Abstract
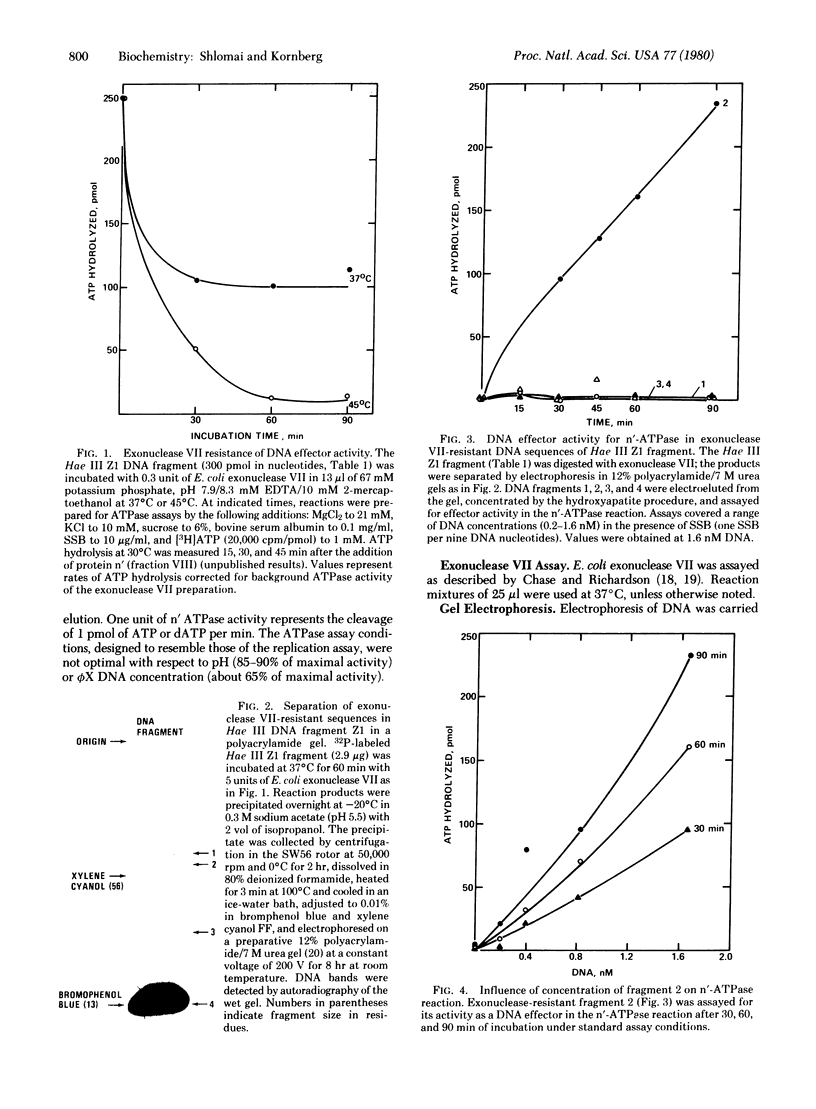
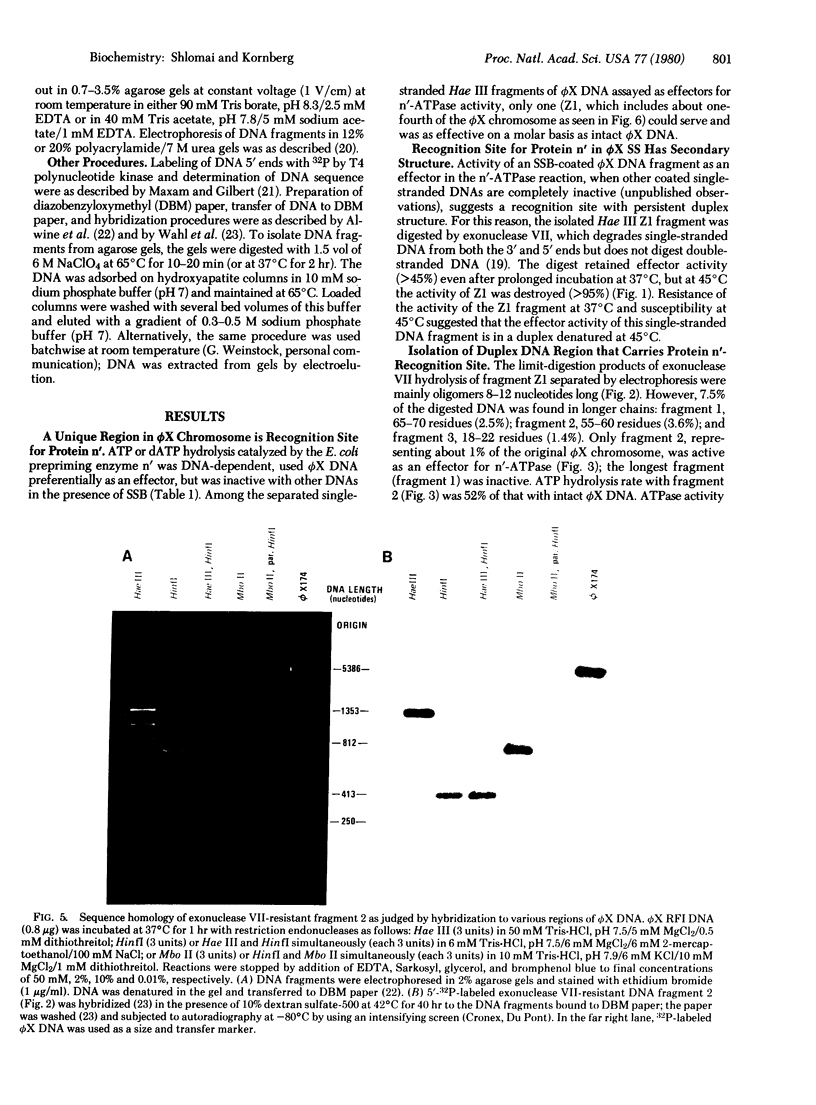
Protein n', a prepriming DNA replication enzyme of Escherichia coli, is a phi X174 DNA-dependent ATPase. Restriction of phi X174 DNA have led to the identification of a 55-nucleotide fragment that carries the protein n' recognition sequence. Molecular hybridization and sequence analysis have located this sequence within the untranslated region between genes F and G, a map location analogous to that of the unique complementary strand origin of phage G4 DNA. Within the 55-nucleotide fragment is a sequence of 44 nucleotides that forms a stable hairpin structure. This duplex may be the signal for protein n' to initiate the prepriming events that led to the start of phi X174 complementary DNA strand replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché J. P., Rowen L., Kornberg A. The RNA primer synthesized by primase to initiate phage G4 DNA replication. J Biol Chem. 1978 Feb 10;253(3):765–769. [PubMed] [Google Scholar]
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
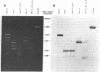
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Mechanism of action. J Biol Chem. 1974 Jul 25;249(14):4553–4561. [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Purification and properties. J Biol Chem. 1974 Jul 25;249(14):4545–4552. [PubMed] [Google Scholar]
- Crea R., Kraszewski A., Hirose T., Itakura K. Chemical synthesis of genes for human insulin. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5765–5769. doi: 10.1073/pnas.75.12.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Harbers B., Hours C., Denhardt D. T. The mechanism of replication of phiX174 DNA. XII. Non-random location of gaps in nascent phiX174 RF II DNA. J Mol Biol. 1975 Nov 25;99(1):107–123. doi: 10.1016/s0022-2836(75)80162-8. [DOI] [PubMed] [Google Scholar]
- Fiddes J. C., Barrell B. G., Godson G. N. Nucleotide sequences of the separate origins of synthesis of bacteriophage G4 viral and complementary DNA strands. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1081–1085. doi: 10.1073/pnas.75.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider K., Kornberg A. Conversion of the M13 viral single strand to the double-stranded replicative forms by purified proteins. J Biol Chem. 1974 Jul 10;249(13):3999–4005. [PubMed] [Google Scholar]
- Kornberg A. Aspects of DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):1–9. doi: 10.1101/sqb.1979.043.01.003. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Martin D. M., Godson G. N. G4 DNA replication. I. Origin of synthesis of the viral and complementary DNA strands. J Mol Biol. 1977 Dec 5;117(2):321–335. doi: 10.1016/0022-2836(77)90130-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMacken R., Kornberg A. A multienzyme system for priming the replication of phiX174 viral DNA. J Biol Chem. 1978 May 10;253(9):3313–3319. [PubMed] [Google Scholar]
- McMacken R., Ueda K., Kornberg A. Migration of Escherichia coli dnaB protein on the template DNA strand as a mechanism in initiating DNA replication. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4190–4194. doi: 10.1073/pnas.74.10.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. R., Shlomai J., Kobori J., Bates D. L., Rowen L., McMacken R., Ueda K., Kornberg A. Enzymatic conversion of single-stranded phiX174 and G4 circles to duplex forms: discontinuous replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):289–293. doi: 10.1101/sqb.1979.043.01.035. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Sims J., Dressler D. Site-specific initiation of a DNA fragment: nucleotide sequence of the bacteriophage G4 negative-strand initiation site. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3094–3098. doi: 10.1073/pnas.75.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Griffith J., Geider K., Schaller H., Kornberg A. Initiation of deoxyribonucleic acid synthesis. VII. A unique location of the gap in the M13 replicative duplex synthesized in vitro. J Biol Chem. 1974 May 25;249(10):3049–3054. [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- Weiner J. H., McMacken R., Kornberg A. Isolation of an intermediate which precedes dnaG RNA polymerase participation in enzymatic replication of bacteriophage phi X174 DNA. Proc Natl Acad Sci U S A. 1976 Mar;73(3):752–756. doi: 10.1073/pnas.73.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Conversion of phiX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4120–4124. doi: 10.1073/pnas.71.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechel K., Bouché J. P., Kornberg A. Replication of phage G4. A novel and simple system for the initiation of deoxyribonucleic acid synthesis. J Biol Chem. 1975 Jun 25;250(12):4684–4689. [PubMed] [Google Scholar]