP) were never transfected. Blue fluorescence shows nuclei labeled with DAPI. The scale bar = 200 μm.
Acknowledgements
Supported by grants from the Research Service of the Veterans Administration, the NIH/NIDCD (RO1DC000139), and the National Organization for Hearing Research (NOHR). Julie Lightner formatted the manuscript and Dr. Alain Dabdoub provided critical comments. Their assistance is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed M, Wong EYM, Sun J, Xu J, Wang F, Xu P-X. Eya1-Six1 interaction is sifficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Devel Cell. 2012;22:377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270:8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- Artinger KB, Fedtsova N, Rhee JM, Bronner-Fraser M, Turner E. Placodal origin of Brn-3-expressing cranial sensory neurons. J Neurobiol. 1998;36:572–585. doi: 10.1002/(sici)1097-4695(19980915)36:4<572::aid-neu10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Atchley WR, Fitch WM. A natural classification of the basic helix-loop-helix class of transcription factors. Proc Natl Acad Sci U S A. 1997;94:5172–5176. doi: 10.1073/pnas.94.10.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea TC, Williams J, Smallwood P, Shi M, Motajo O, Nathans J. Combinatorial expression of Brn3 transcription factors in somatosensory neurons: genetic and morphologic analysis. J Neurosci. 2012;32:995–1007. doi: 10.1523/JNEUROSCI.4755-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts SA, Shoemaker CR, Raphael Y. Notch signaling and Hes labeling in the normal and drug-damaged organ of Corti. Hear Res. 2009;249:15–22. doi: 10.1016/j.heares.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Boeda B, Weil D, Petit C. A specific promoter of the sensory cells of the inner ear defined by transgenesis. Hum Mol Genet. 2001;10:1581–1589. doi: 10.1093/hmg/10.15.1581. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Collin RW, Chellappa R, Pauw RJ, Vriend G, Oostrik J, van Drunen W, Huygen PL, Admiraal R, Hoefsloot LH, Cremers FP, Xiang M, Cremers CW, Kremer H. Missense mutations in POU4F3 cause autosomal dominant hearing impairment DFNA15 and affect subcellular localization and DNA binding. Hum Mutat. 2008;29:545–554. doi: 10.1002/humu.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Axelos M, Bardet C, Atanassova R, Chaubet N, Lescure B. Modular organization and development activity of an Arabidopsis thaliana EF-1 alpha gene promoter. Mol Gen Genet. 1993;238:428–436. doi: 10.1007/BF00292002. [DOI] [PubMed] [Google Scholar]
- Dazert S, Schick B, Hartensuer R, Volkenstein S, Aletsee C, Hansen S, Shehata-Dieler WE, Eigenthaler M, Walter U, Ryan AF, Brors D. Hearing development and spiral ganglion neurite growth in VASP deficient mice. Brain Res. 2007;1178:73–82. doi: 10.1016/j.brainres.2007.06.041. [DOI] [PubMed] [Google Scholar]
- Driver EC, Kelley MW. Transfection of mouse cochlear explants by electroporation. Curr Protoc Neurosci. 2010;4 doi: 10.1002/0471142301.ns0434s51. Unit 4.34.1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dydensborg AB, Rose AA, Wilson BJ, Grote D, Paquet M, Giguere V, Siegel PM, Bouchard M. GATA3 inhibits breast cancer growth and pulmonary breast cancer metastasis. Oncogene. 2009;28:2634–2642. doi: 10.1038/onc.2009.126. [DOI] [PubMed] [Google Scholar]
- Ellenberger T, Fass D, Arnaud M, Harrison SC. Crystal structure of transcription factor E47: E-box recognition by a basic region helix-loop-helix dimer. Genes Dev. 1994;8:970–980. doi: 10.1101/gad.8.8.970. [DOI] [PubMed] [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- Fisher DE, Parent LA, Sharp PA. Myc/Max and other helix-loop-helix/leucine zipper proteins bend DNA toward the minor groove. Proc Natl Acad Sci U S A. 1992;89:11779–11783. doi: 10.1073/pnas.89.24.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S, Cherny L, Sohmer H. Thyroxine affects physiological and morphological development of the ear. Hear Res. 1996;97:19–29. [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? Bioessays. 2006;28:1181–1193. doi: 10.1002/bies.20502. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Eberl DF, Beisel KW. The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cell Mol Life Sci. 2010;67:3089–3099. doi: 10.1007/s00018-010-0403-x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit R, Krizhanovsky V, Ben-Arie N. Math1 controls cerebellar granule cell differentiation by regulating multiple components of the Notch signaling pathway. Development. 2004;131:903–913. doi: 10.1242/dev.00982. [DOI] [PubMed] [Google Scholar]
- Gross J, Angerstein M, Fuchs J, Stute K, Mazurek B. Expression analysis of prestin and selected transcription factors in newborn rats. Cell Mol Neurobiol. 2011;31:1089–1101. doi: 10.1007/s10571-011-9708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid T, Kakar SS. PTTG/securin activates expression of p53 and modulates its function. Mol Cancer. 2004;3:18. doi: 10.1186/1476-4598-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Mooseker MS, Corey DP. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol. 1997;137:1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Hendershot TJ, Liu H, Clouthier DE, Shepherd IT, Coppola E, Studer M, Firulli AB, Pittman DL, Howard MJ. Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression for development of neural crest-derived noradrenergic sympathetic ganglion neurons. Dev Biol. 2008;319:179–191. doi: 10.1016/j.ydbio.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzano R, Dror AA, Montcouquiol M, Ahmed ZM, Ellsworth B, Camper S, Friedman TB, Kelley MW, Avraham KB. Lhx3, a LIM domain transcription factor, is regulated by Pou4f3 in the auditory but not in the vestibular system. Eur J Neurosci. 2007;25:999–1005. doi: 10.1111/j.1460-9568.2007.05332.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Huang J, Feng L, Fukudome S, Hamajima Y, Lin J. Sonic hedgehog (SHH) promotes the differentiation of mouse cochlear neural progenitors via the Math1-Brn3.1 signaling pathway in vitro. J Neurosci Res. 2010;88:927–935. doi: 10.1002/jnr.22286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahori A, Fraidenraich D, Basilico C. A conserved enhancer element that drives FGF4 gene expression in the embryonic myotomes is synergistically activated by GATA and bHLH proteins. Dev Biol. 2004;270:525–537. doi: 10.1016/j.ydbio.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240:52–56. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26:550–558. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kawabata I, Umeda T, Yamamoto K, Okabe S. Electroporation-mediated gene transfer system applied to cultured CNS neurons. Neuroreport. 2004;15:971–975. doi: 10.1097/00001756-200404290-00008. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama T, Mao CA, Cho JH, Fu X, Pan P, Mu X, Klein WH. Overlapping spatiotemporal patterns of regulatory gene expression are required for neuronal progenitors to specify retinal ganglion cell fate. Vision Res. 2011;51:251–259. doi: 10.1016/j.visres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klisch TJ, Xi Y, Flora A, Wang L, Li W, Zoghbi HY. In vivo Atoh1 targetome reveals how a proneural transcription factor regulates cerebellar development. Proc Natl Acad Sci U S A. 2011;108:3288–3293. doi: 10.1073/pnas.1100230108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko LJ, Engel JD. DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol. 1993;13:4011–4022. doi: 10.1128/mcb.13.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Soreq L, Kliminski V, Ben-Arie N. Math1 target genes are enriched with evolutionarily conserved clustered E-box binding sites. J Mol Neurosci. 2006;28:211–229. doi: 10.1385/JMN:28:2:211. [DOI] [PubMed] [Google Scholar]
- Lawoko-Kerali G, Rivolta MN, Holley M. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J Comp Neurol. 2002;442:378–391. doi: 10.1002/cne.10088. [DOI] [PubMed] [Google Scholar]
- Ledent V, Paquet O, Vervoort M. Phylogenetic analysis of the human basic helix-loop-helix proteins. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-6-research0030. RESEARCH0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JH, Cook AL, Van Gele M, Boyle GM, Inglis KJ, Speleman F, Sturm RA. Proneural and proneuroendocrine transcription factor expression in cutaneous mechanoreceptor (Merkel) cells and Merkel cell carcinoma. Int J Cancer. 2002;101:103–110. doi: 10.1002/ijc.10554. [DOI] [PubMed] [Google Scholar]
- LoTurco J, Manent JB, Sidiqi F. New and improved tools for in utero electroporation studies of developing cerebral cortex. Cereb Cortex. 2009;19(Suppl 1):i120–125. doi: 10.1093/cercor/bhp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Transcriptional regulation of neuronal phenotype in mammals. J Physiol. 2006;575:379–387. doi: 10.1113/jphysiol.2006.113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich SM, Xia A, Mathes EL, Wang VY, Oghalai JS, Fritzsch B, Zoghbi HY. Atoh1-lineal neurons are required for hearing and for the survival of neurons in the spiral ganglion and brainstem accessory auditory nuclei. J Neurosci. 2009;29:11123–11133. doi: 10.1523/JNEUROSCI.2232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Dulon D, Pak K, Mullen LM, Li Y, Erkman L, Ryan AF. Regulation of POU4F3 gene expression in hair cells by 5′ DNA in mice. Neuroscience. 2011;197:48–64. doi: 10.1016/j.neuroscience.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JM, Visvader JE. LIM-domain-binding protein 1: a multifunctional cofactor that interacts with diverse proteins. EMBO Rep. 2003;4:1132–1137. doi: 10.1038/sj.embor.7400030. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merika M, Orkin SH. DNA-binding specificity of GATA family transcription factors. Mol Cell Biol. 1993;13:3999–4010. doi: 10.1128/mcb.13.7.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Takako N, Hamada M, Maeda A, Fujioka Y, Kuroha T, Huber RE, Hasegawa SL, Rao A, Yamamoto M, Takahashi S, Lim KC, Engel JD. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development. 2006;133:3871–3881. doi: 10.1242/dev.02553. [DOI] [PubMed] [Google Scholar]
- Murata J, Tokunaga A, Okano H, Kubo T. Mapping of notch activation during cochlear development in mice: implications for determination of prosensory domain and cell fate diversification. J Comp Neurol. 2006;497:502–518. doi: 10.1002/cne.20997. [DOI] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334:339–358. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan LS, Jagutpal SS, Cadge BA, Woo P, Dawson SJ. Identification and functional analysis of common sequence variants in the DFNA15 gene, Brn-3c. Gene. 2007;400:89–97. doi: 10.1016/j.gene.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113(Pt 22):3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- Ono K, Nakagawa T, Kojima K, Matsumoto M, Kawauchi T, Hoshino M, Ito J. Silencing p27 reverses post-mitotic state of supporting cells in neonatal mouse cochleae. Mol Cell Neurosci. 2009;42:391–398. doi: 10.1016/j.mcn.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Palaparti A, Baratz A, Stifani S. The Groucho/transducin-like enhancer of split transcriptional repressors interact with the genetically defined amino-terminal silencing domain of histone H3. J Biol Chem. 1997;272:26604–26610. doi: 10.1074/jbc.272.42.26604. [DOI] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Duncan JS, Kopecky B, Fritzsch B. A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS One. 2012;7:e30358. doi: 10.1371/journal.pone.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2011;275:66–80. doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Kopecky B, Beisel K, Soukup G, Fritzsch B. Stem cells and molecular strategies to restore hearing. Panminerva Med. 2008;50:41–53. Review. [PMC free article] [PubMed] [Google Scholar]
- Rivolta MN, Holley MC. GATA3 is downregulated during hair cell differentiation in the mouse cochlea. J Neurocytol. 1998;27:637–647. doi: 10.1023/a:1006951813063. [DOI] [PubMed] [Google Scholar]
- Sage C, Huang M, Vollrath MA, Brown MC, Hinds PW, Corey DP, Vetter DE, Chen ZY. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc Natl Acad Sci U S A. 2006;103:7345–7350. doi: 10.1073/pnas.0510631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer D, Sage C, Corey DP, Pingault V. Gene expression profiling identifies Hes6 as a transcriptional target of ATOH1 in cochlear hair cells. FEBS Lett. 2007a;581:4651–4656. doi: 10.1016/j.febslet.2007.08.059. [DOI] [PubMed] [Google Scholar]
- Scheffer D, Sage C, Plazas PV, Huang M, Wedemeyer C, Zhang DS, Chen ZY, Elgoyhen AB, Corey DP, Pingault V. The alpha1 subunit of nicotinic acetylcholine receptors in the inner ear: transcriptional regulation by ATOH1 and co-expression with the gamma subunit in hair cells. J Neurochem. 2007b;103:2651–2664. doi: 10.1111/j.1471-4159.2007.04980.x. [DOI] [PubMed] [Google Scholar]
- Sekine A, Akiyama Y, Yanagihara K, Yuasa Y. Hath1 up-regulates gastric mucin gene expression in gastric cells. Biochem Biophys Res Commun. 2006;344:1166–1171. doi: 10.1016/j.bbrc.2006.03.238. [DOI] [PubMed] [Google Scholar]
- Shashikant CS, Bieberich CJ, Belting HG, Wang JC, ly MA, Ruddle FH. Regulation of Hoxc-8 during mouse embryonic development: identification and characterization of critical elements involved in early neural tube expression. Development. 1995;121:4339–4347. doi: 10.1242/dev.121.12.4339. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Loftus JM, Slapnick SM. Tissue culture of the organ of Corti. Acta Otolaryngol Suppl. 1993;502:3–36. [PubMed] [Google Scholar]
- Sun XH, Baltimore D. An inhibitory domain of E12 transcription factor prevents DNA binding in E12 homodimers but not in E12 heterodimers. Cell. 1991;64:459–470. doi: 10.1016/0092-8674(91)90653-g. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev Growth Differ. 2008;50:507–511. doi: 10.1111/j.1440-169X.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- Vahava O, Morell R, Lynch ED, Weiss S, Kagan ME, Ahituv N, Morrow JE, Lee MK, Skvorak AB, Morton CC, Blumenfeld A, Frydman M, Friedman TB, King MC, Avraham KB. Mutation in transcription factor POU4F3 associated with inherited progressive hearing loss in humans. Science. 1998;279:1950–1954. doi: 10.1126/science.279.5358.1950. [DOI] [PubMed] [Google Scholar]
- Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406:419–422. doi: 10.1038/35019088. [DOI] [PubMed] [Google Scholar]
- Wang HC, Perry SS, Sun XH. Id1 attenuates Notch signaling and impairs T-cell commitment by elevating Deltex1 expression. Mol Cell Biol. 2009;29:4640–4652. doi: 10.1128/MCB.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierstra I. Sp1: emerging roles--beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun. 2008;372:1–13. doi: 10.1016/j.bbrc.2008.03.074. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Chen ZY, Zhou L, O’Malley BW, Jr., Klein W, Nathans J. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci U S A. 1997;94:9445–9450. doi: 10.1073/pnas.94.17.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Maklad A, Pirvola U, Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003;4:2. doi: 10.1186/1471-2202-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Yang XO, Angkasekwinai P, Zhu J, Peng J, Liu Z, Nurieva R, Liu X, Chung Y, Chang SH, Sun B, Dong C. Requirement for the basic helix-loop-helix transcription factor Dec2 in initial TH2 lineage commitment. Nat Immunol. 2009;10:1260–1266. doi: 10.1038/ni.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhai SQ, Shou J, Song W, Sun JH, Guo W, Zheng GL, Hu YY, Gao WQ. Isolation, growth and differentiation of hair cell progenitors from the newborn rat cochlear greater epithelial ridge. J Neurosci Methods. 2007;164:271–279. doi: 10.1016/j.jneumeth.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Zhao LD, Guo WW, Lin C, Li LX, Sun JH, Wu N, Ren LL, Li XX, Liu HZ, Young WY, Gao WQ, Yang SM. Effects of DAPT and Atoh1 overexpression on hair cell production and hair bundle orientation in cultured Organ of Corti from neonatal rats. PLoS One. 2011;6:e23729. doi: 10.1371/journal.pone.0023729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zuo J, Treadaway J, Buckner TW, Fritzsch B. Visualization of alpha9 acetylcholine receptor expression in hair cells of transgenic mice containing a modified bacterial artificial chromosome. Proc Natl Acad Sci U S A. 1999;96:14100–14105. doi: 10.1073/pnas.96.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]