Abstract
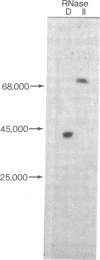
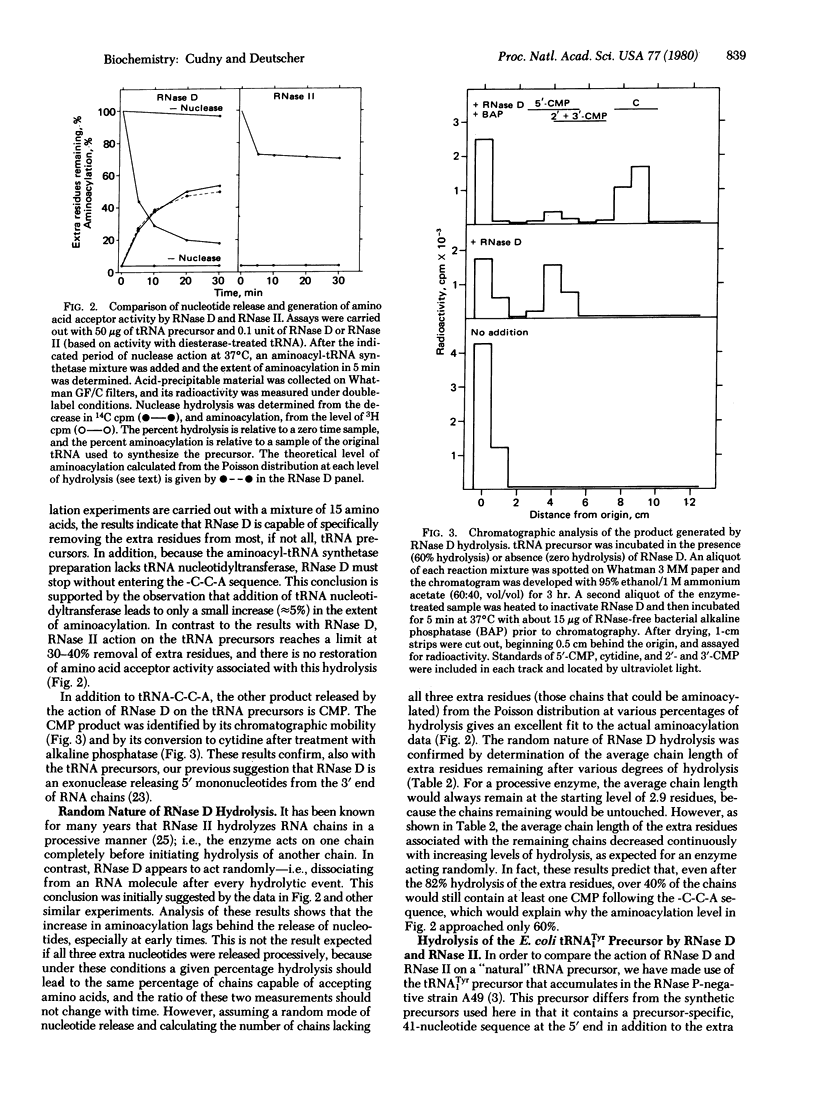
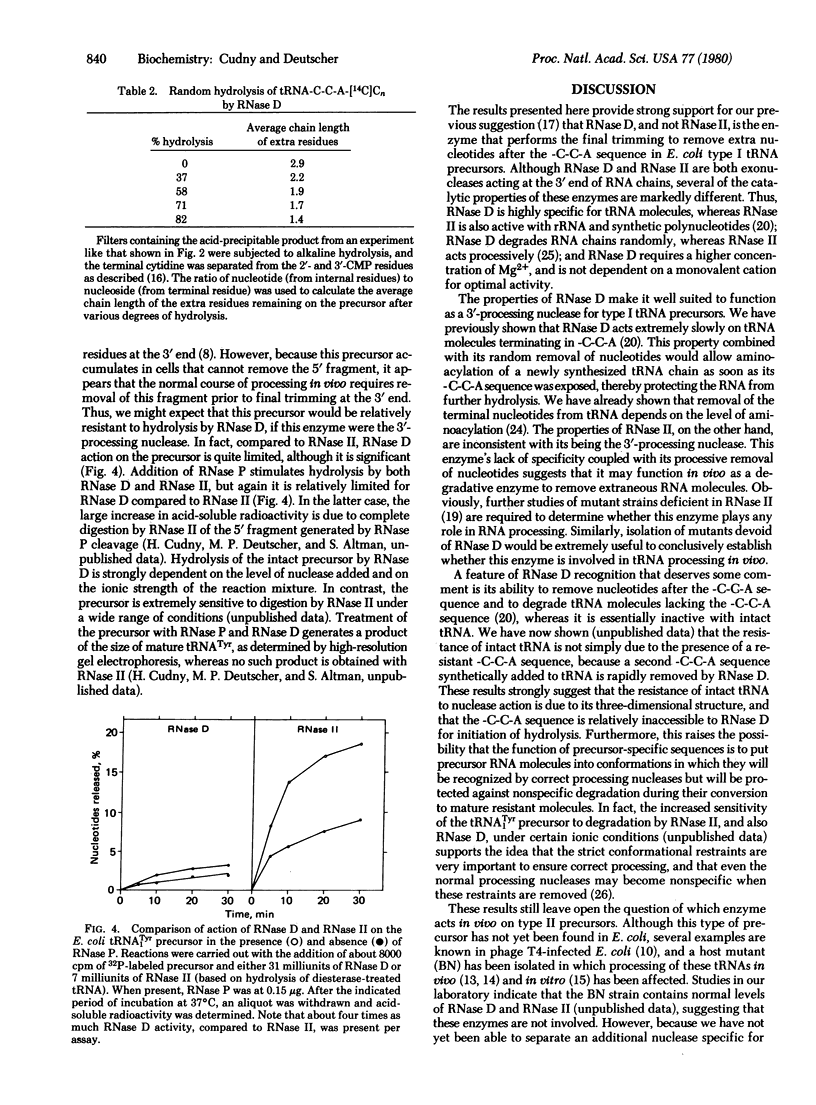
Escherichia coli RNase D and RNase II have been purified to homogeneity and compared for their ability to remove extra nucleotides following the -C-C-A sequence in tRNA precursors. RNase D and RNase II are single-chain proteins with molecular weights of 38,000 and 78,000, respectively. Both enzymes require a divalent cation for activity on tRNA precursors, but, in addition, RNase II is stimulated by monovalent cations. RNase D specifically removes mononucleotide residues from a mixture of tRNA precursors to generate amino acid acceptor activity for essentially all amino acids. Although RNase II can also remove precursor-specific residues, no amino acid acceptor activity is recovered. Similarly, RNase D action on the E. coli tRNATyr precursor is limited, whereas RNase II causes extensive degradation. In contrast to the processive mode of hydrolysis by RNase II, RNase D removes nucleotides randomly and slows down greatly at the -C-C-A sequence, thereby allowing the tRNA to be aminoacylated and protected from further degradation. These results suggest that RNase D is the 3'-processing nuclease in vivo and that RNase II is a nonspecific degradative enzyme. The importance of RNA conformation for correct processing is also discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Smith J. D. Tyrosine tRNA precursor molecule polynucleotide sequence. Nat New Biol. 1971 Sep 8;233(36):35–39. doi: 10.1038/newbio233035a0. [DOI] [PubMed] [Google Scholar]
- Bikoff E. K., LaRue B. F., Gefter M. L. In vitro synthesis of transfer RNA. II. Identification of required enzymatic activities. J Biol Chem. 1975 Aug 25;250(16):6248–6255. [PubMed] [Google Scholar]
- Chang S., Carbon J. The nucleotide sequence of a precursor to the glycine- and threonine-specific transfer ribonucleic acids of Escherichia coli. J Biol Chem. 1975 Jul 25;250(14):5542–5555. [PubMed] [Google Scholar]
- Deutscher M. P., Evans J. A. Transfer RNA nucleotidyltransferase repairs all transfer RNAs randomly. J Mol Biol. 1977 Feb 5;109(4):593–597. doi: 10.1016/s0022-2836(77)80093-4. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Ghosh R. K. Preparation of synthetic tRNA precursors with tRNA nucleotidyltransferase. Nucleic Acids Res. 1978 Oct;5(10):3821–3829. doi: 10.1093/nar/5.10.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P., Lin J. J., Evans J. A. Transfer RNA metabolism in Escherichia coli cells deficient in tRNA nucleotidyltransferase. J Mol Biol. 1977 Dec 25;117(4):1081–1094. doi: 10.1016/s0022-2836(77)80014-4. [DOI] [PubMed] [Google Scholar]
- Ghosh R. K., Deutscher M. P. Identification of an Escherichia coli nuclease acting on structurally altered transfer RNA molecules. J Biol Chem. 1978 Feb 25;253(4):997–1000. [PubMed] [Google Scholar]
- Ghosh R. K., Deutscher M. P. Purification of potential 3' processing nucleases using synthetic tRNA precursors. Nucleic Acids Res. 1978 Oct;5(10):3831–3842. doi: 10.1093/nar/5.10.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Kasai T., Schlessinger D. Purification and some novel properties of Escherichia coli RNase II. J Biol Chem. 1977 Dec 25;252(24):8945–8949. [PubMed] [Google Scholar]
- Kitamura N., Ikeda H., Yamada Y., Ishikura H. Processing by ribonuclease II of the tRNATyr precursor of Escherichia coli synthesized in vitro. Eur J Biochem. 1977 Feb 15;73(1):297–306. doi: 10.1111/j.1432-1033.1977.tb11319.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maisurian A. N., Buyanovskaya E. A. Isolation of an Escherichia coli strain restricting bacteriophage suppressor. Mol Gen Genet. 1973 Feb 2;120(3):227–229. doi: 10.1007/BF00267154. [DOI] [PubMed] [Google Scholar]
- Morse J. W., Deutscher M. P. Letter: Apparent non-involvement of transfer RNA nucleotidyltransferase in the biosynthesis of Escherichia coli suppressor transfer RNAs. J Mol Biol. 1975 Jun 15;95(1):141–144. doi: 10.1016/0022-2836(75)90342-3. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Singer M. F. The processive degradation of individual polyribonucleotide chains. I. Escherichia coli ribonuclease II. J Biol Chem. 1968 Mar 10;243(5):913–922. [PubMed] [Google Scholar]
- Robertson H. D., Altman S., Smith J. D. Purification and properties of a specific Escherichia coli ribonuclease which cleaves a tyrosine transfer ribonucleic acid presursor. J Biol Chem. 1972 Aug 25;247(16):5243–5251. [PubMed] [Google Scholar]
- Schedl P., Primakoff P. Mutants of Escherichia coli thermosensitive for the synthesis of transfer RNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2091–2095. doi: 10.1073/pnas.70.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl P., Roberts J., Primakoff P. In vitro processing of E. coli tRNA precursors. Cell. 1976 Aug;8(4):581–594. doi: 10.1016/0092-8674(76)90226-9. [DOI] [PubMed] [Google Scholar]
- Schmidt F. J., McClain W. H. An Escherichia coli ribonuclease which removes an extra nucleotide from a biosynthetic intermediate of bacteriophage T4 proline transfer RNA. Nucleic Acids Res. 1978 Nov;5(11):4129–4139. doi: 10.1093/nar/5.11.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Schmidt F. J., Foss K., McClain W. H. A mutant of escherichia coli defective in removing 3' terminal nucleotides from some transfer RNA precursor molecules. Cell. 1975 Aug;5(4):389–400. doi: 10.1016/0092-8674(75)90058-6. [DOI] [PubMed] [Google Scholar]
- Sekiya T., Contreras R., Takeya T., Khorana H. G. Total synthesis of a tyrosine suppressor transfer RNA gene. XVII. Transcription, in vitro, of the synthetic gene and processing of the primary transcript to transfer RNA. J Biol Chem. 1979 Jul 10;254(13):5802–5816. [PubMed] [Google Scholar]
- Shimura Y., Sakano H., Nagawa F. Specific ribonucleases involved in processing of tRNA precursors of Escherichia coli. Partial purification and some properties. Eur J Biochem. 1978 May;86(1):267–281. doi: 10.1111/j.1432-1033.1978.tb12308.x. [DOI] [PubMed] [Google Scholar]
- Singer M. F., Tolbert G. Purification and properties of a potassium-activated phosphodiesterase (RNAase II) from Escherichia coli. Biochemistry. 1965 Jul;4(7):1319–1330. doi: 10.1021/bi00883a016. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Venegas A., Weinberg F., Bishop R., Rutter W. J. Structure of yeast phenylalanine-tRNA genes: an intervening DNA segment within the region coding for the tRNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):190–194. doi: 10.1073/pnas.75.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]