Abstract
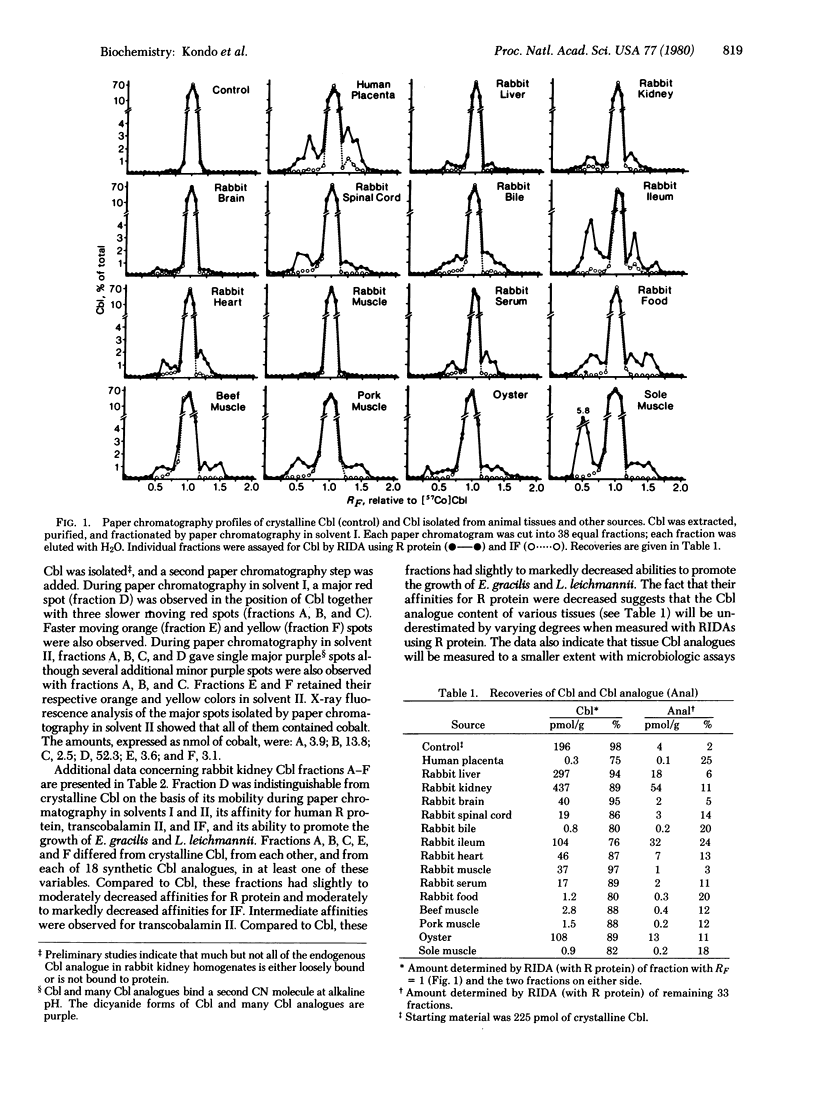
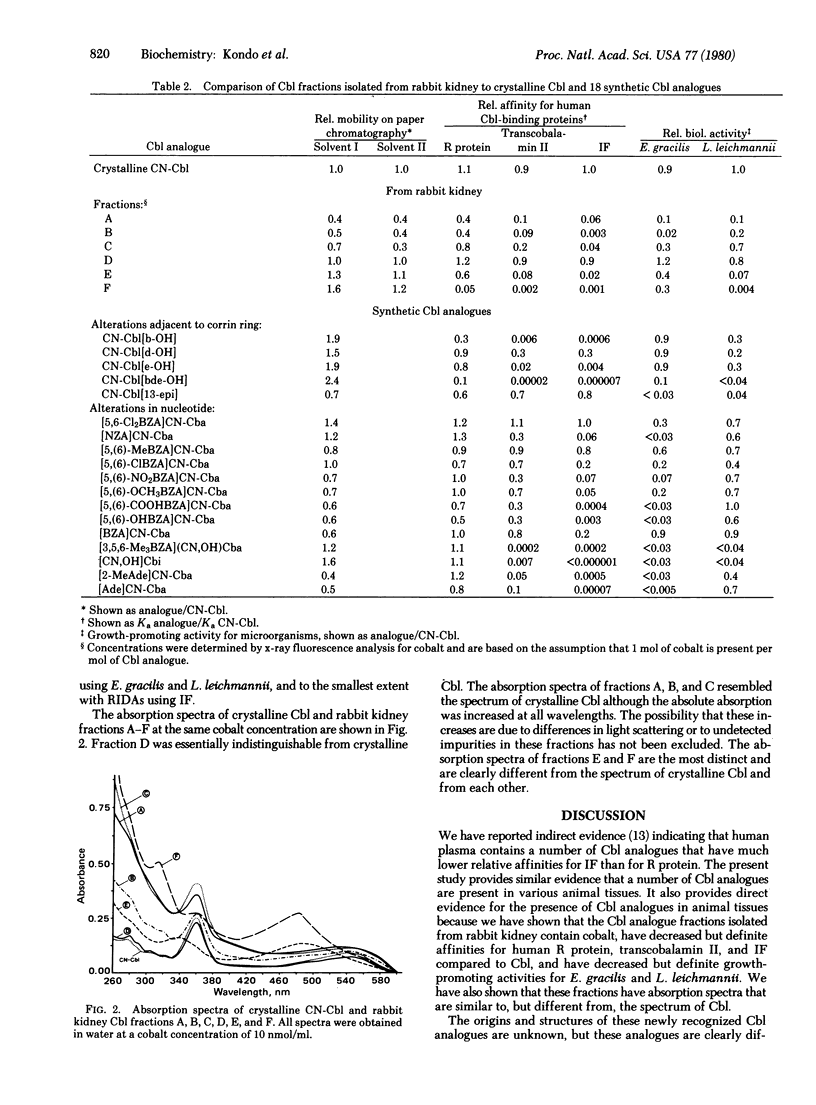
Cobalamin (Cbl, vitamin B-12) has been extracted and isolated from a number of animal tissues by using (i) reverse-affinity chromatography on R protein-Sepharose followed by adsorption to and elution from charcoal-coated agarose and (ii) paper chromatography. Radioisotope dilution assays showed that only 75-97% of the Cbl chromatographed in the position of crystalline Cbl. The remaining 3-25% was present in a number of slower and faster moving fractions. This suggested that Cbl analogues are present in animal tissues because appropriate controls ruled out the possibility that this material was artifactually derived from Cbl during the extraction and purification procedures. With a large-scale isolation from rabbit kidney, the material in five such fractions contained cobalt and had absorption spectra that were similar to but different from the spectrum of Cbl, indicating that they were Cbl analogues. Compared to Cbl, these Cbl analogues had decreased but definite affinities for Cbl-binding proteins with the following order of strength of binding: R protein > transcobalamin II > intrinsic factor. Compared to Cbl, they also had decreased but definite growth-promoting activity for two microorganisms, Euglena gracilis and Lactobacillus leichmannii, which require Cbl for growth. These Cbl analogues differed from each other and from 18 synthetic Cbl analogues, including the most common Cbl analogues synthesized by microorganisms, in at least one of the above features. These studies indicate that animal tissues contain a number of Cbl analogues whose origins, structures, and biologic activities remain to be determined.
Keywords: vitamin B-12, intrinsic factor, R protein, cobalt
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. H. Human vitamin B12 transport proteins. Prog Hematol. 1975;9:57–84. [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Burger R. L., Schneider R. J., Mehlman C. S., Allen R. H. Human plasma R-type vitamin B12-binding proteins. II. The role of transcobalamin I, transcobalamin III, and the normal granulocyte vitamin B12-binding protein in the plasma transport of vitamin B12. J Biol Chem. 1975 Oct 10;250(19):7707–7713. [PubMed] [Google Scholar]
- Cooper B. A., Whitehead V. M. Evidence that some patients with pernicious anemia are not recognized by radiodilution assay for cobalamin in serum. N Engl J Med. 1978 Oct 12;299(15):816–818. doi: 10.1056/NEJM197810122991506. [DOI] [PubMed] [Google Scholar]
- Fenton W. A., Rosenberg L. E. Improved techniques for the extraction and chromatography of cobalamins. Anal Biochem. 1978 Oct 1;90(1):119–125. doi: 10.1016/0003-2697(78)90014-3. [DOI] [PubMed] [Google Scholar]
- Frenkel E. P., McCall M. S., White J. D. Recognition and resolution of errors in the radioisotopic assay of serum vitamin B12. Am J Clin Pathol. 1970 Jun;53(6):891–903. doi: 10.1093/ajcp/53.6.891. [DOI] [PubMed] [Google Scholar]
- Gottlieb C. W., Retief F. P., Herbert V. Blockade of vitamin B12-binding sites in gastric juice, serum and saliva by analogues and derivatives of vitamin B12 and by antibody to intrinsic factor. Biochim Biophys Acta. 1967 Aug 29;141(3):560–572. doi: 10.1016/0304-4165(67)90185-7. [DOI] [PubMed] [Google Scholar]
- HELGELAND K., JONSEN J., LALAND S. New pigments derived from vitamin B12 by a strain of Aerobacter aerogenes present in river mud. Biochem J. 1961 Nov;81:260–265. doi: 10.1042/bj0810260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippe E., Haber E., Olesen H. Nature of vitamin B 12 binding. II. Steric orientation of vitamin B 12 on binding and number of combining sites of human intrinsic factor and the transcobalamins. Biochim Biophys Acta. 1971 Jul 25;243(1):75–82. [PubMed] [Google Scholar]
- Ing S. Y., Pfiffner J. J. Microbial degradation of corrinoids. V. Further observations on the chromophore in pigments derived from vitamin B 12 by Pseudomonas rubescens. Arch Biochem Biophys. 1968 Nov;128(2):281–284. doi: 10.1016/0003-9861(68)90033-7. [DOI] [PubMed] [Google Scholar]
- Kolhouse J. F., Allen R. H. Absorption, plasma transport, and cellular retention of cobalamin analogues in the rabbit. Evidence for the existence of multiple mechanisms that prevent the absorption and tissue dissemination of naturally occurring cobalamin analogues. J Clin Invest. 1977 Dec;60(6):1381–1392. doi: 10.1172/JCI108899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhouse J. F., Allen R. H. Isolation of cobalamin and cobalamin analogs by reverse affinity chromatography. Anal Biochem. 1978 Feb;84(2):486–490. doi: 10.1016/0003-2697(78)90067-2. [DOI] [PubMed] [Google Scholar]
- Kolhouse J. F., Kondo H., Allen N. C., Podell E., Allen R. H. Cobalamin analogues are present in human plasma and can mask cobalamin deficiency because current radioisotope dilution assays are not specific for true cobalamin. N Engl J Med. 1978 Oct 12;299(15):785–792. doi: 10.1056/NEJM197810122991501. [DOI] [PubMed] [Google Scholar]
- PERLAMN D., BARRETT J. B. Biosynthesis of cobalamin analogues by propionibacterium arabinosum. Can J Microbiol. 1958 Feb;4(1):9–15. doi: 10.1139/m58-002. [DOI] [PubMed] [Google Scholar]
- Tortolani G., Bianchini P., Mantovani V. Separation and determination of cobalamins on an SP-Sephadex column. J Chromatogr. 1971 Dec 23;53(3):577–579. doi: 10.1016/s0021-9673(01)98517-6. [DOI] [PubMed] [Google Scholar]



