Abstract
High fluence-rate blue light (BL) rapidly inhibits hypocotyl growth in Arabidopsis, as in other species, after a lag time of 30 s. This growth inhibition is always preceded by the activation of anion channels. The membrane depolarization that results from the activation of anion channels by BL was only 30% of the wild-type magnitude in hy4, a mutant lacking the HY4 BL receptor. High-resolution measurements of growth made with a computer-linked displacement transducer or digitized images revealed that BL caused a rapid inhibition of growth in wild-type and hy4 seedlings. This inhibition persisted in wild-type seedlings during more than 40 h of continuous BL. By contrast, hy4 escaped from the initial inhibition after approximately 1 h of BL and grew faster than wild type for approximately 30 h. Wild-type seedlings treated with 5-nitro-2-(3-phenylpropylamino)-benzoic acid, a potent blocker of the BL-activated anion channel, displayed rapid growth inhibition, but, similar to hy4, these seedlings escaped from inhibition after approximately 1 h of BL and phenocopied the mutant for at least 2.5 h. The effects of 5-nitro-2-(3-phenylpropylamino)-benzoic acid and the HY4 mutation were not additive. Taken together, the results indicate that BL acts through HY4 to activate anion channels at the plasma membrane, causing growth inhibition that begins after approximately 1 h. Neither HY4 nor anion channels appear to participate greatly in the initial phase of inhibition.
The importance of BL for plant growth and development was recognized at least one and a half centuries ago, when several investigators noted that the “rays of high refrangibility” (i.e. shorter wavelengths of the visible spectrum) were the most effective in causing phototropism (for review, see Vines, 1886). It is now apparent that BL affects many aspects of photomorphogenesis, including the process of de-etiolation, a major developmental juncture leading to the transformation of an emerging seedling into a photoautotrophic organism (Parks and Hangarter, 1994; Senger and Schmidt, 1994; Short and Briggs, 1994).
A conspicuous feature of de-etiolation is the suppression of stem elongation by light. This inhibition of growth is induced by both the red and blue regions of the spectrum through the action of multiple photoreceptor systems. The phytochromes govern the growth response to the red region of the spectrum and have the capacity to affect the sensitivity to the blue region (Casal and Boccalandro, 1995; Ahmad and Cashmore, 1997). Responsiveness to the blue region of the spectrum rests primarily with a BL-specific system that was first distinguished from the phytochromes by recognizing that it caused inhibition only seconds after the onset of irradiation as opposed to the several minutes required for red light (Meijer, 1968; Gaba and Black, 1979). Studies of the BL-specific system, performed primarily with cucumber and pea seedlings, found that BL inhibits stem elongation after a lag time as short as 15 s (Laskowski and Briggs, 1989; Spalding and Cosgrove, 1989), and results from a reduction in the rate of wall stress relaxation (Cosgrove, 1988; Kigel and Cosgrove, 1991).
The rapid decrease in growth rate induced by BL is always preceded by a depolarization of the hypocotyl cell plasma membrane in irradiated tissue (Spalding and Cosgrove, 1989). Recent studies have shown that BL activates anion channels in a Ca2+-independent manner to depolarize the plasma membrane (Cho and Spalding, 1996; Lewis et al., 1997). A potent blocker of these anion channels, NPPB, blocks the depolarization and also a portion of BL-induced growth inhibition (Cho and Spalding, 1996). An examination of the time course of growth inhibition could reveal when NPPB, and by extension, anion channels, act in the mechanism controlling hypocotyl growth.
Photomorphogenic mutants altered in their capacity to respond specifically to BL could also be useful tools in attempting to further define the role of an anion channel in the signal cascade that embodies this growth response to BL. The best candidate in this regard is the hy4 mutant of Arabidopsis. Growth of its hypocotyl is relatively uninhibited by light because of a defect in the perception of BL specifically (Koornneef et al., 1980). Studies of the HY4 gene and the encoded protein support the proposal that HY4 functions as a BL receptor controlling several characteristics of a developing seedling and an adult plant (Ahmad and Cashmore, 1993; Jackson and Jenkins, 1995; Lin et al., 1995a, 1995b).
An important question to be addressed is whether the early, rapid electrophysiological responses to BL are affected by a mutation in HY4. We have used high-resolution measuring techniques to study the effect of this BL receptor deficiency on the magnitude and kinetics of the BL-induced electrophysiological and growth responses of Arabidopsis hypocotyls. Parallel kinetic studies of the BL-induced growth of NPPB-treated seedlings were also conducted to address further the suggested role of anion channels in this growth response. Our results indicate that at least two BL photoreceptors, operating over very different time frames, coordinately control hypocotyl elongation during de-etiolation. HY4 appears to affect a longer-term phase of growth via the regulation of anion channels, whereas an initial, rapid, and transient phase of growth regulation is mediated by a presently unidentified photoreceptor.
MATERIALS AND METHODS
Plant Growth Conditions
Seeds of the Landsberg ecotype of Arabidopsis, either wild-type or an allele of hy4 (hy4–2.23N; Koornneef et al., 1980) recently shown to be null for detectable gene product (Ahmad and Cashmore, 1997), were surface sterilized before sowing on 1% agar (w/v) containing 1 mm each of KCl and CaCl2. Where used, NPPB (Calbiochem) was diluted to 20 μm from a 50 mm stock prepared in DMSO. For surface electrode measurements and rapid-growth assays, multiple seeds were embedded just below the surface of this solidified medium that filled capless 1.5-mL microcentrifuge tubes. For Vm measurements and long-term growth assays, seeds were embedded in the same agar medium filling 100- × 15-mm Petri dishes. These tubes or sealed plates were placed in humid boxes and stored in darkness at 4°C for 2 d. Germination was promoted by exposing the seeds to red light (2 μmol m−2 s−1) for 0.5 h at 23°C, and subsequent growth proceeded in complete darkness until seedlings were ready for a particular assay. All subsequent manipulations of seedlings occurred in dim green light (0.002 μmol m−2 s−1). Fluence rates were measured with a radiometer (model IL1700, International Light, Newburyport, MA) equipped with either an SED 033 or an SED 005 quantum sensor (International Light).
Electrophysiology
For direct measurement of Vm using intracellular microelectrodes, 4-d-old seedlings (approximately 1 cm tall) were gently mounted horizontally on nearly solidified 1% agar containing 1 mm KCl and 1 mm CaCl2, covered with a small amount of solution containing the same salts but no agar, and were allowed to recover for at least 2 h before being transferred to the stage of a microscope (Diaphot-TMD, Nikon) and visualized for impalement with dim green light (0.3 μmol m−2 s−1) provided by filtering the output of the microscope illuminator through two layers of green gel (Roscolux no. 90, Rosco, Port Chester, NY) and one layer of amber gel (Roscolux no. 21, Rosco). A hypocotyl cell in the rapidly growing region (approximately 2 mm below the hook) was impaled with a conventional glass microelectrode filled with 1 m KCl. A Ag/AgCl wire connected the microelectrode to the headstage of a patch-clamp amplifier (model 200A, Axon Instruments, Foster City, CA) set in the current-clamp mode. The electrical potential difference between the intracellular microelectrode and a Ag/AgCl reference electrode that contacted the liquid medium via a 1 m KCl/agar salt bridge was low-pass filtered at 5 Hz with a tunable 8-pole Bessel filter (902LPF, Frequency Devices, Haverhill, MA) and digitized at 20 Hz using Axotape software (Axon Instruments). A 20-s pulse of actinic BL (80 μmol m−2 s−1) was delivered to the seedling by placing one layer of Roscolux no. 85 gel in the illuminator light path before removing the green and amber filters.
Surface electrode measurements were performed using 4-d-old seedlings as described previously (Cho and Spalding, 1996). BL (100 μmol m−2 s−1) was produced by filtering the light from a xenon arc lamp (XBO 450W/OFR, Osram Sylvania, Inc., Versailles, KY) through a 450-nm interference filter (10-nm bandwidth, Corion, Holliston, MA). This actinic light was conducted to the seedling in an adjoining darkroom via a liquid light guide. The custom computer-controlled apparatus for measuring electric signals and delivering light was previously described in detail (Spalding, 1995).
Hypocotyl Growth
Rapid changes in seedling growth rate were measured with an electronic displacement transducer in a darkroom. A microcentrifuge tube containing at least one straight, vertical, 3-d-old seedling (<1 cm tall) was placed in a transparent cylinder (to maintain high RH) with a small hole in the top through which a human hair, attached and counterbalanced on the displacement transducer (LVDT no. DC-E050, Lucas Control Systems Products, Hampton, VA), was inserted and looped around the apical hook of the seedling. The transducer output voltage, directly proportional to seedling position, was continuously acquired digitally at 1 Hz by the computerized apparatus described above, which also administered the treatments of either blue (450 nm, 10-nm bandwidth, Corion) or UV-A (360 nm, 10-nm bandwidth, Corion) radiation. The digitized displacement data were differentiated to produce a growth rate that was smoothed, normalized to the average rate obtained during the 2 min of dark preceding the light pulse, and averaged with the results of independent trials by software (Origin version 4.0, Microcal Software, Inc., Northampton, MA).
For long-term growth measurements, approximately 10 2-d-old seedlings (3–5 mm tall) growing in a darkroom on vertical plates were photographed at intervals before and after the onset of constant BL (approximately 80 μmol m−2 s−1) emitted from two fluorescent tubes (F20T12/BB, Philips Lighting, Somerset, NJ). A camera equipped with a 55-mm macro lens and extension tubes produced an image on 35-mm photographic emulsion (T-Max 100, Eastman Kodak) that was 1.8 times actual size. Photographic images used to determine growth in darkness were taken under dim green light (4 μmol m−2 s−1; Spalding, 1995). Negative images were scanned to produce high-resolution digital images (5 μm pixel−1). Where indicated, digital images of the seedlings were also acquired with a CCD (charge-coupled device) camera (DIC-N, World Precision Instruments, Sarasota, FL) equipped with a close-focus zoom lens (D52,274, Edmund Scientific, Barrington, NJ) and interfaced with a computer, thus obviating film development and scanning. The image resolution for this apparatus was 5.7 μm pixel−1. Seedling growth could be monitored under either IR radiation (290 μmol m−2 s−1, no detectable emission below 825 nm) produced by four light-emitting diodes (948 nm, 50-nm bandwidth, no. 276–143c, Radio Shack, Fort Worth, TX), or BL as described above. Seedlings were only exposed to IR radiation during initial focusing and image capturing for growth rate determination in darkness. These seedlings grew at the same rate (approximately 0.2 mm h−1) as those in complete darkness and displayed no detectable signs of de-etiolation. Determination of seedling length from all digital images was accomplished using Photoshop version 4.0 (Adobe Systems, Inc., San Jose, CA) and Image Tool version 1.28 (University of Texas Health Science Center, San Antonio). Growth rates are reported relative to the average rate in darkness.
RESULTS
Impaired Electrophysiological Response to BL in hy4
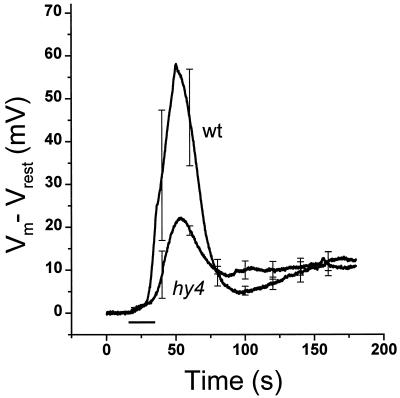
Changes in Vm induced by a 20-s BL pulse and measured in hypocotyl cells of either etiolated hy4 or wild-type seedlings using intracellular microelectrodes are shown in Figure 1. The resting Vm in darkness for both genotypes ranged from −150 mV to −200 mV, and was subtracted from each digitally acquired trace to obtain ΔVm. For wild-type seedlings depolarization in response to a BL pulse began almost immediately after the onset of irradiation, attaining a maximum change of 60 mV from the resting potential approximately 40 s after the onset of BL. The membrane subsequently repolarized to near the initial resting level approximately 90 s after the response began. In hy4 the magnitude of the depolarization was reduced to approximately 40% of the wild-type response.
Figure 1.
Response of Vm to BL in etiolated wild-type and hy4 seedlings. The plots show the averaged responses of three wild-type (wt) and hy4 seedlings. The value of Vm prior to light treatment (Vrest) was generally between −150 and −200 mV. Error bars are se shown at 20-s intervals. The horizontal bar shows the timing of the 20-s BL pulse.
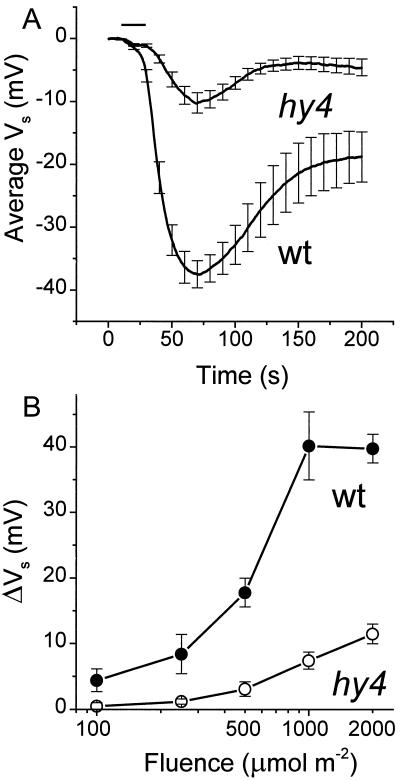
The change in Vm induced by BL was also measured with surface-contact electrodes. The recorded signal, termed Vs, is equivalent in time course and similar in magnitude, but opposite in polarity when compared with measurements made with intracellular microelectrodes (Spalding and Cosgrove, 1989, 1993). Although not a direct measure of Vm within a single cell, it provides a less invasive and technically simpler method than impaling cells with intracellular microelectrodes, thus facilitating the generation of a larger data set. Figure 2A shows that BL began to depolarize the membranes of wild-type and hy4 seedlings approximately 3 s after the onset of irradiation, followed by a peak change in voltage approximately 60 s later. The magnitude of the hy4 response was approximately 30% of the wild type. The fluence-response relationships for the BL-induced depolarizations measured in this manner for wild-type and mutant seedlings are shown in Figure 2B. The response of hy4 ranged from 11% to 29% of the wild-type response over the tested fluence range. The plateau of the sigmoidal wild-type curve at the higher fluence may indicate that the depolarization saturates at 1000 μmol m−2. However, this may not represent true response saturation since photons in the latter portion of a 20-s pulse may have arrived after the signaling process had progressed to a stage where it was independent of BL. The measurement of BL-induced membrane depolarization using either impalement with intracellular microelectrodes or surface-contact electrodes produced similar results; specifically, the depolarization induced by BL was greatly reduced in hy4 relative to wild type. Given the evidence that HY4 is a photoreceptor (Ahmad and Cashmore, 1993; Lin et al., 1995a, 1995b) and what is known about the depolarization mechanism (Cho and Spalding, 1996), the present results indicate that absorption of BL by HY4 quickly leads to the activation of anion channels. However, the significant response detected in this null allele of hy4 indicates that a photoreceptor other than HY4 also contributes to anion-channel activation by BL.
Figure 2.
Membrane depolarizations induced by BL and measured with surface-contact electrodes. A, Averaged responses of etiolated wild-type (wt) and hy4 seedlings. Error bars are se shown at 10-s intervals. The timing of the 20-s pulse of BL is indicated by the horizontal bar. B, Fluence-response analysis. The peak magnitude of changes in Vs induced by 450 nm BL (100 μmol m−2 s−1) was plotted versus the photon fluence, which was varied by changing the exposure time. Each point represents the mean ± se of between 6 and 20 measurements.
Two Phases of Growth Inhibition Induced by BL
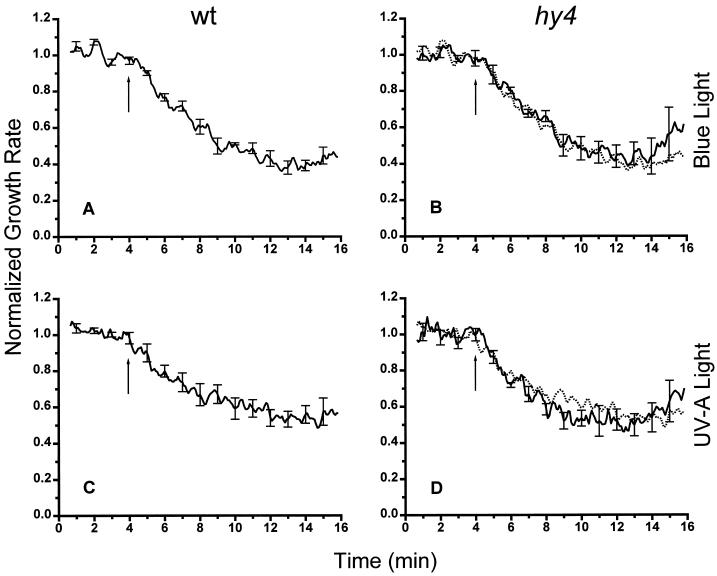
The electrophysiological response to BL was previously proposed to be related to the ensuing rapid inhibition of growth (Spalding and Cosgrove, 1989). Therefore, our finding that the depolarization was significantly impaired in hy4 (Figs. 1 and 2) led us to predict that this mutant would not display a normal rapid growth response. Figure 3A shows that a 20-s pulse of monochromatic BL decreased hypocotyl growth rate in wild-type seedlings approximately 30 s after the onset of the light pulse as determined with an electronic displacement transducer. Growth rate continued to decrease for approximately 10 min, transiently reaching a rate that was approximately 40% of the dark rate, before recovering by a slower and more variable time course (recovery not shown). Surprisingly, hy4 yielded a response to BL that was essentially indistinguishable from wild type (Fig. 3B). Thus, a photoreceptor other than HY4 is responsible for the rapid growth inhibition. Figure 3, C and D, demonstrates that this unknown photoreceptor is also sensitive to monochromatic 360-nm irradiation (UV-A), since this treatment induced similar rapid responses in the wild type and hy4, with magnitude and kinetics that were comparable to 450-nm light.
Figure 3.
Rapid inhibition of hypocotyl elongation in wild-type (wt) and hy4 seedlings in response to BL or UV-A irradiation. A seedling growing while attached to an electronic displacement transducer received a 20-s pulse of BL (50 μmol m−2 s−1) or UV-A radiation (20 μmol m−2 s−1) at the times indicated by the upward arrows. The average dark growth rate, equal to 1 on the ordinate, was approximately 0.2 mm h−1 for each of the five independent experiments averaged in each panel. Growth rate curves for the wild type are replotted as dashed lines in B and D that show the hy4 responses. Error bars given at 1-min intervals represent se.
Three important points are established by the results in Figures 1–3. First, anion-channel activation does not correlate well with rapid inhibition of hypocotyl elongation in response to either BL or UV-A irradiation. Second, the normal induction of a rapid growth response in hy4 suggests that a different photoreceptor, one capable of sensing both UV-A and BL, controls this response. And third, the rapid inhibition of growth in Figure 3 must be a transient phase because the hypocotyl of hy4 is conspicuously longer than wild type after days of growth under light, the phenotype for which it was originally isolated (Koornneef et al., 1980).
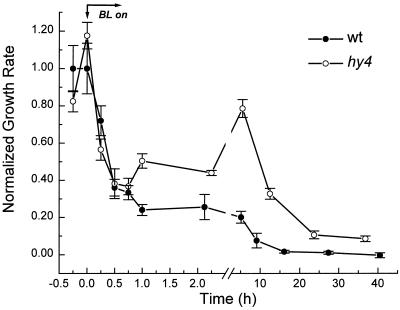
Reconciling the long-hypocotyl phenotype of hy4 with the observations in Figure 3 required characterizing the entire time course of growth inhibition induced by continuous BL in hy4 and wild-type seedlings. For this purpose, a photographic method was used in lieu of the displacement transducer because long-term light treatments typically caused the apical hook to open, leading to slippage along or detachment from the transducer. Figure 4 shows the average growth response of hy4 and wild-type seedlings over an extended period of BL treatment. Wild-type seedlings showed a distinct and persistent reduction in the elongation rate beginning within 15 min of irradiation and continuing for the next 40 h. The same initial inhibition was observed in hy4 seedlings, thereby providing independent confirmation of the rapid growth measurements described earlier for these two genotypes. However, in contrast to wild type, growth inhibition in hy4 was transient. The growth of hy4 seedlings began accelerating after approximately 1 h of BL and peaked approximately 5 h later. Growth rate gradually slowed toward the low wild-type level over the next 37 h. These results demonstrate that HY4 controls hypocotyl elongation after approximately 1 h of continuous BL without a detectable contribution to the initial, rapid inhibition. Integrating the growth rate versus time curves in Figure 4 revealed that hy4 displayed 4.4 times more growth than the wild type during the BL period, consistent with its hallmark long-hypocotyl phenotype.
Figure 4.
Long-term growth responses of wild-type (wt) and hy4 seedlings to continuous BL. Growth rate relative to the average dark rate (0.25 and 0.26 mm h−1 for wild type and mutant, respectively) was determined from photographic sequences. n = 5 for each genotype ± se.
Anion Channels and the Long-Term Phase of Growth Inhibition
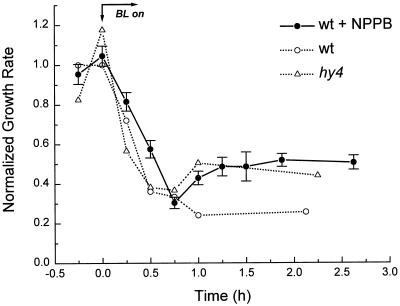
If HY4 acts through anion channels to inhibit growth, then one would predict either the anion-channel blocker NPPB or a mutation in HY4 to have similar effects. Figure 5 shows the time course of BL-induced growth inhibition for seedlings grown on NPPB determined from photographs taken at 15-min intervals. Neither the magnitude of the initial phase of growth inhibition nor its time course was greatly affected by NPPB, although the inhibition may be slightly less rapid in NPPB-treated seedlings. However, unlike the nontreated wild type, seedlings grown on NPPB did not remain inhibited by BL. Instead, they began growing faster approximately 1 h after the onset of irradiation, similar to hy4. This increased growth rate of NPPB-treated wild-type seedlings persisted for at least 1.5 h. Thus, NPPB treatment resulted in a phenocopy of this photoreceptor mutant over the initial 2.5 h of irradiation.
Figure 5.
Effect of 20 μm NPPB on the growth response of wild-type (wt) seedlings to continuous BL. The growth rates were determined from photographs and are expressed relative to the average dark rate, which was 0.15 mm h−1. Each point represents the mean ± se of between 35 and 80 measurements. Growth curves for the wild type and hy4 are replotted from Figure 4 as dashed lines for comparison.
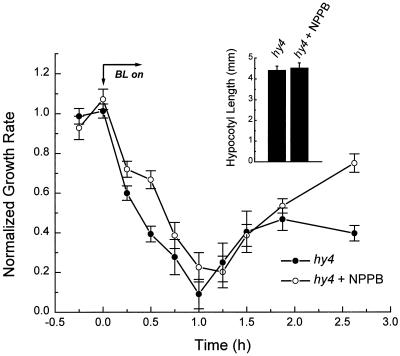
Another predicted property of a linear transduction scheme in which HY4 leads to the activation of anion channels is that the effect of a mutation in HY4 and the effect of NPPB should not be additive. Figure 6 shows the effect of NPPB on the growth response of hy4 seedlings after the onset of continuous BL. The pattern of the hy4 growth response in the presence of NPPB was very similar to the hy4 control. Growth rate declined rapidly after the onset of BL, although possibly less rapidly in NPPB-treated seedlings, as was seen previously in Figure 5. Growth rate resumed in both treated and nontreated seedlings after approximately 1 h of BL. The time course of growth rate increase after 1 h of BL was very similar for both populations until the end of the experiment, when NPPB-treated seedlings grew more rapidly. However, this growth rate difference must not persist since hy4 seedlings treated with NPPB were very similar in length to nontreated mutant seedlings after 6 d of growth in BL (Fig. 6, inset). This result is consistent with the NPPB-sensitive component (anion channels) being dependent on HY4, and inconsistent with the alternative that NPPB and the HY4 mutation affect growth in BL for different reasons.
Figure 6.
Effect of 20 μm NPPB on the growth response of hy4 seedlings in continuous BL. Seedling lengths and growth rates were determined from digital images acquired directly using a CCD camera. Growth rates are expressed relative to the average dark rate, which was 0.20 and 0.25 mm h−1 for treated and nontreated seedlings, respectively. Each point represents the mean ± se of 19 measurements. Bars in the figure inset represent the average hypocotyl length ± se of 9 NPPB-treated and 13 nontreated seedlings after growth for 6 d in continuous BL.
DISCUSSION
The present study has demonstrated both genetically and pharmacologically that the control of stem elongation in response to BL is composed of at least two temporally distinct phases. The null allele of hy4 used here showed an initial rapid growth response to BL or UV-A irradiation that was indistinguishable from the wild type and persisted for approximately 1 h (Figs. 3 and 4), thereby demonstrating that the HY4 photoreceptor does not control this response. Which BL/UV-A photoreceptor mediates this rapid growth inhibition is not yet known. We have conducted preliminary tests of mutants at other loci responsible for BL-regulated growth responses (nph1, Liscum and Briggs [1995]; cry2, Lin et al. [1998]) and the indication is that products encoded at these loci are not important for the rapid-growth response (data not shown). However, the very recent report that a cry1/cry2 double mutant does not exhibit phototropism, whereas the single mutants do (Ahmad et al., 1998), suggests that these two BL photoreceptors have overlapping functions in certain responses to BL. These phenomena may also include the rapid-growth response described here. Another candidate photoreceptor for the rapid-growth response is zeaxanthin, which has been suggested to function as a BL photoreceptor regulating stomatal opening (Zeiger and Zhu, 1998).
The surge in growth following the initial inhibition displayed by hy4 was transient, reaching a peak rate after 6 h of BL and slowly declining thereafter (Fig. 4). What caused the slow decline in growth rate in the latter portions of the hy4 time course is not known. It may have been due to a developmental, age-dependent process or to a slower-developing inhibitory influence of a non-HY4 photosensory system such as phytochrome. Whether the mechanism responsible for this slow decline also operates in wild-type seedlings, though undetectably due to the preceding inhibitory influence of HY4, cannot be determined from the present results.
Considering the present data together with those in Cho and Spalding (1996), we propose that BL, acting through HY4, leads within seconds to anion-channel activation. This plasma membrane phenomenon is somehow causally related to growth inhibition that begins approximately 1 h later, but not to the earlier rapid-growth inhibition as previously proposed (Spalding and Cosgrove, 1989). This interpretation is independently supported by our pharmacological studies (Figs. 5 and 6; Cho and Spalding, 1996) and by the genetic dissection of the BL-induced electrophysiological and growth responses reported here. A variant of this interpretation is that the growth-inhibiting mechanism responsible for the initial, rapid phase is converted or adapted to a persistent form by the HY4-dependent activation of anion channels. Inhibiting the action of HY4 either by mutation or by pharmacologically blocking the anion channels reveals the transient nature of the primary, nonadapted growth-inhibition mechanism.
One observation not readily explained by the above interpretation is that NPPB-treated wild-type seedlings are considerably shorter than hy4 after 4 to 6 d of growth in BL (compare Cho and Spalding [1996] with Fig. 6, inset). This indicates that sometime after approximately 2.5 h of BL, NPPB-treated seedlings must fail to phenocopy hy4 and begin to grow more slowly. Perhaps HY4 has a later-developing inhibitory effect on growth that does not depend on anion channels, and therefore is insensitive to NPPB. Alternatively, seedlings may develop resistance to NPPB over days.
The present work can be compared to recent studies by Wang and Iino (1998), which showed that fluences of BL similar to those used here caused protoplasts of Arabidopsis hypocotyls to shrink transiently with kinetics very similar to the initial, rapid phase of growth inhibition (Fig. 3). However, unlike the initial phase of growth inhibition, protoplast shrinking was blocked by NPPB and did not occur in hy4. In these respects, BL-induced protoplast shrinkage is actually similar to, and perhaps an osmotic consequence of, anion-channel activation (Figs. 1 and 2). The transience of the membrane depolarization and protoplast shrinkage does not necessarily mean that the anion channels are only transiently activated. They may remain activated throughout the 1-h lag time leading to the HY4-dependent phase of growth inhibition. Membrane repolarization and the regaining of protoplast volume may be accomplished by compensatory changes in the activation of other ion transporters at the plasma membrane during persistent anion-channel activation.
How rapid, HY4-mediated anion-channel activation causes a phase of growth inhibition that was detected after approximately 1 h of BL is a goal of future work. Evidence from genetic and photobiological studies has indicated that active phytochrome is required for the normal HY4-mediated control of hypocotyl length (Casal and Boccalandro, 1995; Ahmad and Cashmore, 1997). The requirement for a phytochrome-generated signal at some stage in the HY4-mediated response pathway may be responsible for the observed 1-h lag. This is consistent with the observation that protoplasts would not shrink in response to BL without a Pfr signal that had been generated 30 min earlier (Wang and Iino, 1998). Now that we have identified the appropriate time frames, experiments incorporating other mutants may be designed to elucidate the mechanism by which HY4, anion channels, and phytochrome exert control of hypocotyl elongation.
ACKNOWLEDGMENT
We thank Claudia Lipke for her assistance with the photography.
Abbreviations:
- BL
blue light
- NPPB
5-nitro-2-(3-phenylpropylamino)-benzoic acid
- Vm
membrane potential
Footnotes
This work was supported by the National Aeronautics and Space Administration/National Science Foundation Network for Research on Plant Sensory Systems (grant no. IBN-9416016) and a grant to the University of Wisconsin from the Department of Energy/National Science Foundation/U.S. Department of Agriculture Collaborative Program on Research in Plant Biology (grant no. BIR 92-20331).
LITERATURE CITED
- Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature (London) 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Cashmore AR. The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J. 1997;11:421–427. doi: 10.1046/j.1365-313x.1997.11030421.x. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Jarillo JA, Smirnova O, Cashmore AR. Cryptochrome blue-light photoreceptors of Arabidopsis implicated in phototropism. Nature. 1998;392:720–723. doi: 10.1038/33701. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Boccalandro H. Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta. 1995;197:213–218. doi: 10.1007/BF00202639. [DOI] [PubMed] [Google Scholar]
- Cho MH, Spalding EP. An anion channel in Arabidopsis hypocotyls activated by blue light. Proc Natl Acad Sci USA. 1996;93:8134–8138. doi: 10.1073/pnas.93.15.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. Mechanism of rapid suppression of cell expansion in cucumber hypocotyls after blue-light irradiation. Planta. 1988;176:109–116. [PubMed] [Google Scholar]
- Gaba V, Black M. Two separate photoreceptors control hypocotyl growth in green seedlings. Nature. 1979;278:51–54. [Google Scholar]
- Jackson JA, Jenkins GI. Extension-growth responses and expression of flavonoid biosynthesis genes in the Arabidopsis hy4 mutant. Planta. 1995;197:233–239. doi: 10.1007/BF00202642. [DOI] [PubMed] [Google Scholar]
- Kigel J, Cosgrove DJ. Photoinhibition of stem elongation by blue and red light. Effects on hydraulic and cell wall properties. Plant Physiol. 1991;95:1049–1056. doi: 10.1104/pp.95.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Laskowski MJ, Briggs WR. Regulation of pea epicotyl elongation by blue light. Fluence response relationships and growth distribution. Plant Physiol. 1989;89:293–298. doi: 10.1104/pp.89.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BD, Karlin-Neumann G, Davis RW, Spalding EP. Calcium-activated anion channels and membrane depolarizations induced by blue light and cold in Arabidopsis seedlings. Plant Physiol. 1997;114:1327–1334. doi: 10.1104/pp.114.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Ahmad M, Gordon D, Cashmore AR. Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensivity to blue, UV-A, and green light. Proc Natl Acad Sci USA. 1995a;92:8423–8427. doi: 10.1073/pnas.92.18.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Robertson DE, Ahmad M, Raibekas AA, Jorns MS, Dutton PL, Cashmore AR. Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science. 1995b;269:968–970. doi: 10.1126/science.7638620. [DOI] [PubMed] [Google Scholar]
- Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore AR. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc Natl Acad Sci USA. 1998;95:2686–2690. doi: 10.1073/pnas.95.5.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR. Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell. 1995;7:473–485. doi: 10.1105/tpc.7.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer G. Rapid growth inhibition of gherkin hypocotyls in blue light. Acta Bot Neerl. 1968;17:9–14. [Google Scholar]
- Parks BM, Hangarter RP. Blue light sensory systems in plants. Cell Biol. 1994;5:347–353. doi: 10.1006/scel.1994.1041. [DOI] [PubMed] [Google Scholar]
- Senger H, Schmidt W. Blue-light and UV-receptors. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 301–325. [Google Scholar]
- Short TW, Briggs WR. The transduction of blue light signals in higher plants. Annu Rev Plant Physiol. 1994;45:143–171. [Google Scholar]
- Spalding EP. An apparatus for studying rapid electrophysiological responses to light demonstrated on Arabidopsis leaves. Photochem Photobiol. 1995;62:934–939. doi: 10.1111/j.1751-1097.1995.tb09159.x. [DOI] [PubMed] [Google Scholar]
- Spalding EP, Cosgrove DJ. Large plasma-membrane depolarization precedes rapid blue-light-induced growth inhibition in cucumber. Planta. 1989;178:407–410. [PubMed] [Google Scholar]
- Spalding EP, Cosgrove DJ. Influence of electrolytes on growth, phototropism, nutation and surface potential in etiolated cucumber seedlings. Plant Cell Environ. 1993;16:445–451. doi: 10.1111/j.1365-3040.1993.tb00891.x. [DOI] [PubMed] [Google Scholar]
- Vines SH. Lectures on the Physiology of Plants. Cambridge, UK: Cambridge University Press; 1886. [Google Scholar]
- Wang X, Iino M. Interaction of cryptochrome 1, phytochrome, and ion fluxes in blue-light-induced shrinking of Arabidopsis hypocotyl protoplasts. Plant Physiol. 1998;117:1265–1279. doi: 10.1104/pp.117.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E, Zhu J. Role of zeaxanthin in blue light photoreception and the modulation of light-CO2 interactions in guard cells. J Exp Bot. 1998;49:433–442. [Google Scholar]