SUMMARY
The evolutionarily ancient arm of the E2f family of transcription factors consisting of the two atypical members E2f7 and E2f8 is essential for murine embryonic development. However, the critical tissues, cellular processes and molecular pathways regulated by these two factors remain unknown. Using a series of fetal and placental lineage-specific cre mice we show that E2F7/E2F8 functions in extra-embryonic trophoblast lineages are both necessary and sufficient to carry fetuses to term. Expression profiling and biochemical approaches exposed the canonical E2F3a activator as a key family member that antagonizes E2F7/E2F8 functions. Remarkably, the concomitant loss of E2f3a normalized placental gene expression programs, corrected placental defects and fostered the survival of E2f7/E2f8 deficient embryos to birth. In summary, we identified a placental transcriptional network tightly coordinated by activation and repression through two distinct arms of the E2F family that is essential for extra-embryonic cell proliferation, placental development and fetal viability.
INTRODUCTION
Cells respond to external growth stimuli by activating signaling cascades that carry them through the cell cycle to generate two genetically identical daughter cells. A critical step in these proliferative signaling cascades involves the activation of G1-specific cyclin-dependent kinases (Cdks), the phosphorylation of retinoblastoma (Rb) and Rb-related pocket proteins, and the accumulation of E2F transcriptional activity (Frolov and Dyson, 2004). The execution of E2F-dependent transcription late in G1 phase is believed to be the final event in Cdk-mediated mitogenic signaling that commits cells to S phase entry. Subsequent waves of E2F-mediated repression are thought to coordinate the completion of remaining phase-specific events and successful cell divisions. This classic paradigm of E2F-mediated gene activation and repression in the control of cell cycle progression is based almost exclusively on the analyses of invertebrates and overexpression strategies in mammalian cell culture systems (Dimova and Dyson, 2005; Frolov et al., 2001). However, analyses of mice deficient for various E2F family members have revealed a spectrum of tissue specific phenotypes that are inconsistent with the rigid view of E2Fs as universal factors required to coordinate cell cycle dependent gene expression programs (Chen et al., 2009; Chong et al., 2009b; Cloud et al., 2002; Danielian et al., 2008; Field et al., 1996; Humbert et al., 2000; Kinross et al., 2006; Li et al., 2003; Li et al., 2008; Lindeman et al., 1998; Murga et al., 2001; Pohlers et al., 2005; Rempel et al., 2000; Tsai et al., 2008; Yamasaki et al., 1996). These findings suggest that E2F family members either have different functions or perform similar functions but in a tissue-specific manner. It is also possible that the ablation of individual family members is insufficient to expose how their combined activities might be coordinated in vivo.
The E2F family consists of nine related proteins (DeGregori and Johnson, 2006) that based on sequence conservation and structure-function studies have been conveniently divided into transcription activators and repressors. Canonical E2F activators, consisting of E2F1, E2F2, E2F3a and E2F3b, have transactivation domains and associate with co-activator proteins to robustly induce RNA polymerase II-dependent gene expression (Danielian et al., 2008; Trimarchi and Lees, 2002). E2F repressors fall into two subclasses, with E2F4, E2F5 and E2F6 in one subclass (canonical) and E2F7 and E2F8 in the other (atypical). While E2F4–6 mediated repression is responsive to Cdk signaling and involves the recruitment of histone deacetylases (HDACs), polycomb group proteins as well as Mga and Max to E2F-target promoters (Attwooll et al., 2004), the mechanisms of how E2F7/E2F8 mediate repression is essentially unknown but appears to be independent of Cdk mediated phosphorylation of pocket proteins. To make matters more complex, it is now clear that at least in some developmental contexts, E2F activators can also function to repress gene expression, but the molecular basis for such plasticity is not completely understood (Chen et al., 2009; Chong et al., 2009b; Trikha et al., 2011; Wenzel et al., 2011).
Unlike other E2F family members, E2F7/E2F8 associate with DNA independent of dimerization with DP1/DP2 proteins, and instead utilize two tandem DNA-binding domains to recognize and bind target DNA sequences. These two atypical E2Fs also lack amino acid sequences typically used to physically interact with Rb related proteins, and thus may function outside the canonical Cdk-Rb-E2F pathway. Previous work showed that embryos lacking E2f7 and E2f8 exhibit widespread apoptosis and die by E11.5 (Li et al., 2008). In the current study, we developed extra-embryonic lineage-specific cre mice to explore E2F7/E2F8 functions during development. Using mouse genetic, biochemical and bioinformatic approaches we identified two antagonistic arms of the E2F program, one regulated by E2F7/E2F8 and the second by E2F3a, that coordinate the G1-S transcriptional output necessary for balancing cell proliferation and differentiation in the placenta. Ablation of the repressive E2F7/E2F8 arm in trophoblast cell lineages was sufficient to incite ectopic proliferation and disrupt placental architecture and function, which inevitably led to embryonic death by E11.5. Remarkably many of the phenotypes observed in E2f7−/−;E2f8−/− embryos, including their early lethality, was suppressed by the concomitant ablation of the E2f3a activator. These findings provide a mechanism for how canonical and atypical E2F pathways coordinate the control of transcriptional programs essential for mammalian cell proliferation and development.
RESULTS
Loss of E2f7 and E2f8 Leads to Profound Placental Defects
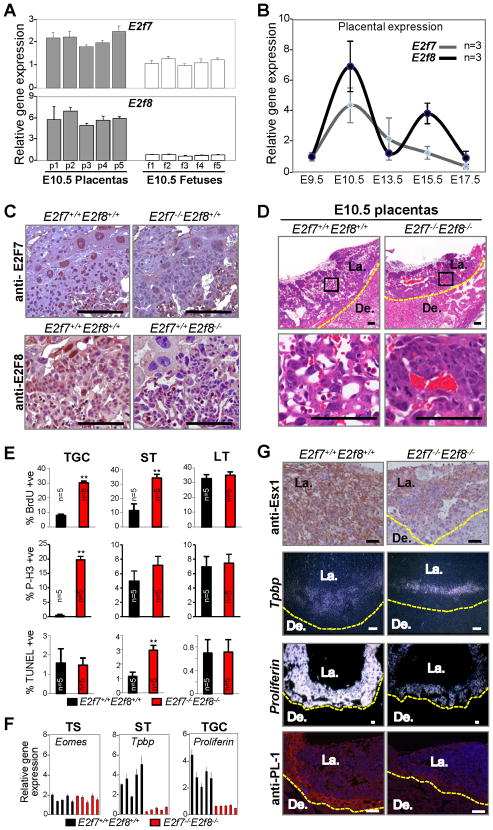
Previous studies using gene knockout approaches in mice showed that E2F7 and E2F8 are essential for embryonic development, but the tissues, cellular processes and molecular pathways that they regulate are poorly defined (Li et al., 2008). We reasoned that identification of the tissues and cells where E2F7 and E2F8 functions are most critical for embryonic development and viability might provide valuable insight into their physiological function. Expression analysis demonstrated that E2f7 and E2f8 mRNA levels are relatively high in placental versus fetal tissues (Figure 1A), with peak expression at E10.5 (Figure 1B). Interestingly, a second wave of placental E2f8 expression coincided with the proliferation of glycogen trophoblast cells at E15.5 (Coan et al., 2006). Immunohistochemistry (IHC) on placental sections showed E2F7 and E2F8 proteins in the three major trophoblast lineages, labyrinth trophoblasts (LT), spongiotrophoblasts (ST) and trophoblast giant cells (TGC) (Figure 1C). The observation that E2F7 and E2F8 proteins were highly expressed in some cells but undetectable in others likely reflects their cell cycle dependent expression (de Bruin et al., 2003; Di Stefano et al., 2003; Maiti et al., 2005).
Figure 1. Profound Placental Defects in E2f7−/−E2f8−/− Embryos.
(A) Quantitative RT-PCR analysis of E2f7 and E2f8 expression in E10.5 wild type placentas (gray) and fetuses (white). (B) Quantitative RT-PCR analysis of E2f7 (grey) and E2f8 (black) expression in wild type placentas at different stages of embryonic development. (C) E2F7 (top) and E2F8 (bottom) protein expression as identified by immunohistochemistry (IHC) in E10.5 placental sections with the indicated genotypes. (D) Hematoxylin and Eosin (H&E) of E10.5 placental sections. Bottom panels are high magnification views of representative boxed areas in top panels. (E) Quantification of BrdU, TUNEL and P-H3 positive trophoblast cells in E10.5 E2f7+/+E2f8+/+ (black) and E2f7−/−E2f8−/− (red) placentas (** p<0.01). TGC, trophoblast giant cells; ST, spongiotrophoblasts; LT, labrynthine trophoblasts. (F) Quantitative RT-PCR analysis for trophoblast lineage markers in E10.5 E2f7+/+E2f8+/+ (black) and E2f7−/−E2f8−/− (red) placentas. (G) Qualitative analysis of differentiation markers in major trophoblast lineages in placentas. Top panel, representative IHC analysis of LT-specific Esx1 immunohistochemistry staining (E10.5); middle two panels, RNA in situ hybridization analysis of ST-specific Tpbp (E10.5) and TGC-specific Proliferin (E9.5); and lower panel, immunofluorescent detection of TGC-specific Placental Lactogen 1 (E10.5). Scale bars, 100 μm. Yellow dotted line demarks junctional zone from decidua. De., Decidua; La., Labyrinth.
The above findings prompted us to examine placentas of E2f7−/−;E2f8−/− embryos. Histological examination of double mutant placentas revealed severely compromised tissue architecture (Figure 1D, right panels). Wild type placentas typically have a well organized labyrinth of trophoblast cells with the vasculature arranged as a network of maternal sinusoids juxtaposed to fetal-derived blood vessels (Figure 1D, left panels). In contrast, E2f7−/−;E2f8−/− placentas were overall smaller and had abnormally large clusters of densely packed trophoblast cells that failed to effectively invade into the maternal decidua. The vascular network was poorly formed and maternal sinusoids were rarely found adjacent to fetal blood vessels. DNA replication and mitotic markers were elevated in mutant STs and TGCs as determined by IHC using anti-BrdU and anti-phopho-histone3 antibodies (Figure 1E, top two panels and S1A). An increased number of apoptotic STs was also observed in double mutant placentas (Figure 1E, lower panel and S1A). Moreover, quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis showed that double mutant placentas have normal expression of trophoblast stem (TS) cell-specific markers (Eomes and Cdx2) but reduced levels of ST- and TGC-specific markers (Tpbp, Pdgf and Proliferin, Csf1r, Pl-1, Prp, respectively; Figure 1F and S1B). In situ hybridization confirmed lower expression of Tpbp and Proliferin in mutant STs and TGCs, respectively, and IHC showed lower levels of Esx1 and PL-1 proteins in mutant LTs and TGCs, respectively (Figure 1G). Therefore, in addition to the fetal defects previously characterized (Li et al., 2008), E2f7−/−;E2f8−/− embryos exhibit a severely compromised placenta that is associated with ectopic proliferation, apoptosis and altered differentiation of multiple extra-embryonic cell lineages.
Loss of E2f7 and E2f8 disrupts a distinct gene expression program in the placenta
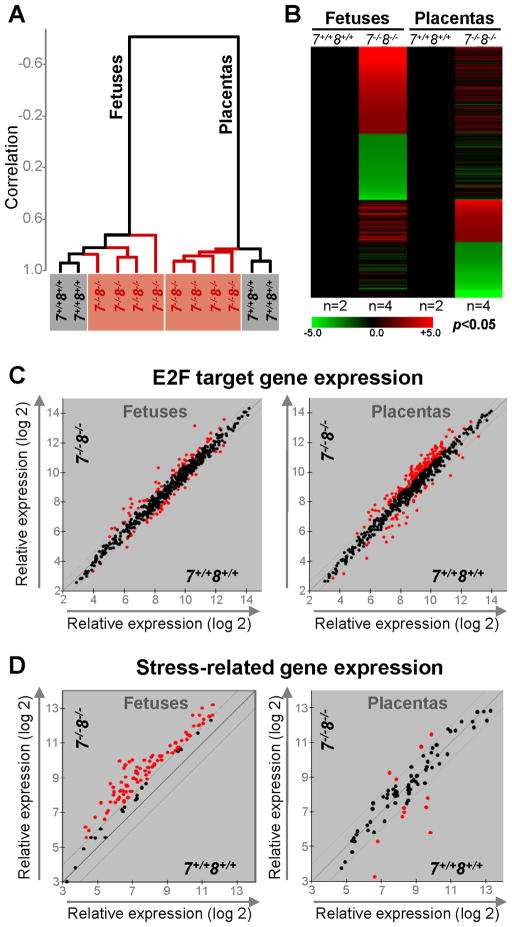
We reasoned that changes in gene expression would directly underlie many of the phenotypes caused by loss of the two atypical E2F transcription factors. We thus performed global gene expression profiling (Affymetrix Mouse Genome 430 2.0) in wild type and double mutant E10.5 placentas and fetuses. Unsupervised clustering analysis of gene expression separated samples into two groups, with fetuses clustering in one group and placentas clustering in the other (Figure 2A). Samples within each group could be further clustered based on the genotype of the tissue. Heat maps illustrate that most differentially expressed genes in double mutant placentas are distinct from those in mutant fetuses (Figure 2B). For example, expression of many E2F target genes, as identified by previous gene expression, reporter and chromatin immunoprecipitation (ChIP) assays, was significantly increased in double mutant placentas but not in mutant fetuses (Figures 2C). On the other hand, there was a significant increase in the expression of genes related to nutritional and hypoxic stress responses in mutant fetuses but not in mutant placentas, including a marked induction of HIF-1 α targets (Figure 2D and Table S1).
Figure 2. Loss of E2f7 and E2f8 in the Placenta Elicits an Intense Stress Response in the Fetus.
(A) Dendogram of unsupervised hierarchical clustering analysis of E10.5 fetal and placental global gene expression profiles using centered correlation and average linkage. (B) Heat maps of microarray probes that showed a >2-fold misregulation in E2f7−/−;E2f8−/− fetuses or placentas relative to E2f7+/+;E2f8+/+ counterparts (p<0.05). n, number of samples analyzed per genetic group. (C) Scatter plot analyses of E2F target gene expression in fetuses and placentas of indicated genotypes using 1.5-fold cutoff (red, >1.5-fold deregulation). and (D) Scatter plot analyses of stress-related gene expression in fetuses and placentas of indicated genotypes using 2-fold cutoff (red, >2-fold deregulation).
E2F7 and E2F8 Functions are Essential in Trophoblast Progenitor Cells
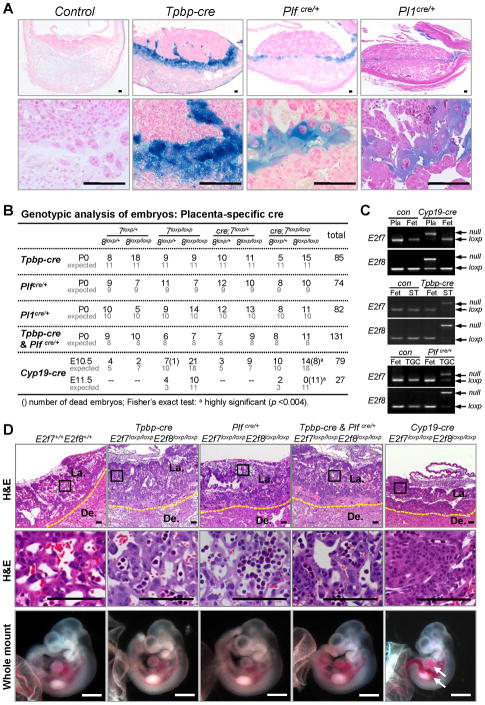
The above findings led us to hypothesize that a primary defect in E2F-target expression in mutant placentas may lead to placental dysfunction and underlie the profound stress response, fetal growth retardation and mid-gestation lethality observed in E2f7−/−;E2f8−/− embryos. To test this possibility we assessed the consequences of ablating E2f7 and E2f8 in either placental or fetal compartments to the overall development of embryos. First, we analyzed the consequences of ablating E2f7 and E2f8 in specific extra-embryonic cell lineages. Our initial focus was on STs and TGCs since dramatic defects in these cell types were observed in E2f7−/−;E2f8−/− placentas (Figure 1E–G). To this end, Tpbp-cre transgenic mice were used to target gene deletion in STs (Simmons et al., 2007). We also generated and used Plfcre/+ and Pl1cre/+ knockin mice to target cre-mediated recombination in TGCs (Figure 3A and unpublished data). Ablation of E2f7 and E2f8 in either or both of these cell lineages (ST and TGC) resulted in live and phenotypically normal fetuses at every embryonic stage analyzed, including at birth (Figures 3B, 3D and data not shown). Placentas appeared well vascularized without any evidence of architectural disruption (Figure 3D). The specific ablation of E2f7 and E2f8 in STs and TGCs was confirmed by laser capture microdissection (LCM) of the appropriate extra-embryonic cell lineages and PCR genotyping (Figure 3C). To ablate these E2fs in the entire placenta we interbred E2f7loxp/loxp;E2f8loxp/loxp and Cyp19-cre mice, which express cre in all trophoblast cells, including in undifferentiated trophoblast progenitor cells as early as E6.5 (Wenzel and Leone, 2007). Strikingly, these intercrosses failed to yield any live Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp embryos past E10.5 (Figure 3B). Gross and histological examination of live E10.5 Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp embryos revealed a similar collapse of the labyrinth-like placental architecture along with vascular dilation, hemorrhage and growth retardation in associated fetuses (with intact E2f7 and E2f8) as observed in E2f7−/−;E2f8−/− embryos (Figure 3D and S2A–B).
Figure 3. Extra-embryonic Functions of E2F7 and E2F8 are Essential for Embryonic Survival.
(A) X-gal staining showing lineage specific expression of cre recombinase in Tpbp-cre, Plfcre/+ and Pl1cre/+ mice heterozygous for Rosa26loxp reporter allele. (B) Genotypic analysis of embryos derived from intercrosses of Tpbp-cre, Plfcre, Pl1cre and Cyp19-cre with E2f7loxp/loxp;E2f8loxp/loxp mice. For the Tpbp-cre, Pl1cre & Plfcre experiment, cre refers to presence of both cre alleles in heterozygosity. (C) Representative PCR genotyping analyses of E2f7 and E2f8. Genomic DNA was isolated from E10.5 whole placentas (Pla) or laser capture microdissected spongiotrophoblasts (ST) or giant cells (TGC) in Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp (Cyp19-cre), Tpbp-cre;E2f7loxp/loxp;E2f8loxp/loxp (Tpbp-cre) and Plfcre/+;E2f7loxp/loxp;E2f8loxp/loxp (Plfcre/+) respectively, along with cre negative controls (con) and whole fetuses (Fet). (D) Representative low and high magnification images of H&E stained E10.5 placental sections (top two rows) and gross appearance of associated fetuses (bottom) with the indicated genotypes. Arrows indicate dilated blood vessels and hemorrhagic areas. Histology scale bars, 100 μm; whole mount scale bars, 1 mm. De., Decidua; La., Labyrinth. Yellow dotted line demarks junctional zone from decidua.
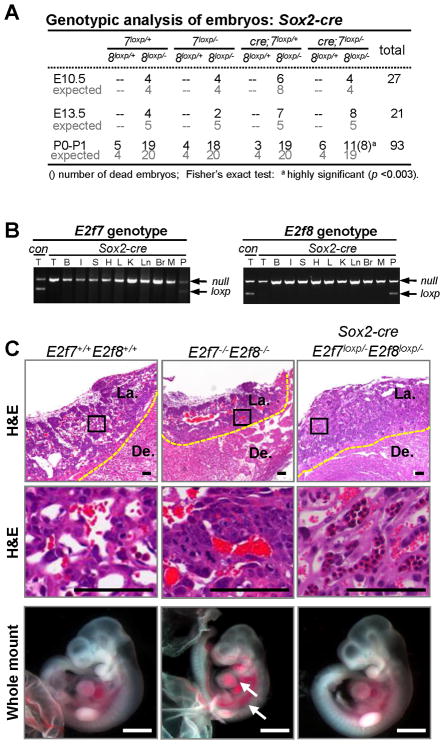
To determine whether fetal functions of E2F7 and E2F8 are also required for embryonic development, E2f7loxp/loxp;E2f8loxp/loxp and Sox2-cre;E2f7+/−;E2f8+/− mice were interbred and progeny analyzed at various stages of embryonic and postnatal development. Transgenic Sox2-cre mice express cre in all cells of the inner cell mass (embryo proper) with no expression in the trophoblast or extra-embryonic lineages (Hayashi et al., 2002). From these intercrosses we recovered live Sox2-cre;E2f7loxp/−;E2f8loxp/− fetuses at every embryonic stage analyzed, including at birth (P0), although most of these newborn pups died within their first day of life (Figure 4A and S3C). The specific deletion of E2f7 and E2f8 in fetal tissues was confirmed by PCR genotyping and X-Gal staining of Sox2-cre;E2f7loxp/−;E2f8loxp/−;Rosa26+/loxp embryonic tissues (Figure 4B and S3A–B). Double mutant E10.5 fetuses supplied with a wild type placenta (Sox2-cre;E2f7loxp/−;E2f8loxp/−) appeared normal and lacked the severe developmental phenotypes characteristic of E10.5 E2f7−/−;E2f8−/− embryos (Figure 4C). Together, these data suggest that the disruption of E2f7 and E2f8 in trophoblast progenitor cells is most likely the defining event causing mid-gestation lethality of E2f7−/−;E2f8−/− embryos. From these genetic analyses, we conclude that extra-embryonic functions of E2f7 and E2f8 are necessary and sufficient for embryonic development.
Figure 4. Loss of E2f7 and E2f8 in the Embryonic Compartment is Compatible with Intrauterine Life of the Fetus.
(A) Genotypic analysis of embryos derived from intercrosses of E2f7loxp/loxp;E2f8loxp/loxp and Sox2-cre;E2f7+/−;E2f8+/− mice. (B) Representative PCR genotyping analyses of E2f7 and E2f8 in different organs of Sox2-cre; E2f7loxp/−;E2f8loxp/− P0 pups. T, tail; B, bladder; I, intestine; S, stomach; H, heart; L, liver; K, kidney; Ln, lung; Br, brain; M, muscle; P, placenta. (C) Representative low and high magnification images of H&E stained E10.5 placental sections (top two rows) and gross appearance of associated fetuses (bottom) with the indicated genotypes. Note that wild type and double mutant controls are shown for comparison. Arrows indicate dilated vasculatures and hemorrhagic areas. H&E scale bars, 100 μm; whole mount scale bars, 1 mm. De., Decidua; La., Labyrinth. Yellow dotted line demarks junctional zone from decidua.
Identification of E2F7 and E2F8 target genes
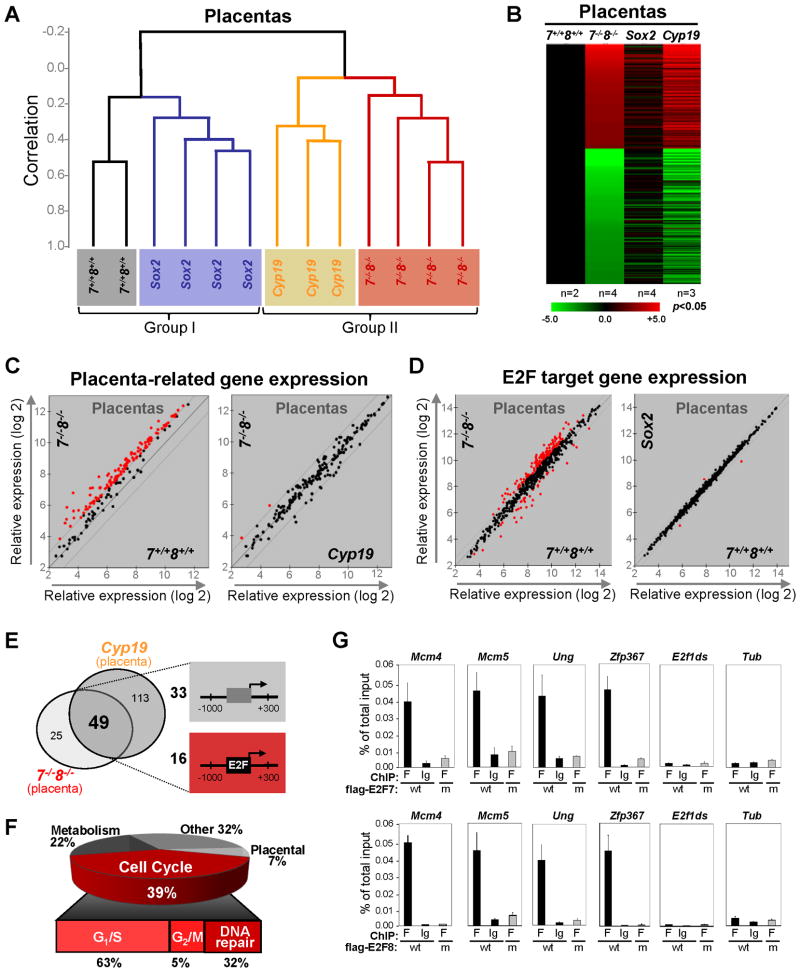
In an attempt to reveal the molecular events regulated by E2f7 and E2f8 we compared gene expression profiles in E10.5 placentas derived from four genetic groups, E2f7+/+;E2f8+/+, E2f7−/−;E2f8−/−, Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp and Sox2-cre;E2f7loxp/−;E2f8loxp/−. As expected, unsupervised clustering of gene expression separated the four placental cohorts into two groups based on the presence or absence of E2F7/E2F8 proteins, with placentas from E2f7+/+;E2f8+/+ and Sox2-cre cohorts clustering together in one group (Group I), and doubly deficient placentas from E2f7−/−;E2f8−/− and Cyp19-cre cohorts clustering in the other (Group II; Figure 5A). The heat maps and scatter plots shown in Figures 5B–D further highlight the similar expression profiles between E2f7−/−;E2f8−/− and Cyp19-cre placentas and between E2f7+/+;E2f8+/+ and Sox2-cre placentas.
Figure 5. Sox2-cre Fetuses and Cyp19-cre Placentas Recapitulate Molecular Events in their Germline-deleted Counterparts.
(A) Dendogram of unsupervised hierarchical clustering analysis of gene expression from E10.5 placentas with the indicated genotypes. Note the segregation of placentas based on the genotypes. Sox2, Sox2-cre;E2f7loxp/−;E2f8loxp/−; Cyp19, Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp; 7−/−;8−/−, E2f7−/−;E2f8−/−; 7+/+;8+/+, E2f7+/+;E2f8+/+. (B) Heat maps of genes with >2-fold misregulation in Cyp19, Sox2 or E2f7−/−;E2f8−/− placentas relative to E2f7+/+;E2f8+/+ placentas (p<0.05). n, number of placentas analyzed per genetic group. (C) Scatter plot analyses of placenta-related gene expression and E2F target gene expression in placentas with indicated genotypes using a 2-fold cutoff (red, >2-fold deregulation). (D) Scatter plot analyses of E2F target gene expression in placentas with indicated genotypes using a 1.5-fold cutoff (red, >1.5-fold deregulation). (E) Venn diagram depicting the overlap in genes significantly upregulated (>2-fold, p<0.05) in E2f7−/−E2f8−/− and Cyp19 placentas (left panel). Schematic diagram of two types of representative promoters are depicted on the right (−1000bp to +300bp relative to transcriptional start site) of the 49 genes shared between two placental sets. 16 of 49 promoters have at least one E2F binding site conserved between mice and humans. (F) Gene ontology of the 49 overlapping genes in E. (G) Chromatin immunoprecipitation (ChIP) assays confirming promoter occupancy by E2F7 and E2F8 in a subset of the 16 potential E2F targets from E. HEK293 cells overexpressing either flag-tagged versions of wild type E2F7 and E2F8 (wt), or DNA binding mutant E2F7 and E2F8 (m) were used. Quantitative RT-PCR (normalized to 1% of input) was performed using primers specific to the E2F binding sites in target gene promoters as well as to irrelevant sequences in the tubulin promoter (Tub) and in the E2f1 downstream coding region (E2f1ds).
Given the known roles of E2F7 and E2F8 in transcriptional repression, we focused on the analysis of upregulated genes in placentas lacking E2f7/E2f8. The Venn diagram shown in Figure 5E depicts the overlap of genes upregulated in E2f7−/−;E2f8−/− and Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp placentas (p>0.05 and >2-fold). Querying the TFsearch engine revealed that 16 out of the 49 promoters derepressed in E2f7/E2f8-deficient placentas contain consensus E2F binding sites that are conserved between mouse and human (Figure 5E, Table S3 and S4). Most of these 16 potential targets encode proteins with functions related to the control of G1-S specific events, whereas derepressed targets lacking canonical E2F binding sites encode proteins known to be associated with metabolic and placental processes (Figure 5F and Tables S3). Although ChIP studies of E2F7/8 target genes have been performed in human cells, to our knowledge, none of the available antibodies are ChIP-grade in mouse cells. As a preliminary means of validating candidate E2F7/8 target genes, we overexpressed flag-tagged versions of E2F7 and E2F8 in human embryonic kidney cells (HEK 293) and performed ChIP assays using anti-flag antibodies. These assays showed that anti-flag, but not control IgG antibodies, could co-immunoprecipitate promoter sequences of most of the 16 genes with E2F binding elements, but could not co-immunoprecipitate irrelevant sequences lacking E2F binding sites (downstream (ds) extronic sequences of E2f1 and Tubulin (Tub)) (Figure 5G). Moreover, parallel ChIP assays with HEK 293 cells expressing mutant versions of E2F7 and E2F8 lacking DNA-binding capacity confirmed the specificity of these assays (Figure 5G). From these results we conclude that many of the placental E2F target genes containing E2F binding sites in their promoters identified here by expression profiling represent good candidate targets of E2F7 and E2F8. Whether targets lacking canonical E2F bindings may be directly regulated by E2F7/E2F8, as it would appear for E2F1 (Cao et al., 2011; Rabinovich et al., 2008), remains to be determined.
Cell Non-autonomous Functions of E2F7 and E2F8 in the Placenta Dictate Molecular Events in the Fetus
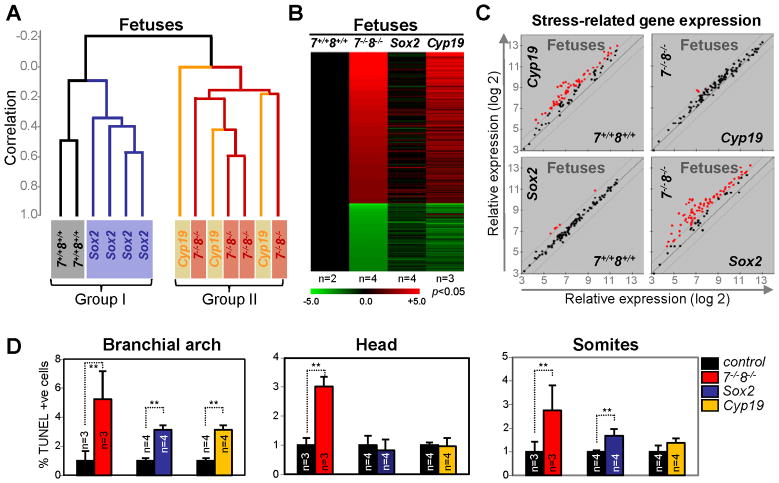
We then evaluated the extent to which E2F7 and E2F8 in the placenta influences gene expression in fetal tissues. Once again, unsupervised clustering analysis of E10.5 fetal expression profiles derived from E2f7+/+;E2f8+/+, E2f7−/−;E2f8−/−, Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp and Sox2-cre;E2f7loxp/−;E2f8loxp/− embryos separated samples into two groups, with E2f7+/+;E2f8+/+ and Sox2-cre;E2f7loxp/−;E2f8loxp/− fetuses clustering in one group (Group I) and E2f7−/−;E2f8−/− and Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp fetuses clustering in the other (Group II, Figure 6A). This clustering is remarkable given that samples within each of the two groups represent fetuses that have opposite genotypes. The common feature among samples within each group is the presence (Group I) or absence (Group II) of E2F7/E2F8 proteins in their associated placentas.
Figure 6. Cell Autonomous and Non-cell Autonomous Functions of E2f7 and E2f8 in the Fetus.
(A) Dendogram of unsupervised hierarchical clustering analysis of gene expression from of E10.5 fetuses with the indicated genotypes. Note that the segregation of these fetuses is based on the genotype of their placentas. Sox2, Sox2-cre;E2f7loxp/−;E2f8loxp/−; Cyp19, Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp; 7−/−;8−/−, E2f7−/−;E2f8−/−; 7+/+;8+/+, E2f7+/+;E2f8+/+. (B) Heat maps of genes with >2-fold misexpression in Sox2, Cyp19 and E2f7−/−;E2f8−/− fetuses relative to E2f7+/+;E2f8+/+ fetuses (p<0.05). n, number of fetuses analyzed per genetic group. (C) Scatter plot analyses of stress-related gene expression in the fetuses with indicated genetic groups using a 2-fold cutoff (red, >2-fold deregulation). (D) Quantification of apoptotic (TUNEL-positive cells) in E10.5 fetal tissues. E2f7−/−E2f8−/− (red, previously published in Li et al., 2008, included here for comparison); Sox2-cre;E2f7loxp/−;E2f8loxp/− (blue); Cyp19-cre; E2f7loxp/loxp;E2f8loxp/loxp (yellow); littermate controls (black, E2f7+/+E2f8+/+, E2f7loxp/−;E2f8loxp/− or E2f7loxp/loxp;E2f8loxp/loxp, respectively; ** p<0.02).
Consistent with these observations, the marked over-representation of genes related to nutritional and hypoxic stress responses (42 of 88 highly upregulated genes) in E2f7−/−;E2f8−/− embryos was almost completely alleviated in E2f7/E2f8 deficient fetuses supplied with a wild type placenta (Sox2-cre;E2f7loxp/−;E2f8loxp/−). Conversely, loss of E2f7 and E2f8 in the placenta (Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp) was sufficient to drive the expression of stress related programs in otherwise wild type fetuses, highlighting the fact that vast majority of the molecular and cellular events in double mutant fetuses are an indirect consequence to placental defects (Figure 6B–C and S4A).
E2F7 and E2F8 functions in the placenta also contribute to the massive programmed cell death observed in E2f7−/−;E2f8−/− fetuses. In contrast to E2f7−/−;E2f8−/− fetuses where apoptosis is rampant in all tissues examined, TUNEL assays identified few apoptotic cells in head regions and a moderate but significant number of apoptotic cells in the branchial arch and somites of either Sox2-cre;E2f7loxp/−;E2f8loxp/− or Cyp19-cre;E2f7loxp/loxp;E2f8loxp/loxp fetuses (Figure 6D). While these data clearly demonstrate that placental failure strongly contributes to programmed cell death in associated fetuses, they also identify cell autonomous functions of E2F7 and E2F8 that promote cell survival.
As described in Figure 4, supplying a wild type placenta to E2f7/E2f8 deficient fetuses (Sox2-cre;E2f7loxp/−;E2f8loxp/−) carried embryos to term, but these rescued pups inevitably died within a few days of life. Examination of organs in one-day old Sox2-cre rescued pups revealed significant ectopic proliferation and apoptosis of pulmonary epithelial cells, which was confirmed by TTF-1 and CC10 specific IF to represent Clara cells (bronchioles; Figure S3D–F). While the hypercellularity of pulmonary cells was obvious upon visual inspection of H&E stained lung sections (Figure S3G), other organs did not appear overtly hyperplastic (data not shown). Based on these observations, we propose that proliferative imbalances leading to the cellular crowding of airways, defective pulmonary function, lung collapse and asphyxiation may be the major cause of lethality in Sox2-cre rescued new-born pups, but further studies are needed to fully evaluate this and other possibilities.
Loss of E2f3a Rescued Placental Defects and Lethality of E2f7−/−;E2f8−/− Fetuses
We then considered potential mechanisms for how E2F7 and E2F8 might regulate gene expression. The observation that E2F target genes are upregulated in tissues lacking E2f7 and E2f8 suggested a simple mechanism involving transcriptional repression. However, it remained possible that other E2Fs could participate in regulating the same target genes, particularly in the absence of E2F7 and E2F8 function. Indeed, recent gene knockout studies of E2F family members in mice have revealed incredible cooperation, redundancy and cross-regulation among E2F family members (Chen et al., 2009; Chong et al., 2009b; Li et al., 2008; Trikha et al., 2011; Tsai et al., 2008).
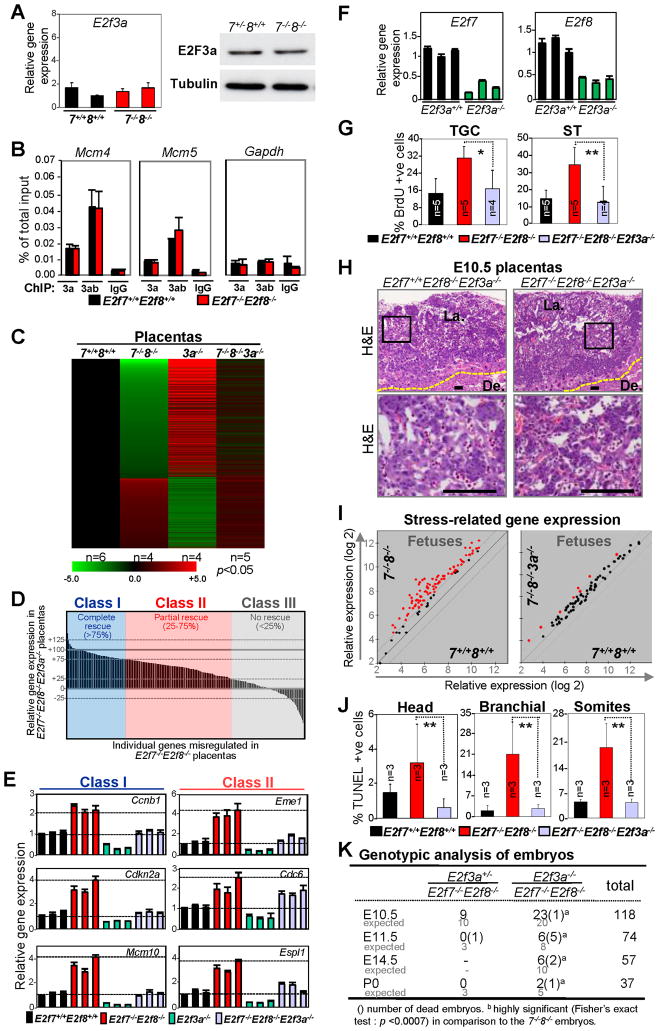
Given the established role for E2F3a in extra-embryonic tissues (Chong et al., 2009a), we explored the functional relationship between E2F7/E2F8 and E2F3a in placental development. Quantitative RT-PCR and Western blot assays showed that the levels of E2f3a mRNA and its protein product were not altered in E2f7−/−E2f8−/− placental tissues (Figure 7A), suggesting that E2f3a is unlikely to be a direct target of E2F7/E2F8-mediated repression. Moreover, ChIP assays demonstrated significant occupancy of E2F3a on E2F7/E2F8 target promoters, which was not affected by the absence of E2f7 and E2f8 (Figure 7B, red bars). We then entertained the possibility that E2F3a and E2F7/E2F8 may act in parallel to regulate the expression of target promoters and thus analyzed gene expression profiles in E2f3a−/− animals. Remarkably, greater than ninety percent of targets differentially expressed in E2f7/E2f8-deficient tissues were also differentially expressed in E2f3a-deficient tissues, but in the opposite direction (Figure 7C and S5A), suggesting that E2F3a and E2F7/E2F8 activate and repress, respectively, an overlapping set of targets.
Figure 7. Loss of E2f3a Rescues Placental Defects, Fetal Apoptosis and Prolongs Viability of E2f7−/−E2f8−/− Embryos.
(A) Quantitative RT-PCR of E2f3a mRNA levels in E10.5 E2f7+/+;E2f8+/+ (7+/+8+/+; n=2) and E2f7−/−;E2f8−/− (7−/−8−/−; n=2) placentas (left panel); western blot analyses of lysates from E10.5 E2f7+/+;E2f8+/+ (7+/+8+/+) and E2f7−/−;E2f8−/− (7−/−8−/−) placentas (right panel). (B) ChIP assays using cell lysates from proliferating E2f7+/+;E2f8+/+ (black) and E2f7−/−;E2f8−/− (red) mouse embryonic fibroblasts using antibodies against E2F3a (3a), total E2F3 (3ab) and normal rabbit IgG (IgG). Quantitive PCR (normalized to 1% of input) was performed with primers flanking the E2F binding sites on the indicted promoters. (C) Heat maps of genes with >2-fold misexpression in E2f7−/−;E2f8−/− (7−/−8−/−), E2f3a−/− (3a−/−), or E2f7−/−;E2f8−/−;E2f3a−/− (7−/−8−/−3a−/−) placentas relative to E2f7+/+;E2f8+/+ (7+/+8+/+) counterparts (p<0.05). (D) Waterfall plot representing degree of gene expression rescue in E2f7−/−;E2f8−/−;E2f3a−/− placentas for 285 genes (>2-fold, p<0.05) that are deregulated in E2f7−/−;E2f8−/− placentas. Data shown as percentage change in average expression between E2f7−/−;E2f8−/−;E2f3a−/− and E2f7−/−;E2f8−/− placentas relative to E2f7+/+;E2f8+/+ placentas. Three classes of targets are noted, with Class I representing genes that are rescued by more than 75% relative to wild type levels, Class II representing genes that are rescued by 25–75%, and Class III representing genes that are rescued by less than 25%. (E) Quantitative RT-PCR confirmation of expression changes in a subset of genes in D Values are normalized to Gapdh levels and wild type control sample with lowest expression was normalized to 1. (F) Relative expression of E2f7 and E2f8 in E2f3a+/+ and E2f3a−/− E10.5 whole placentas by quantitative RT-PCR assays. Values are normalized to Gapdh levels and wild type control sample with lowest expression was normalized to 1. (G) Quantification of BrdU-positive cells in trophoblast giant cells (TGC) and spongiotrophoblast cell (ST) lineages with the indicated genotypes. (* p<0.02, ** p<0.003). (H) Representative low and high magnification H&E images of E10.5 placental tissues with the indicated genotypes. Scale bars, 100 μm. De., Decidua; La., Labyrinth. Yellow dotted line demarks junctional zone from decidua. (I) Scatter plot analyses of stress-related gene expression in the fetuses with indicated genetic groups using a 2-fold cutoff (red, >2-fold deregulation). (J) Quantification of TUNEL-positive cells in the indicated tissues of E10.5 E2f7−/−;E2f8−/−E2f3a−/−, E2f7−/−;E2f8−/− and E2f7+/+;E2f8+/+ fetuses (** p<0.003). (K) Genotypic analysis of embryos derived from intercrosses of E2f7+/−;E2f8+/−;E2f3a+/− and/or E2f7+/−;E2f8−/−;E2f3a+/− mice at the indicated stages of development.
To rigorously test the hypothesis that E2F3a and E2F7/E2F8 represent two antagonistic arms of the same E2F program, we interbred E2f7+/−;E2f8+/− and E2f3a−/− animals and analyzed gene expression in the resulting progeny. Surprisingly, expression profiling showed that a significant number of differentially expressed genes shared between E2f7/E2f8 doubly deficient and E2f3a deficient E10.5 placentas, including most E2F target genes, were expressed to normal or near-normal levels in triple deficient embryos (Figure 7C and S5B). The water fall plot in figure 7D depicts the extent to which loss of E2f3a normalizes the expression of 285 gene targets, with some targets being completely rescued (Class I), others partially rescued (Class II) and a small subset not rescued at all (Class III). Quantitative RT-PCR confirmed the complete or partial rescue of gene expression to wild type levels (Figure 7E). We interpret these results to mean that the high levels of E2F target genes observed in E2f7/E2f8 doubly deficient cells is due to the additive effects of derepression and E2F3a-mediated activation.
Interestingly, loss of E2f3a led to downregulation of E2f7 and E2f8 mRNA levels, suggesting that E2F3a may directly activate the expression of these two atypical repressors (Figure 7F). Indeed previous work has demonstrated that E2f7/E2f8 expression is regulated by E2F binding sites in their promoters (de Bruin et al., 2003; Maiti et al., 2005). These results suggest that E2F3a mediated accumulation of E2F7/E2F8 proteins later in S-phase coordinates the timely activation and repression of E2F targets during cell cycle. This coordinated control of E2F target expression by E2F3a and E2F7/E2F8 is of biological importance because loss of E2f3a significantly suppressed the ectopic DNA replication caused by a deficiency in E2f7 and E2f8 (Figure 7G). Indeed, E2f7−/−;E2f8−/−;E2f3a−/− placentas appeared normal with well-organized labyrinthine architecture and vasculature (Figure 7H). These placentas were apparently functional since associated fetuses lacked any signs of an acute stress response and had no evidence of ectopic programmed cell death (Figure 7I–J). Remarkably, we were able to recover live E2f7−/−;E2f8−/−;E2f3a−/− embryos at every embryonic stage analyzed, including at birth (Figure 7K and S5C). Together, these findings suggest that E2F3a is a key modulator that antagonizes E2F7 and E2F8 mediated repression through activation of the same transcriptional program, which is critical for placental development.
DISCUSSION
The atypical repressors E2F7 and E2F8 form the most ancient arm of the E2F family of transcription factors. The classic repressors E2F4–6, unlike E2F7 and E2F8, associate with pocket proteins and dimerization proteins (DP1/DP2) and are widely viewed as the major E2F repressive arm that drives cell cycle exit and differentiation. In this study we demonstrate that the function of atypical repressors in extra-embryonic tissues is essential for repressing a broad spectrum of E2F targets that control trophoblast proliferation, placental development and embryonic viability. Importantly, these studies uncover a transcriptional program that is coordinated by two opposing arms of the E2F family, the activating E2Fs and the two atypical E2Fs, and highlight an unanticipated feed-forward loop between these two arms to ensure orderly cell cycle progression.
The placenta is one physiological context that has provided insightful clues into the functions of the Cdk-Rb-E2F pathway in mammals (Geng et al., 2003; Kohn et al., 2003; Kohn et al., 2004; Kozar et al., 2004; Malumbres et al., 2004; Parisi et al., 2003; Wenzel et al., 2007). For example, genetic ablation of p57KIP, p21CIP1, Cyclin E1/E2, Rb and DP1, has been shown to result in extra-embryonic defects that contribute to the lethality of mutant embryos (Geng et al., 2003; Kohn et al., 2004; Wenzel et al., 2007). Previous work from our lab demonstrated that the targeted disruption of E2f7 and E2f8 in mice leads to lethality by embryonic age 11.5, and consistent with their high expression in placental tissues, we now show that double mutant embryos exhibit severe placental abnormalities. Using tissue specific gene ablation strategies we identified disruption of E2F7 and E2F8 functions in trophoblast progenitor cells of the placenta as the likely underlying cause of lethality in E2f7−/−;E2f8−/− embryos. Indeed, supplying mutant fetuses with a wild type placenta was sufficient to carry these embryos to birth and beyond. It would thus appear that two parallel E2F regulatory pathways, one dependent (E2F1–3) and the other independent (E2F7–8) on the canonical Cdk-Rb-E2F axis, are critically important in extra-embryonic trophoblast lineages. Thus, one reason for the increasing complexity of the metazoan E2F gene family during evolution, from three E2Fs in worms and two in flies to at least eight in mammals, may reflect divergent cell cycle requirements in extra-embryonic versus fetal tissues (van den Heuvel and Dyson, 2008; Winn et al., 2011).
Previous work showed that the early lethality of E2f7−/−;E2f8−/− embryos is accompanied by massive apoptosis throughout the fetus. Data presented here demonstrates that defective placental function contributes to fetal programmed cell death. Additional genetic studies, however, showed that the concomitant loss of E2f1 or p53 is able to suppress apoptosis in multiple organs of E2f7/E2f8 deficient embryos, but this occurs without correcting placental abnormalities or extending fetal viability past E11.5 (unpublished results; Li et al., 2008). Thus, cell autonomous mechanisms involving E2f1 and p53 and cell non-autonomous mechanisms resulting from placental abnormalities both contribute to the massive ectopic apoptosis observed in E2f7−/−;E2f8−/− fetuses.
Elegant work in flies has demonstrated antagonistic roles for dE2f1 and dE2f2 in the control of cell proliferation and larval development (Ambrus et al., 2007; Cayirlioglu et al., 2003; Frolov et al., 2001; Frolov et al., 2003; Weng et al., 2003). While it was shown that in some cases dE2F1 and dE2F2 regulate the same target genes (including many cell cycle regulatory genes) through activation and repression, respectively, by and large these two fly E2Fs regulate distinct sets of targets whose functions converge, in ways not completely understood, on key developmental processes (Cayirlioglu et al., 2003; Dimova et al., 2003; Stevaux et al., 2005). It is generally viewed that mammalian activators (E2F1, E2F2 and E2F3) and classic repressors (E2F4, E2F5 and E2F6) also work together to orchestrate the expression of E2F target genes during the cell cycle. However, surprisingly little evidence in vivo exists to support the notion that these two subsets of mammalian E2Fs regulate the same set of target genes or even function in the same tissues. Indeed, mice deficient for each E2F activator or canonical repressor display phenotypes with distinct tissue involvement that are conspicuously devoid of major cell cycle alterations (Chen et al., 2009; Chong et al., 2009b; Cloud et al., 2002; Danielian et al., 2008; Field et al., 1996; Humbert et al., 2000; Kinross et al., 2006; Li et al., 2003; Lindeman et al., 1998; Murga et al., 2001; Pohlers et al., 2005; Rempel et al., 2000; Yamasaki et al., 1996). While redundant functions among family members may explain the absence of clear-cut cell cycle related phenotypes in these mice, it is possible and perhaps likely that the expected outcomes were overly reliant on previous results obtained from cell culture systems. We show here that a single activator (E2F3a) and the two atypical repressors (E2F7/E2F8) are utilized in vivo to co-regulate cell proliferation. Using systematic analyses of global gene expression profiles and biochemical approaches, we demonstrate that E2F7 and E2F8 directly repress a subset of G1-S genes that control S-phase entry in trophoblast lineages. Surprisingly, microarray gene expression analysis of E2f3a deficient placentas revealed a significant decrease in most of these same target genes. This observation is remarkable given the divergent nature of their DNA binding domains, with E2F3a utilizing inter-molecular interactions with its partner protein DP (Zheng et al., 1999) and E2F7/E2F8 utilizing both inter- and intra-molecular interactions between its two DNA binding domains (Di Stefano et al., 2003; Logan et al., 2005; Maiti et al., 2005). The fact that expression of target genes in E2f7, E2f8 and E2f3a triple deficient placentas was partially or completely restored to wild type levels, and even more surprising that these mutant embryos now survived to term, suggests that these three E2Fs coordinately regulate the expression of genes that are physiologically meaningful for embryonic development.
But how might these two arms of E2Fs co-regulate gene expression in vivo? Two possibilities may be ruled out based on key observations. First, bioinformatics analysis of target promoter sequences identified here shows that most targets only have a single E2F consensus binding site, and therefore activators and atypical repressors are unlikely to simultaneously bind and co-regulate these target promoters. Second, competitive binding of E2F activators and repressors to the same consensus sequences is also unlikely since the expression and binding of E2F3a protein to these sequences was unaffected by the genetic inactivation of E2f7 and E2f8. Several clues suggest instead that the basis for how E2F3a and E2F7/E2F8 proteins coordinate gene expression is dependent on their temporal occupancy on target promoters during the cell cycle. Studies performed in multiple cell lines have shown that E2F3a protein levels peak at G1/S, followed by a precipitous drop in early-mid S phase (Ishida et al., 2001; Leone et al., 1998). In contrast, levels of E2F7 and E2F8 begin to increase in mid-late S phase, peak in G2 and decline as cells approach M and the next G1 phase (de Bruin et al., 2003; Maiti et al., 2005). The cell cycle dependent accumulation of E2F7 and E2F8 proteins in cell lines is consistent with our IHC of placentas showing that ~30% of trophoblast nuclei stain strongly positive for these two proteins at any given time point. The sequential accumulation of E2F3a followed by E2F7/E2F8 proteins during the cell cycle is likely no coincidence, since we show here that loss of E2f3a leads to a decrease in E2f7 and E2f8 expression. These observations support a mechanism where the loading of E2F3a on target promoters at G1/S leads to their activation, including that of E2f7 and E2f8. As atypical E2F protein levels increase in mid-to-late S phase and E2F3a protein is targeted for degradation (Leone et al., 1998), G1/S targets become efficiently repressed by E2F7 and E2F8, leading to their decline by G2/M. Thus, the E2F3a-dependent activation of E2f7 and E2f8 may be viewed as a mechanism to ensure that rhythmic waves of E2F-dependent activation and repression drive cell cycle dependent gene expression. Whether E2F3a directly activates E2f7 and E2f8 promoters is not yet clear, but certainly is an attractive possibility.
While not all questions have been answered, it is certain from our analyses of single, double and triple knockout mice that activator and atypical repressor arms of the E2F program coordinately regulate cell cycle dependent gene expression in vivo, and that this has a profound impact on cell proliferation and embryonic development. We thus suggest a paradigm for how E2F targets are regulated, which contrary to current belief does not generally involve classical E2F repressors, but rather involves repression by the most ancient and ironically termed ‘atypical’ E2Fs. In this view, cell cycle dependent gene expression requires the balanced and timely interplay between Rb-regulated E2F activators and Rb-independent atypical repressors.
EXPERIMENTAL PROCEDURES
Mouse Strains and Genotyping
Transgenic mice used for this study were maintained in a mixed 129SvEv; C57BL/6; FVB background. Allele-specific (E2f7/8, Rosa26, PlfCre, and Pl1Cre) and transgene specific primers (Sox2-cre and Cyp19-cre) were used for PCR genotyping (Hayashi et al., 2002; Li et al., 2008; Soriano, 1999; Wenzel and Leone, 2007); manuscript in preparation).
Histology, Immunostaining and Quantification
Standard protocols were used for preparation of 5μm thick paraffin embedded sections of placentas and for hematoxylin and eosin staining. For immunohistochemistry, primary antibodies against E2F7 (ab56022, Abcam), E2F8 (custom-made polyclonal antibody raised against a peptide representing amino acids 576–595 of murine E2F8, Quality Controlled Biochemicals), Esx1 (sc-133566, Santa Cruz), P-H3 (06–570, Millipore), BrdU (MO-0744, DAKO), TTF-1 (sc-13040, Santa Cruz), CC10 (sc-25555, Santa Cruz) and PL-1 (a gift from Dr. F. Talamantes) were used. Pregnant mice at 10.5 days postcoitum were given intraperitoneal injections of BrdU (100 μg/grams of body weight) 30 min prior to harvesting. Detection of primary antibodies was done using species specific biotinylated secondary antibodies along with Vectastain Elite ABC reagent (Vector labs) and DAB peroxidase substrate kit (Vector labs) or fluorophore (Alexa Fluor, Invitrogen) conjugated secondary antibodies. Nuclear counterstaining was done using either hematoxylin or DAPI. Apoptotic cells were detected using TUNEL (S7101, Millipore) assays, performed according to the manufacturer’s protocol. Images of immunostained sections were captured using Eclipse 50i (Nikon) and Axioskop 40 (Zeiss) microscopes and positive cells were quantified using Metamorph Imaging 6.1 software. Three sections per sample and at least three different samples for each genotype were analyzed. Data is reported as the average ± SD of percentage of positive cells.
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using Qiagen RNA miniprep columns with in-column DNase treatment according to the manufacturer’s protocol. Reverse transcription of total RNA was performed using Superscript III reverse transcriptase (Invitrogen) and RNAse Inhibitor (Roche) as described by the manufacturer. Quantitative PCR was performed using SYBR Green reaction mix (BioRad) and the BioRad iCycler PCR machine. All reactions were performed in triplicate and relative amounts of cDNA were normalized to Gapdh. Data is reported as the average ± SD fold induction.
In situ Hybridization
In situ hybridization was performed on E9.5 (Proliferin) and E10.5 (Tpbp) placenta sections using a previously reported protocol (Christensen et al., 2002) modified (deparaffinization in xylene and Proteinase K digestion) for paraffin-embedded sections. Plasmids for Proliferin and Tpbp (gifts from Dr. J. Rossant) were linearized with HindIII and XbaI, respectively, to generate templates for riboprobe synthesis. Hybridizations were performed with 1×107 dpm/slide of radiolabeled probes generated by in vitro transcription with either T7 (Proliferin) or T3 (Tpbp) RNA polymerase (Roche) using both 35S-CTP and 35S-UTP. Autoradiography emulsion NTB (Kodak) was applied to the slides and the emulsion was exposed for 1 day (Proliferin) or 3 days (Tpbp) before being developed.
Affymetrix Microarray Analysis
Total RNA was isolated using Qiagen RNA miniprep columns according to the manufacturer’s protocol. Global gene expression analyses were performed on Affymetrix Mouse Genome 430 2.0 arrays at the Ohio State University Comprehensive Cancer Center (www.osuccc.osu.edu/microarray/). Expression values were adjusted by quantile normalization and log2 transformation with RMAExpress and were analyzed using BRB-ArrayTools 3.7.0 (http://linus.nci.nih.gov/BRB-ArrayTools.html). Class comparison was used to select genes differentially expressed at a significance level of p<0.05. Probes with a >2-fold misexpression in E2f7−/−;E2f8−/− when compared to E2f7+/+;E2f8+/+ were used and the average relative expression level of each genetic group was used to generate heatmaps. Clustering and scatter plot analyses were performed by using functions of BRB Array Tools. Promoter sequences of each gene (−1000bp to +300bp relative to transcriptional start site) were obtained from UCSC genome browser (http://genome.ucsc.edu/). TFSearch (http://www.cbrc.jp/research/db/TFSEARCH.html) aided in the identification of genes containing E2F consensus binding sites (Heinemeyer et al., 1998).
Chromatin Immunoprecipitation (ChIP)
The EZ CHIP assay kit (Upstate Biotech) was used as described by the manufacturer. Primary antibodies used were anti-flag (M2, Sigma) and normal mouse IgG (Oncogene) antibodies [E2F7 and E2F8 ChIP] or anti-E2F3a (N-20, Santa Cruz), anti-E2F3 (C-18, Santa Cruz) and normal rabbit IgG (Oncogene) [E2F3a ChIP]. Quantitive PCR was performed on de-crosslinked and column purified (Qiaquick, Qiagen) DNA fractions using the Bio-Rad iCycler system with primers specific for the indicated promoter regions. Reactions were performed in triplicate and normalized using the threshold cycle number for the 1% of total input sample. Data is reported as the average ± SD fold change.
Statistical Analysis
Pairwise comparisons of quantifications from histology and immunohistochemistry samples were evaluated by two-tailed Student’s T-test. Statistical analysis of viability of embryos harvested from timed pregnancies was done by Fisher’s exact test.
Supplementary Material
HIGHLIGHTS.
E2F7/8 in trophoblast progenitor cells are necessary and sufficient for embryogenesis
E2F activators and atypical repressors coordinate gene expression
Development of extra-embryonic lineage-specific cre mice
Acknowledgments
We are grateful for technical assistance provided by J. Moffitt, L. Rawahneh, J. Opavska, K. Wolken and B. Kemmenoe. We also thank Dr. F. Talamantes for generously providing PL-1 antibodies, Dr. J. Rossant for pG-Proliferin and pKS-Tpbp plasmids, Dr. Kathryn Wikenheiser-Brokamp for help with analyzing lung phenotypes and Dr. Mark Parthun and Dr. Stephen Osmani for critical reading of manuscript. This work was funded by NIH grants to G.L. (R01CA85619, R01CA82259, R01HD047470, P01CA097189). H-Z.C. and T.P. are recipients of Pelotonia Fellowships. G.L. is the recipient of The Pew Charitable Trust Scholar Award and the Leukemia & Lymphoma Society Scholar Award.
Footnotes
GEO Accession Code for microarray raw files
GSE30488
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrus AM, Nicolay BN, Rasheva VI, Suckling RJ, Frolov MV. dE2F2-independent rescue of proliferation in cells lacking an activator dE2F1. Molecular and Cellular Biology. 2007;27:8561–8570. doi: 10.1128/MCB.01068-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwooll C, Lazzerini Denchi E, Helin K. The E2F family: specific functions and overlapping interests. EMBO J. 2004;23:4709–4716. doi: 10.1038/sj.emboj.7600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao AR, Rabinovich R, Xu M, Xu X, Jin VX, Farnham PJ. Genome-wide analysis of transcription factor E2F1 mutant proteins reveals that N- and C-terminal protein interaction domains do not participate in targeting E2F1 to the human genome. J Biol Chem. 2011;286:11985–11996. doi: 10.1074/jbc.M110.217158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayirlioglu P, Ward WO, Key SCS, Duronio RJ. Transcriptional. repressor functions of Drosophila E2F1 and E2F2 cooperate to inhibit genomic DNA synthesis in ovarian follicle cells. Molecular and Cellular Biology. 2003;23:2123–2134. doi: 10.1128/MCB.23.6.2123-2134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Pacal M, Wenzel P, Knoepfler PS, Leone G, Bremner R. Division and apoptosis of E2f-deficient retinal progenitors. Nature. 2009;462:925–929. doi: 10.1038/nature08544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JL, Tsai SY, Sharma N, Opavsky R, Price R, Wu L, Fernandez SA, Leone G. E2f3a and E2f3b contribute to the control of cell proliferation and mouse development. Mol Cell Biol. 2009a;29:414–424. doi: 10.1128/MCB.01161-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JL, Wenzel PL, Saenz-Robles MT, Nair V, Ferrey A, Hagan JP, Gomez YM, Sharma N, Chen HZ, Ouseph M, et al. E2f1–3 switch from activators in progenitor cells to repressors in differentiating cells. Nature. 2009b;462:930–934. doi: 10.1038/nature08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen RN, Weinstein M, Tassava RA. Expression of fibroblast growth factors 4, 8, and 10 in limbs, flanks, and blastemas of Ambystoma. Dev Dyn. 2002;223:193–203. doi: 10.1002/dvdy.10049. [DOI] [PubMed] [Google Scholar]
- Cloud JE, Rogers C, Reza TL, Ziebold U, Stone JR, Picard MH, Caron AM, Bronson RT, Lees JA. Mutant mouse models reveal the relative roles of E2F1 and E2F3 in vivo. Molecular and Cellular Biology. 2002;22:2663–2672. doi: 10.1128/MCB.22.8.2663-2672.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan PM, Conroy N, Burton GJ, Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn. 2006;235:3280–3294. doi: 10.1002/dvdy.20981. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Friesenhahn LB, Faust AM, West JC, Caron AM, Bronson RT, Lees JA. E2f3a and E2f3b make overlapping but different contributions to total E2f3 activity. Oncogene. 2008;27:6561–6570. doi: 10.1038/onc.2008.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin A, Maiti B, Jakoi L, Timmers C, Buerki R, Leone G. Identification and characterization of E2F7, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem. 2003;278:42041–42049. doi: 10.1074/jbc.M308105200. [DOI] [PubMed] [Google Scholar]
- DeGregori J, Johnson D. Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Curr Mol Med. 2006;6:739–748. doi: 10.2174/1566524010606070739. [DOI] [PubMed] [Google Scholar]
- Di Stefano L, Jensen MR, Helin K. E2F7, a novel E2F featuring DP-independent repression of a subset of E2F-regulated genes. EMBO J. 2003;22:6289–6298. doi: 10.1093/emboj/cdg613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005;24:2810–2826. doi: 10.1038/sj.onc.1208612. [DOI] [PubMed] [Google Scholar]
- Dimova DK, Stevaux O, Frolov MV, Dyson NJ. Cell cycle-dependent and cell cycle-independent control of transcription by the Drosophila E2F/RB pathway. Genes Dev. 2003;17:2308–2320. doi: 10.1101/gad.1116703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin WG, Jr, Livingston DM, Orkin SH, Greenberg ME. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- Frolov MV, Dyson NJ. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J Cell Sci. 2004;117:2173–2181. doi: 10.1242/jcs.01227. [DOI] [PubMed] [Google Scholar]
- Frolov MV, Huen DS, Stevaux O, Dimova D, Balczarek-Strang K, Elsdon M, Dyson NJ. Functional antagonism between E2F family members. Genes Dev. 2001;15:2146–2160. doi: 10.1101/gad.903901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov MV, Stevaux O, Moon NS, Dimova D, Kwon EJ, Morris EJ, Dyson NJ. G1 cyclin-dependent kinases are insufficient to reverse dE2F2-mediated repression. Gene Dev. 2003;17:723–728. doi: 10.1101/gad.1031803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, Rideout WM, Bronson RT, Gardner H, Sicinski P. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119(Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, Ignatieva EV, Ananko EA, Podkolodnaya OA, Kolpakov FA, et al. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998;26:362–367. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert PO, Verona R, Trimarchi JM, Rogers C, Dandapani S, Lees JA. E2f3 is critical for normal cellular proliferation. Genes Dev. 2000;14:690–703. [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Huang E, Zuzan H, Spang R, Leone G, West M, Nevins JR. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol. 2001;21:4684–4699. doi: 10.1128/MCB.21.14.4684-4699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinross KM, Clark AJ, Iazzolino RM, Humbert PO. E2f4 regulates fetal erythropoiesis through the promotion of cellular proliferation. Blood. 2006;108:886–895. doi: 10.1182/blood-2005-09-008656. [DOI] [PubMed] [Google Scholar]
- Kohn MJ, Bronson RT, Harlow E, Dyson NJ, Yamasaki L. Dp1 is required for extra-embryonic development. Development. 2003;130:1295–1305. doi: 10.1242/dev.00355. [DOI] [PubMed] [Google Scholar]
- Kohn MJ, Leung SW, Criniti V, Agromayor M, Yamasaki L. Dp1 is largely dispensable for embryonic development. Mol Cell Biol. 2004;24:7197–7205. doi: 10.1128/MCB.24.16.7197-7205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Leone G, DeGregori J, Yan Z, Jakoi L, Ishida S, Williams RS, Nevins JR. E2F3 activity is regulated during the cell cycle and is required for the induction of S phase. Genes Dev. 1998;12:2120–2130. doi: 10.1101/gad.12.14.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FX, Zhu JW, Tessem JS, Beilke J, Varella-Garcia M, Jensen J, Hogan CJ, DeGregori J. The development of diabetes in E2f1/E2f2 mutant mice reveals important roles for bone marrow-derived cells in preventing islet cell loss. Proc Natl Acad Sci U S A. 2003;100:12935–12940. doi: 10.1073/pnas.2231861100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ran C, Li E, Gordon F, Comstock G, Siddiqui H, Cleghorn W, Chen HZ, Kornacker K, Liu CG, et al. Synergistic function of E2F7 and E2F8 is essential for cell survival and embryonic development. Dev Cell. 2008;14:62–75. doi: 10.1016/j.devcel.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman GJ, Dagnino L, Gaubatz S, Xu YH, Bronson RT, Warren HB, Livingston DM. A specific, nonproliferative role for E2F-5 in choroid plexus function revealed by gene targeting. Gene Dev. 1998;12:1092–1098. doi: 10.1101/gad.12.8.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan N, Graham A, Zhao XJ, Fisher R, Maiti B, Leone G, La Thangue NB. E2F-8: an E2F family member with a similar organization of DNA-binding domains to E2F-7. Oncogene. 2005;24:5000–5004. doi: 10.1038/sj.onc.1208703. [DOI] [PubMed] [Google Scholar]
- Maiti B, Li J, de Bruin A, Gordon F, Timmers C, Opavsky R, Patil K, Tuttle J, Cleghorn W, Leone G. Cloning and characterization of mouse E2F8, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem. 2005;280:18211–18220. doi: 10.1074/jbc.M501410200. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Murga M, Fernandez-Capetillo O, Field SJ, Moreno B, Borlado LR, Fujiwara Y, Balomenos D, Vicario A, Carrera AC, Orkin SH, et al. Mutation of E2F2 in mice causes enhanced T lymphocyte proliferation, leading to the development of autoimmunity. Immunity. 2001;15:959–970. doi: 10.1016/s1074-7613(01)00254-0. [DOI] [PubMed] [Google Scholar]
- Parisi T, Beck AR, Rougier N, McNeil T, Lucian L, Werb Z, Amati B. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 2003;22:4794–4803. doi: 10.1093/emboj/cdg482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlers M, Truss M, Frede U, Scholz A, Strehle M, Kuban RJ, Hoffmann B, Morkel M, Birchmeier C, Hagemeier C. A role for E2F6 in the restriction of male-germ-cell-specific gene expression. Current Biology. 2005;15:1051–1057. doi: 10.1016/j.cub.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Rabinovich A, Jin VX, Rabinovich R, Xu X, Farnham PJ. E2F in vivo binding specificity: comparison of consensus versus nonconsensus binding sites. Genome Res. 2008;18:1763–1777. doi: 10.1101/gr.080622.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel RE, Saenz-Robles MT, Storms R, Morham S, Ishida S, Engel A, Jakoi L, Melhem MF, Pipas JM, Smith C, et al. Loss of E2F4 activity leads to abnormal development of multiple cellular lineages. Molecular Cell. 2000;6:293–306. doi: 10.1016/s1097-2765(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304:567–578. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stevaux O, Dimova DK, Ji JY, Moon NS, Frolov MV, Dyson NJ. Retinoblastoma family 2 is required in vivo for the tissue-specific repression of dE2F2 target genes. Cell Cycle. 2005;4:1272–1280. doi: 10.4161/cc.4.9.1982. [DOI] [PubMed] [Google Scholar]
- Trikha P, Sharma N, Opavsky R, Reyes A, Pena C, Ostrowski MC, Roussel MF, Leone G. E2f1–3 are critical for myeloid development. J Biol Chem. 2011;286:4783–4795. doi: 10.1074/jbc.M110.182733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Opavsky R, Sharma N, Wu L, Naidu S, Nolan E, Feria-Arias E, Timmers C, Opavska J, de Bruin A, et al. Mouse development with a single E2F activator. Nature. 2008;454:1137–1141. doi: 10.1038/nature07066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel S, Dyson NJ. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol. 2008;9:713–724. doi: 10.1038/nrm2469. [DOI] [PubMed] [Google Scholar]
- Weng L, Zhu C, Xu J, Du W. Critical role of active repression by E2F and Rb proteins in endoreplication during Drosophila development. EMBO J. 2003;22:3865–3875. doi: 10.1093/emboj/cdg373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel P, Wu L, de Bruin A, Chong J, Chen W, Dureska G, Sites E, Pan T, Sharma A, Huang K, et al. Rb is critical in a mammalian tissue stem cell population. Genes Dev. 2007;21:85–97. doi: 10.1101/gad.1485307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel PL, Chong JL, Saenz-Robles MT, Ferrey A, Hagan JP, Gomez YM, Rajmohan R, Sharma N, Chen HZ, Pipas JM, et al. Cell proliferation in the absence of E2F1–3. Dev Biol. 2011;351:35–45. doi: 10.1016/j.ydbio.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel PL, Leone G. Expression of Cre recombinase in early diploid trophoblast cells of the mouse placenta. Genesis. 2007;45:129–134. doi: 10.1002/dvg.20276. [DOI] [PubMed] [Google Scholar]
- Winn J, Carter M, Avery L, Cameron S. Hox and a Newly-identified E2F Co-repress Cell Death in Caenorhabditis elegans. Genetics. 2011 doi: 10.1534/genetics.111.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85:537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- Zheng N, Fraenkel E, Pabo CO, Pavletich NP. Structural basis of DNA recognition by the heterodimeric cell cycle transcription factor E2F-DP. Gene Dev. 1999;13:666–674. doi: 10.1101/gad.13.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.