Abstract
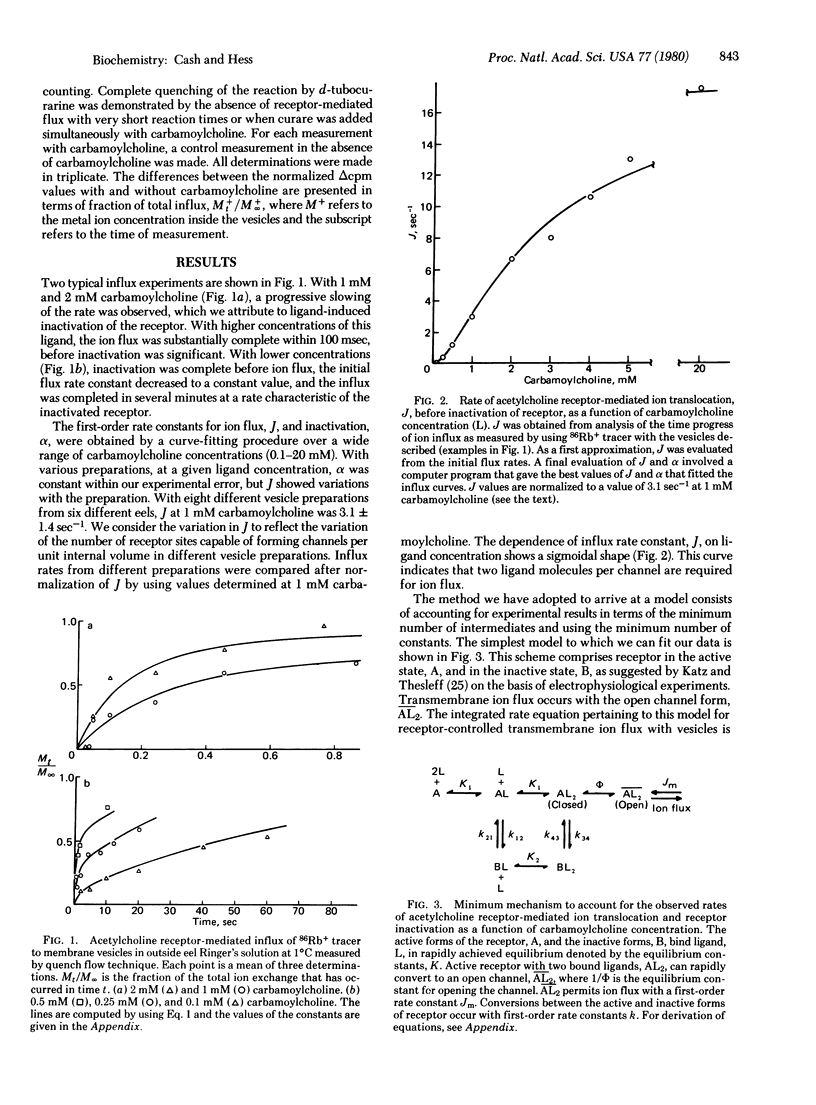
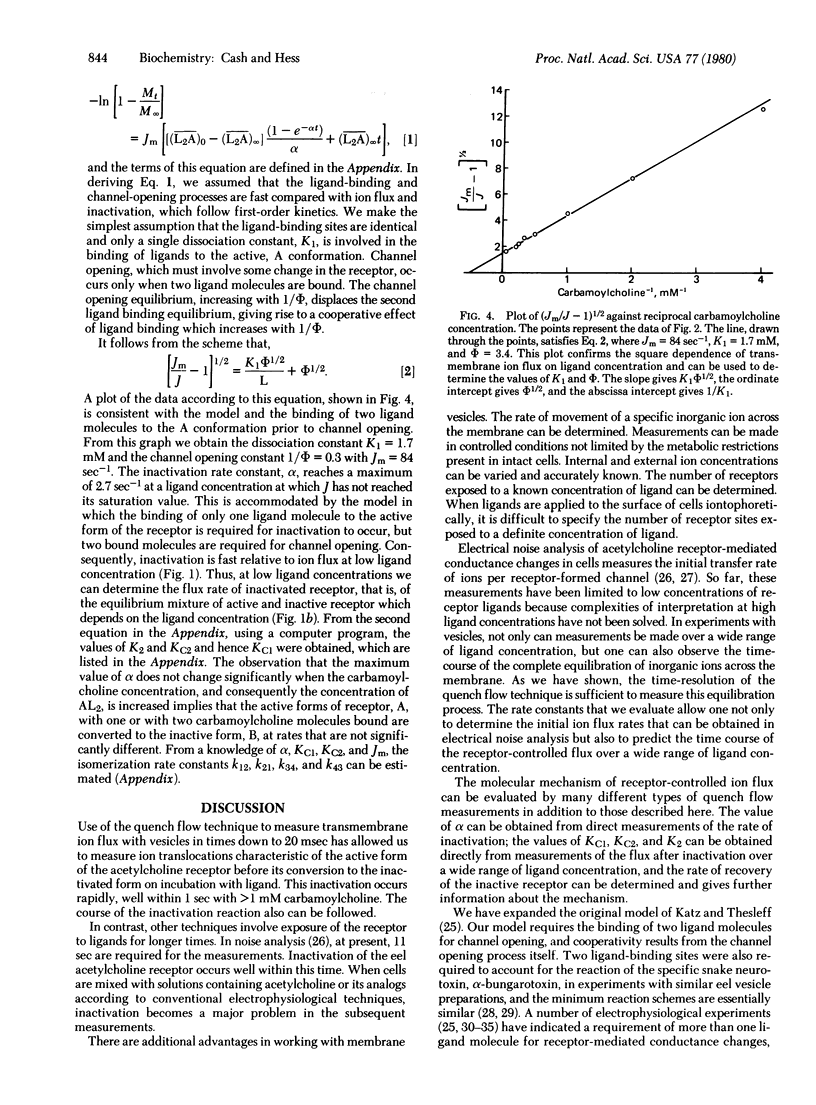
Two molecular processes, the binding of acetylcholine to the membrane-bound acetylcholine receptor protein and the receptor-controlled flux rates of specific inorganic ions, are essential in determining the electrical membrane potential of nerve and muscle cells. The measurements reported establish the relationship between the two processes: the acetylcholine receptor-controlled transmembrane ion flux of 86Rb+ and the concentration of carbamoylcholine, a stable analog of acetylcholine. A 200-fold concentration range of carbamoylcholine was used. The flux was measured in the millisecond-to-minute time region by using a quench flow technique with membrane vesicles prepared from the electric organ of Electrophorus electricus in eel Ringer's solution at pH 7.0 and 1°C. The technique makes possible the study of the transmembrane transport of specific ions, with variable known internal and external ion concentrations, in a system in which a determinable number of receptors is exposed to a known concentration of ligand. The response curve of ion flux to ligand was sigmoidal with an average maximum rate of 84 sec-1. Carbamoylcholine induced inactivation of the receptor with a maximum rate of 2.7 sec-1 and a different ligand dependence so that it was fast relative to ion flux at low ligand concentration but slow relative to ion flux at high ligand concentration. The simplest model that fits the data consists of receptor in the active and inactive states in ligand-controlled equilibria. Receptor inactivation occurs with one or two ligand molecules bound. For channel opening, two ligand molecules bound to the active state are required, and cooperativity results from the channel opening process itself. With carbamoylcholine, apparently, the equilibrium position for the channel opening step is only one-fourth open. The integrated rate equation, based on the model, predicts the time dependence of receptor-controlled ion flux over the concentration range of carbamoylcholine investigated. The values of the constants in the rate equation form the basis for predicting receptor-controlled changes in the transmembrane potential of cells and the conditions leading to transmission of signals between cells.
Keywords: Electrophorus electricus, quench flow technique, carbamoylcholine, d-tubocurarine chloride, membrane vesicles
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Relaxation experiments using bath-applied suberyldicholine. J Physiol. 1977 Jun;268(2):271–289. doi: 10.1113/jphysiol.1977.sp011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger J. E., Fu J. L., Hindy E. F., Silberstein R. L., Hess G. P. Allosteric interactions between the membrane-bound acetylcholine receptor and chemical mediators. Kinetic studies. Biochemistry. 1977 Feb 22;16(4):684–692. doi: 10.1021/bi00623a020. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Density and dose-response curve of acetylcholine receptors in frog neuromuscular junction. Nature. 1975 Feb 20;253(5493):641–643. doi: 10.1038/253641a0. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Jakes R. Demonstration of two reaction pathways for the aminoacylation of tRNA. Application of the pulsed quenched flow technique. Biochemistry. 1975 Jul 29;14(15):3350–3356. doi: 10.1021/bi00686a010. [DOI] [PubMed] [Google Scholar]
- Fu J. L., Donner D. B., Moore D. E., Hess G. P. Allosteric interactions between the membrane-bound acetylcholine receptor and chemical mediators: equilibrium measurements. Biochemistry. 1977 Feb 22;16(4):678–684. doi: 10.1021/bi00623a019. [DOI] [PubMed] [Google Scholar]
- Hammes G. G., Wu C. W. Kinetics of allosteric enzymes. Annu Rev Biophys Bioeng. 1974;3(0):1–33. doi: 10.1146/annurev.bb.03.060174.000245. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Structural and functional properties of the acetylcholine receptor protein in its purified and membrane-bound states. Annu Rev Biochem. 1978;47:317–357. doi: 10.1146/annurev.bi.47.070178.001533. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P. Functional acetylcholine receptor--electroplax membrane microsacs (vesicles): purification and characterization. Proc Natl Acad Sci U S A. 1977 Feb;74(2):482–486. doi: 10.1073/pnas.74.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P., Struve G. E. Apparent cooperative effects in acetylcholine receptor-mediated ion flux electroplax membrane preparations. Biochem Biophys Res Commun. 1976 Apr 5;69(3):830–837. doi: 10.1016/0006-291x(76)90950-5. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P., Struve G. E., Goombs S. E. Acetylcholine-receptor-mediated ion flux in electroplax membrane preparations. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4371–4375. doi: 10.1073/pnas.72.11.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Bulger J. E., Fu J. J., Hindy E. F., Silberstein R. J. Allosteric interactions of the membrane-bound acetylcholine reception: kinetic studies with alpha-bungarotoxin. Biochem Biophys Res Commun. 1975 Jan 2;64(3):1018–1027. doi: 10.1016/0006-291x(75)90149-7. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Cash D. J., Aoshima H. Acetylcholine receptor-controlled ion fluxes in membrane vesicles investigated by fast reaction techniques. Nature. 1979 Nov 15;282(5736):329–331. doi: 10.1038/282329a0. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Lipkowitz S., Struve G. E. Acetylcholine-receptor-mediated ion flux in electroplax membrane microsacs (vesicles): change in mechanism produced by asymmetrical distribution of sodium and potassium ions. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1703–1707. doi: 10.1073/pnas.75.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport. Annu Rev Biochem. 1970;39:561–598. doi: 10.1146/annurev.bi.39.070170.003021. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Land B. R., Podleski T. R., Salpeter E. E., Salpeter M. M. Acetylcholine receptor distribution on myotubes in culture correlated to acetylcholine sensitivity. J Physiol. 1977 Jul;269(1):155–176. doi: 10.1113/jphysiol.1977.sp011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACHMANSOHN D. Metabolism and function of the nerve cell. Harvey Lect. 1953;49:57–99. [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Schimerlik M. I., Quast U., Raftery M. A. Ligand-induced changes in membrane-bound acetylcholine receptor observed by ethidium fluorescence. 3. Stopped-flow studies with histrionicotoxin. Biochemistry. 1979 May 15;18(10):1902–1906. doi: 10.1021/bi00577a008. [DOI] [PubMed] [Google Scholar]
- Schimerlik M., Quast U., Raftery M. A. Ligand-induced changes in membrane-bound acetylcholine receptor observed by ethidium fluorescence. 1. Equilibrium studies. Biochemistry. 1979 May 15;18(10):1884–1890. doi: 10.1021/bi00577a006. [DOI] [PubMed] [Google Scholar]



