Abstract
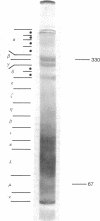
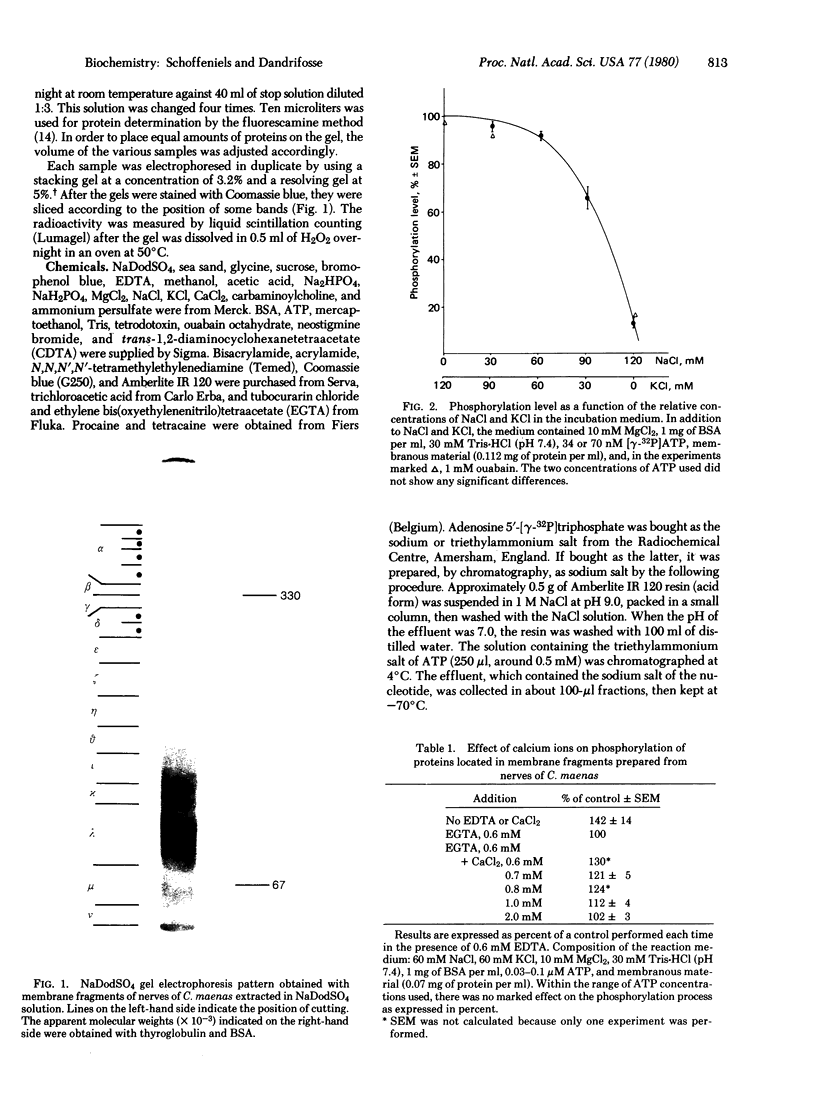
High molecular weight proteins extracted from the walking nerves of the shore crab (Carcinus maenas) exhibit a cycle of phosphorylation-dephosphorylation that is influenced by neurotropic compounds and inorganic ions. The net phosphorylation state of the proteins is increased in the presence of K+ ions and decreased with Na+ ions. In the absence of Mg2+ there is no phosphorylation. Ca2+ ions at low concentrations are necessary for optimal phosphorylation. At high concentration (above 0.1 mM), Ca2+ ions are inhibitory. Neurotropic compounds generally inhibit the phosphorylation process. More specifically, tetrodotoxin and veratridine, depending on the ionic composition of the medium, have opposite effects on the phosphorylation process, a result in agreement with their known physiological action. It is suggested that the high molecular weight components thus identified are part of the sodium permeation sites and that the conductance state of those sites is controlled by a phosphorylation process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., HILL A. V., HOWARTH J. V. The positive and negative heat production associated with a nerve impulse. Proc R Soc Lond B Biol Sci. 1958 Feb 18;148(931):149–187. doi: 10.1098/rspb.1958.0012. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Kemp R. G., Riley W. D., Cooper R. A., Krebs E. G. Activation of skeletal muscle phosphorylase kinase by adenosine triphosphate and adenosine 3',5'-monophosphate. J Biol Chem. 1968 May 10;243(9):2200–2208. [PubMed] [Google Scholar]
- Doherty J. D., Matsumura F. A highly ion-sensitive ATP-phosphorylation system in lobster nerve. Biochem Biophys Res Commun. 1974 Apr 23;57(4):987–992. doi: 10.1016/0006-291x(74)90793-1. [DOI] [PubMed] [Google Scholar]
- Dubois D. M., Schoffeniels E. A molecular model of action potentials. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2858–2862. doi: 10.1073/pnas.71.7.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Diamond I. Phosphorylation of membrane proteins at a cholinergic synapse. Proc Natl Acad Sci U S A. 1977 Jan;74(1):263–267. doi: 10.1073/pnas.74.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M., Rojas E. The binding of tetrodotoxin to nerve membranes. J Physiol. 1971 Feb;213(1):235–254. doi: 10.1113/jphysiol.1971.sp009379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margineanu D. G., Schoffeniels E. Molecular events and energy changes during the action potential. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3810–3813. doi: 10.1073/pnas.74.9.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACHMANSOHN D. Metabolism and function of the nerve cell. Harvey Lect. 1953;49:57–99. [PubMed] [Google Scholar]
- Neumann T. E. Molecular hysteresis and its cybernetic significance. Angew Chem Int Ed Engl. 1973 May;12(5):356–369. doi: 10.1002/anie.197303561. [DOI] [PubMed] [Google Scholar]
- Nonner W., Rojas E., Stämpfli H. Displacement currents in the node of Ranvier. Voltage and time dependence. Pflugers Arch. 1975;354(1):1–18. doi: 10.1007/BF00584499. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Changeux J. P. Evidence for protein phosphorylation and dephosphorylation in membrane fragments isolated from the electric organ of Electrophorus electricus. FEBS Lett. 1977 Feb 15;74(1):71–76. doi: 10.1016/0014-5793(77)80755-2. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]