Abstract
NADP+-isocitrate dehydrogenase (NADP+-IDH; EC 1.1.1.42) is involved in the supply of 2-oxoglutarate for ammonia assimilation and glutamate synthesis in higher plants through the glutamine synthetase/glutamate synthase (GS/GOGAT) cycle. Only one NADP+-IDH form of cytosolic localization was detected in green cotyledons of pine (Pinus spp.) seedlings. The pine enzyme was purified and exhibited molecular and kinetic properties similar to those described for NADP+-IDH from angiosperm, with a higher catalytic efficiency (105 m−1 s−1) than the deduced efficiencies for GS and GOGAT in higher plants. A polyclonal antiserum was raised against pine NADP+-IDH and used to assess protein expression in the seedlings. Steady-state levels of NADP+-IDH were coordinated with GS during seed germination and were associated with GS/GOGAT enzymes during chloroplast biogenesis, suggesting that NADP+-IDH is involved in the provision of carbon skeletons for the synthesis of nitrogen-containing molecules. However, a noncoordinated pattern of NADP+-IDH and GS/GOGAT was observed in advanced stages of cotyledon development and in the hypocotyl. A detailed analysis in hypocotyl sections revealed that NADP+-IDH abundance was inversely correlated with the presence of GS, GOGAT, and ribulose-1,5-bisphosphate carboxylase/oxygenase but was associated with the differentiation of the organ. These results cannot be explained by the accepted role of the enzyme in nitrogen assimilation and strongly suggest that NADP+-IDH may have other, as-yet-unknown, biological functions.
The synthesis of 2-oxoglutarate represents a connecting point between carbon and nitrogen metabolism because this ketoacid provides the carbon skeleton for the assimilation of inorganic nitrogen into amino acids in higher plants through the GS/GOGAT cycle (Miflin and Lea, 1980). In this pathway GS (EC 6.3.1.2) catalyzes the condensation of the ammonium ion and glutamate to produce Gln. In a second step, the amido group of Gln is transferred to 2-oxoglutarate by the GOGAT action (EC 1.4.7.1 and 1.4.1.14) and two glutamate molecules are produced. These two reactions are of relevance in nitrogen metabolism because Gln and glutamate are the donors for the synthesis of major nitrogen compounds in higher plants: amino acids, chlorophyll, polyamines, and nucleic acids. Therefore, physiological processes that demand a large synthesis of nitrogen-containing molecules also require a net supply of 2-oxoglutarate. Reactions catalyzed by glutamate dehydrogenase (EC 1.4.1.3) and aminotransferases (Givan, 1980) may generate the ketoacid from glutamate, but when a net synthesis of the amino acid is required, the 2-oxoglutarate must be supplied by the oxidative decarboxylation of isocitrate, a reaction catalyzed by two different IDHs differing in which pyridine nucleotide cosubstrate they use (NAD+ or NADP+).
Traditionally, the Krebs cycle enzyme NAD+-IDH (EC 1.1.1.41) was considered to be responsible for 2-oxoglutarate supply in amino acid biosynthesis (Bray, 1983); however, NADP+-IDH (EC 1.1.1.42) has recently been regarded as an alternative pathway when large quantities of the ketoacid are required (Chen and Gadal, 1990a; Gálvez and Gadal, 1995). This hypothesis was based on the findings that (a) plant organs exhibit very low NAD+-IDH activity, (b) NADP+-IDH has a higher affinity for substrates, and (c) cytosolic aconitase is present in plants and may produce isocitrate outside of the mitochondria. In angiosperms NADP+-IDH has been studied in a limited number of species, including pea (Omran and Dennis, 1971; Randall and Givan, 1981; Chen et al., 1989a), several members of the Solanaceae (Gálvez et al., 1994; Fieuw et al., 1995; Gallardo et al., 1995), and cucumber (Canino et al., 1996). It occurs as several isozymes located in different subcellular compartments, including the cytosol, chloroplast, mitochondria, and peroxisome (Chen and Gadal, 1990b, and refs. therein; Rasmusson and Moller, 1990; Gálvez et al., 1994; Gallardo et al., 1995; Cornu et al., 1996).
Cytosolic NADP+-IDH represents 90% to 100% of the activity detected in any plant organ (Chen and Gadal, 1990b; Fieuw et al., 1995; Gallardo et al., 1995; Nieri et al., 1995), but little is known about its biological role. It has been suggested that the synthesis of 2-oxoglutarate in the cytosol is associated with the mitochondrial oxidation of l-malate in photosynthetic conditions (Hanning and Heldt, 1993), and the accumulation of cytosolic NADP+-IDH and glutamate during the ripening of tomato fruit has also been reported (Gallardo et al., 1995). In addition, it was reported that tobacco mutants with very low levels of nitrate reductase activity showed a higher expression of genes involved in nitrogen assimilation, including cytosolic NADP+-IDH (Scheible et al., 1997). In spite of the evidence implicating NADP+-IDH in nitrogen assimilation and glutamate biosynthesis, the role of the enzyme in other plant metabolic processes consuming cytosolic NADPH and its possible role in the supply of 2-oxoglutarate to pathways other than the GS/GOGAT cycle remain to be established. Furthermore, very little information is available about the provision of the ketoacid for the GS/GOGAT pathway in trees and especially in gymnosperms.
We describe the purification and characterization of NADP+-IDH from a gymnosperm species. The expression studies during early development in pine suggest that cytosolic NADP+-IDH is involved in not only the biosynthesis of nitrogen compounds but also the differentiation of the hypocotyl. The possible implication of the enzyme in the supply of ketoacids in reactions other than amino acid biosynthesis is discussed.
MATERIALS AND METHODS
Plant Material
Scots pine (Pinus sylvestris L.) and maritime pine (Pinus pinaster cv Aiton) seeds were supplied by Instituto Nacional para la Conservación de la Naturaleza (Madrid, Spain). The seeds were soaked for 24 h in water and germinated in moistened vermiculite at 20°C. Experimental conditions for plant growth and selection of seedling development stages have been described (Cánovas et al., 1991). For in vitro experiments water-soaked maritime pine seeds were sterilized with 10% sodium hypochlorite and the embryos were detached, placed onto Murashige-Skoog medium (Murashige and Skoog, 1962), and incubated under continuous white light (110 μmol photon m−2 s−1). After the first evidence of cotyledon development (cotyledon size of 3 mm), the embryos were harvested, frozen in liquid nitrogen, and stored at −80°C until use.
Protein Extraction
The plant material was extracted with a mortar and pestle in 50 mm Tris-HCl, pH 8.0, 1 mm EDTA, 10 mm MgCl2, and 14 mm 2-mercaptoethanol with washed sea sand on ice. The ratio of plant material to buffer to sand was 1.0:4.0:0.5. The extract was filtered through two layers of muslin and clarified by centrifugation at 22,000g for 15 min. The supernatant was used for protein determination, enzymatic analysis, purification of NADP+-IDH, and western analysis. All steps were performed at 4°C.
Protein Determination, Enzyme Assays, and Kinetic Studies
Protein was estimated by the method of Bradford (1976) using BSA as a standard. NADP+-IDH activity was determined by monitoring the synthesis of NADPH at 340 nm, as described previously (Gallardo et al., 1995). Kinetic studies were performed with purified preparations desalted by gel filtration through a Sephacryl S-300 column. Determination of Km values for NADP+ and 2R,3S-isocitrate was carried out in 40 mm potassium phosphate, pH 7.5, and 5 mm MgCl2 at different concentrations of one substrate and at the saturation concentration of the other substrate (Gallardo et al., 1995). NAD+-IDH activity was measured as described for NADP+-IDH but using NAD+ (1 mm) as the pyridine nucleotide.
Purification of NADP+-IDH from Pine Cotyledons
The major NADP+-IDH form present in Scots pine cotyledons (1–2 cm in size) was purified from 50 g of material following the procedure described by Gallardo et al. (1995) with modifications. Protein extracts were prepared and fractionated by precipitation with ammonium sulfate at 45% to 85% saturation. The precipitates were dissolved and dialyzed against 200 volumes of 10 mm potassium phosphate, pH 7.5, 1 mm MgCl2, and 14 mm 2-mercaptoethanol (standard buffer). The dialyzed extracts were loaded onto a DEAE-cellulose column (21 × 2.5 cm) previously equilibrated in standard buffer. The column was washed with 200 mL of the same buffer, and bound proteins were eluted with a linear gradient of 0 to 0.4 m KCl in standard buffer. The fractions with relevant NADP+-IDH activity were pooled and concentrated by ammonium sulfate precipitation and extensive dialysis. The enzyme preparation was further purified by affinity chromatography on a 2-mL Matrex Red Gel A (Amicon, Danvers, MA) column equilibrated in standard buffer. After the column was washed with 10 mL of buffer, the proteins were eluted by the application of a linear gradient of 0 to 1 mm NADP+ and 0 to 25 mm isocitrate in standard buffer. The active fractions were collected and concentrated by ammonium sulfate precipitation and extensive dialysis.
Estimation of Native Molecular Mass
The molecular mass of pine NADP+-IDH was estimated by gel filtration using a Sephacryl S-300 column (68 × 1.6 cm) previously calibrated with protein markers of known size: urease (483 kD), catalase (240 kD), alcohol dehydrogenase (150 kD), BSA (66 kD), and Cyt c (12.4 kD).
Antiserum Production
Polyclonal antibodies against pine NADP+-IDH were raised in New Zealand White rabbits. Immunization of the animals with 200 μg of purified protein was carried out basically as reported previously (Cantón et al., 1996). After blood specimens were obtained, samples were allowed to clot overnight, and the serum was recovered by centrifugation for 5 min at 4°C and 5000g. The final preparation was divided into aliquots and stored at −80°C until use.
Denaturing Gel Electrophoresis and Western Analysis
Proteins were analyzed by SDS-PAGE using the discontinuous buffer system of Laemmli (1970). After electrophoresis the polypeptides were electrotransferred to a nitrocellulose filter or visualized by standard Coomassie blue or silver staining (Morrissey, 1981). Protein blotting, saturation of blots, incubations with the antisera, and washing steps were performed as described previously (Gallardo et al., 1995). The antisera raised against pine NADP+-IDH, cytosolic GS (Cantón et al., 1996), Fd-GOGAT (García-Gutiérrez et al., 1995), and the large subunit of Rubisco (García-Gutiérrez et al., 1993) were diluted 10,000-, 5,000-, 8,000-, and 500-fold, respectively, in 20 mm Tris and 150 mm NaCl, pH 7.5. The immunocomplexes were detected with a secondary antibody conjugated to peroxidase with a molar peroxidase to antibody ratio of 3.3 (Vector Laboratories, Burlingame, CA). Visualization of immunocomplexes was carried out by assaying peroxidase activity using 3% (v/v) H2O2 and 0.03 mm 4-chloro-1-naphthol as substrates. Molecular masses were estimated relative to prestained protein markers (Sigma).
Chlorophyll Determination
Pigments were extracted from hypocotyl sections in 80% (v/v) acetone and total chlorophyll content was determined as reported previously (Graan and Ort, 1984).
RESULTS
IDH Activities in Scots Pine Tissues
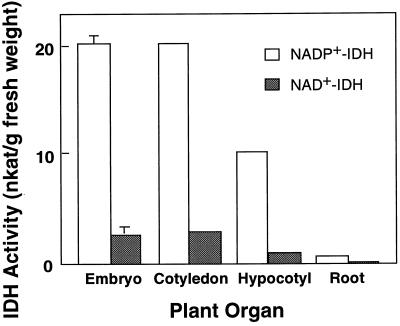
The analysis of IDH activities in pine seedlings revealed that NADP+-IDH activity was notably higher than NAD+-IDH activity in all organs studied (Fig. 1). NADP+-IDH represents approximately 90% of total IDH activity detected in any pine tissue, suggesting that this enzyme has a relevant role in the production of 2-oxoglutarate in pine seedlings. The NADP+-IDH levels were higher in the embryo and cotyledons than in hypocotyls and roots (Fig. 1); however, on a protein-content basis, the activity was higher in hypocotyls and roots (results not shown) and similar (4.5–7.0 nkat/mg protein) to the values described in angiosperm tissues (for review, see Chen and Gadal, 1990b).
Figure 1.
IDH activities in Scots pine seedlings. Values are the means ± se of at least three different experiments. Values of se lower than the thickness of the drawing line are not shown.
As a preliminary step in assessing the relevance of NADP+-IDH in the metabolism of the seedling, we purified and characterized the enzyme from pine cotyledons. This organ was selected as a protein source because of its high NADP+-IDH activity (Fig. 1) and its availability in large quantities from seedlings.
NADP+-IDH Isoenzyme Pattern and Purification from Scots Pine Cotyledons
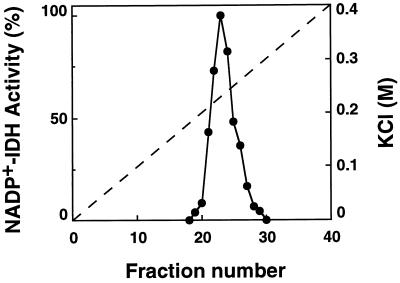
Although NADP+-IDH exists in higher plants as different isoenzymes located in the cytosol, chloroplast, and mitochondria, most of the NADP+-IDH activity reported so far in plant extracts (90%–100%) corresponds to the cytosolic isoenzyme (Chen and Gadal, 1990a; Gálvez et al., 1994; Gallardo et al., 1995). To determine the isoenzyme pattern in pine cotyledons, protein extracts were concentrated by ammonium sulfate precipitation (0%–90% saturation) and fractionated by ion-exchange chromatography on a DEAE-cellulose column. The elution profile of NADP+-IDH showed the presence of a single peak that eluted from the column at 0.22 m KCl, indicating that most of the activity detected in the tissue corresponds to the same isoenzyme (Fig. 2).
Figure 2.
Elution profile of NADP+-IDH activity from pine cotyledon on a DEAE-cellulose column. Enzyme activity is expressed as a percentage of the maximum value. The linear gradient of KCl is shown as a broken line.
To identify this isoform, specific antibodies raised against tobacco NADP+-IDH (Gálvez et al., 1994) were used in western analysis of pine extracts. A 46-kD band corresponding to the pine NADP+-IDH subunit (as will be shown) was recognized by an antiserum raised against the cytosolic tobacco NADP+-IDH, but no cross-reaction was observed when the antiserum raised against the chloroplast NADP+-IDH was used (results not shown). Furthermore, the chloroplast NADP+-IDH isoenzyme, which is the second major isoenzyme in angiosperm tissues (Randall and Givan, 1981; Chen and Gadal, 1990b; Gálvez et al., 1994), was not detected in Percoll-purified chloroplasts from pine cotyledons (not shown). Together, the above data suggest that cytosolic NADP+-IDH represents the major NADP+-IDH isoenzyme in pine cotyledons.
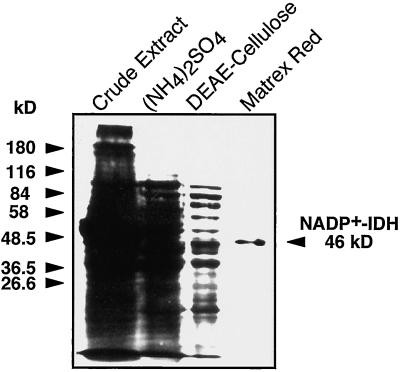
NADP+-IDH was purified following an experimental procedure that consisted of protein fractionation by ammonium sulfate precipitation, ion-exchange chromatography on DEAE-cellulose, and affinity chromatography on Matrex Red Gel A. The saturation range of ammonium sulfate (45%—85%) that precipitated most of NADP+-IDH was established in a pilot experiment. As observed in the ion-exchange chromatography step, the NADP+-IDH activity was also eluted as a single peak from the Matrex Red Gel A at 0.27 mm NADP+ and 6.85 mm isocitrate (not shown). This purification protocol (summarized in Table I) renders a homogeneous preparation, as judged by the presence of a single polypeptide in the final preparation (Fig. 3), with an absolute specificity for NADP+ as the electron acceptor. The enzyme was purified 250-fold, with a recovery of 37.6%, and the specific activity of the final preparation was 451.5 nkat/mg protein (Table I). Based on the data presented in Table I, NADP+-IDH is a relatively abundant enzyme in pine cotyledons and represents about 0.4% of total soluble protein.
Table I.
Purification of NADP+-IDH from maritime pine cotyledons
| Purification Step | Total Activity | Total Protein | Specific Activity | Yield | Purification |
|---|---|---|---|---|---|
| nkat | mg | nkat mg−1 protein | % | -fold | |
| Crude extract | 240 | 133 | 1.8 | 100 | 1 |
| 45% to 85% (NH4)2SO4 | 200 | 12 | 16.6 | 83 | 9.2 |
| DEAE-cellulose | 160 | 21 | 76.1 | 66 | 42.3 |
| Matrex Gel Red A | 90.3 | 0.2 | 451.5 | 37.6 | 250.8 |
Data are representative of five different purifications from 50 g of tissue from seedlings with cotyledons 1 to 2 cm in length.
Figure 3.
Analysis of polypeptides by SDS-PAGE of the purification steps of NADP+-IDH. Samples with 20, 10, 5, and 0.5 μg of proteins corresponding to the crude extract, ammonium sulfate precipitate, DEAE-cellulose, and Matrex Gel Red A, respectively, were separated by SDS-PAGE and stained with silver. The positions of molecular mass standards are indicated on the left. The migration of the NADP+-IDH subunit is marked on the right.
Properties of NADP+-IDH from Pine Cotyledons
The electrophoretic analysis of the purified NADP+-IDH preparation revealed the presence of a polypeptide of 46 kD (Fig. 3). This value is in the range of the reported subunit size for NADP+-IDH in angiosperms (41.0–48.5 kD; Chen and Gadal, 1990b; Gálvez et al., 1994; Gallardo et al., 1995). To estimate the native molecular mass of the pine enzyme, a purified preparation was subjected to gel filtration through a Sephacryl S-300 column previously calibrated with standard proteins of known size. The pine enzyme eluted from the column as a single peak of activity with an apparent molecular mass of 95 kD. Therefore, pine NADP+-IDH seems to be a dimer with a subunit size of 46 kD.
The kinetic properties of the enzyme were also determined using purified preparations that were desalted by extensive dialysis. The initial velocity kinetics were examined with NADP+ and 2R,3S-isocitrate as variable substrates. In both cases graphical representations of enzyme kinetic data according to the Lineweaver-Burk, Eadie-Hofstee, and Hanes equations (Price and Stevens, 1989) denoted the existence of noncooperativity of the enzyme for both substrates assayed (not shown). The Km values of the pine enzyme for 2R,3S-isocitrate and NADP+ were 80 and 10 μm, respectively. The Kcat of the enzyme was 43 s−1 and the ratio Kcat/Km for 2R,3S-isocitrate and NADP+ were 5 × 105 and 4 × 106 m−1 s−1, respectively. These kinetic data are in the value range described or deduced for NADP+-IDH from angiosperms (Chen and Gadal, 1990b, and the refs. therein; Gálvez et al., 1994; Gallardo et al., 1995).
Gln, glutamate, 2-oxoglutarate, ATP, and ADP were tested for inhibitory and stimulatory effects on pine NADP+-IDH enzyme at 0.1, 1.0, and 10 mm. Little modification of enzyme activity was recorded with any of the metabolites assayed at 0.1 and 1.0 mm. Adenine nucleotides and 2-oxoglutarate at 10 mm in the assay mixture inhibited 40% to 50% of the enzyme activity in the presence of 5 mm Mg2+, but such inhibition was less pronounced (30%) when Mg2+ was added to a final concentration of 15 mm. These data are similar to those reported for angiosperms (Omran and Dennis, 1971; Cornu et al., 1996) and Aspergillus niger (Meixener-Monori et al., 1986) NADP+-IDH and suggest that inhibition of the enzyme by nucleotides and 2-oxoglutarate is the result of chelating free Mg2+ and, therefore, of poor regulatory significance.
Production and Characterization of a Polyclonal Antiserum Raised against Scots Pine NADP+-IDH
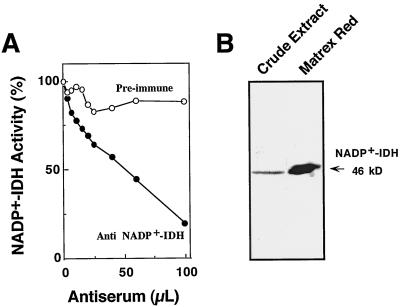
A preparation of the purified enzyme was injected into rabbits to raise an antiserum against pine NADP+-IDH. The specificity of the antiserum was assayed by its ability to decrease the NADP+-IDH activity present in a cotyledon extract. As shown in Figure 4A, NADP+-IDH activity decreased when the extract was incubated with increasing volumes of the antiserum obtained, whereas identical volumes of a preimmune antiserum showed little effect on the enzyme activity. The ability of the antiserum to recognize the enzyme was also assessed by western analysis. Total proteins from cotyledons were subjected to SDS-PAGE, transferred onto a nitrocellulose filter, and probed with dilutions of the antiserum ranging from 1,000 to 10,000. In all cases, the 46-kD polypeptide corresponding to the NADP+-IDH subunit was detected in the crude extract. Figure 4B shows a typical experiment in which an aliquot of the purified enzyme was used as a control in the western blot probed with the antiserum diluted 10,000-fold. Because of the high signal-to-noise ratio, a 10,000-fold dilution of the antiserum was used for protein expression studies.
Figure 4.
Specificity of the antiserum raised against pine NADP+-IDH. A, Inactivation of NADP+-IDH activity following the addition of the antiserum raised against the purified protein. Aliquots of a crude extract containing 0.16 nkat of enzyme activity were incubated with increasing volumes of the specific antiserum (•) or with a preimmune rabbit serum (○) for 4 h. The immunocomplexes formed were recovered by centrifugation at 20,000g for 5 min at 4°C, and enzyme activity was determined in the supernatant. B, Detection of NADP+-IDH by western analysis. Samples of proteins from a cotyledon extract (20 μg) and the purified preparation (0.5 μg) were subjected to SDS-PAGE, electrotransferred to a nitrocellulose filter, and probed with a 10,000-fold dilution of rabbit anti-NADP+-IDH antibodies. The position and size of the immunoreactive bands are indicated on the right.
Accumulation of NADP+-IDH in Germinating Embryos
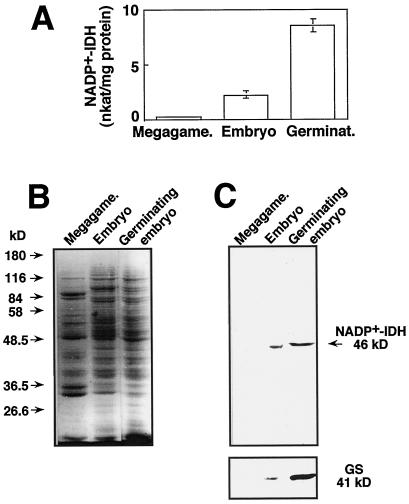
Seed germination is a complex process that requires the synthesis of specific nitrogen compounds to support the postembryonic differentiation and development of plant organs. To investigate whether NADP+-IDH is associated with germination, we evaluated activity and protein levels in water-soaked seeds and in in vitro germinated maritime pine embryos that were collected upon the first evidence of cotyledon development (cotyledon size of 3 mm). Maritime pine was selected for this study instead of Scots pine because of its larger seed and embryo size, which allowed for easier isolation and better manipulation of the embryos for the in vitro culture.
The NADP+-IDH specific activity (Fig. 5A) was very low in the megagametophyte of water-soaked seeds (0.2 ± 0.1 nkat/mg protein), whereas abundant levels were detected in embryos (2.3 ± 0.4 nkat/mg protein). Upon germination the specific activity in the embryos increased about 4-fold (8.6 ± 0.6 nkat/mg protein). A similar pattern of enzyme activity was observed when results were expressed on a fresh-weight basis (not shown). The protein analysis by SDS-PAGE revealed that many of the major polypeptides in the megagametophyte, which correspond to nitrogen-storage proteins, were absent in the embryo and, as a result of the germination, the embryo was enriched in some polypeptides and others became less abundant (Fig. 5B). The relative abundance of NADP+-IDH polypeptide was analyzed in the same protein extracts using the antiserum generated in this work.
Figure 5.
Expression of NADP+-IDH and GS in seeds and in in vitro germinating maritime pine embryos. A, Protein-soluble extracts were prepared from the megagametophyte (Megagame.) and embryos of water-soaked seeds (Embryo) and from in vitro germinating embryos collected upon the first symptoms of development (Germinat.) and the NADP+-IDH was determined. The results are the means ± se of at least three independent experiments. Proteins (20 μg) of the same samples in which enzyme activity was determined were separated by SDS-PAGE and stained with Coomassie blue (B) or blotted onto nitrocellulose filters and probed with the antibodies raised against pine NADP+-IDH or against pine cytosolic GS (C) (Cantón et al., 1996). The migration of protein molecular markers is indicated on the left. The position and size of the immunostained bands are marked on the right.
In good agreement with recorded activity values, the NADP+-IDH polypeptide was not detectable in the megagametophyte, whereas it was clearly evident in water-soaked embryos and its relative abundance increased upon germination (Fig. 5C). Western analysis of cytosolic GS polypeptide showed an expression pattern similar to that of the NADP+-IDH polypeptide, with very low levels in the megagametophyte and a marked increase in the embryo associated with the germination process (Fig. 5C). Expression of Fd-GOGAT was also monitored by western analysis, but the polypeptide was not detectable in water-soaked seeds or in germinating embryos (results not shown).
NADP+-IDH in Developing Scots Pine Seedlings
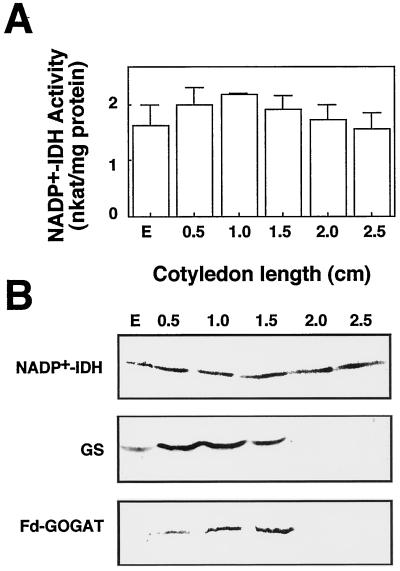
Another significant event in plant development that requires enhanced synthesis of a great number of nitrogen-containing molecules is the genesis of chloroplasts. In conifers, the differentiation of the photosynthetic organelles is a light-independent process that is completed during the development of the cotyledons, the primary photosynthetic organs of the plant (Mariani et al., 1990; Cánovas et al., 1993). To investigate whether NADP+-IDH is associated with the development of the cotyledon, we determined activity and protein level in Scots pine seedlings grown in continuous light and in darkness. In this pine species the chloroplasts are fully developed when the cotyledon size is 1.0 to 1.5 cm (Palomo, 1997). The enzymatic activity was similar in cotyledons of light-grown plantlets at the different stages considered (Fig. 6A). The polypeptide levels of NADP+-IDH, GS, and Fd-GOGAT were abundant in the organ during chloroplast development (stages 0.5–1.5 cm; Fig. 6B). These results are consistent with the view that NADP+-IDH is involved in the supply of carbon skeletons for ammonia assimilation and glutamate biosynthesis during plastid differentiation; however, in later stages of cotyledon development (stages 2.0–2.5 cm; Fig. 6B) NADP+-IDH protein content remained abundant, whereas GS and Fd-GOGAT polypeptide content declined to low levels in the organ. Similar results were found during cotyledon development in dark-grown plants (results not shown).
Figure 6.
Analysis of NADP+-IDH, GS, and Fd-GOGAT during cotyledon development in Scots pine. A, NADP+-IDH activity quantified in protein extracts prepared from embryos of water-soaked seeds (E) and cotyledons of light- and dark-grown seedlings. The results are the means ± se of at least three independent experiments. B, Samples with 20 μg of proteins corresponding to the same extracts in which enzyme activity was determined were analyzed by western blotting with the antisera raised against pine NADP+-IDH, GS (Cantón et al., 1996), and Fd-GOGAT (García-Gutiérrez et al., 1995). Twenty micrograms of protein was loaded per lane in the gels prepared for the detection of NADP+-IDH and GS polypeptides, and 40 μg of protein was loaded per lane for the analysis of Fd-GOGAT.
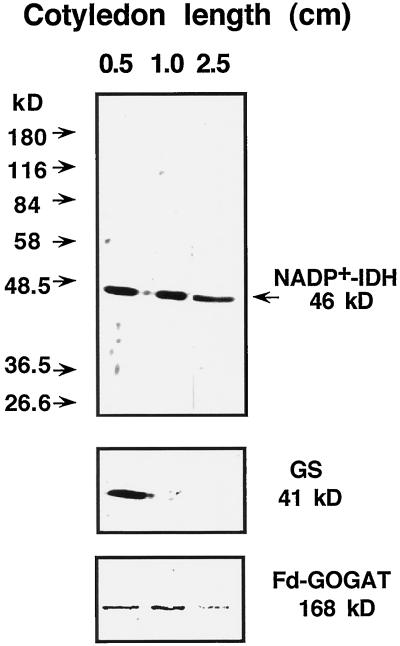
A related polypeptide pattern was observed in the hypocotyls of the seedlings. Protein extracts were prepared from hypocotyls of pine plantlets with a cotyledon size of 0.5, 1.0, and 2.5 cm, and NADP+-IDH, GS, and Fd-GOGAT content were analyzed by western blotting (Fig. 7). The hypocotyl of very young pine seedlings (cotyledon length, 0.5 cm) corresponds to a differentiating organ with a high chlorophyll content (data not shown) and exhibited a relevant content of NADP+-IDH and GS; however, the hypocotyl from older plants (cotyledon length, 2.5 cm) also exhibited high levels of NADP+-IDH but a low GS polypeptide content. The level of Fd-GOGAT polypeptide content was low in the hypocotyl, with levels higher in young than in differentiated hypocotyl.
Figure 7.
NADP+-IDH, GS, and Fd-GOGAT polypeptides in developing Scots pine hypocotyls. Samples of protein extracts prepared from the whole hypocotyl of seedlings with cotyledons 0.5, 2.0, and 2.5 cm in length were analyzed by western blotting for the detection of NADP+-IDH and GS polypeptides. The migration of protein molecular markers is indicated on the left. The position and size of the immunostained bands are marked on the right. Twenty micrograms of protein was loaded per lane in the gels prepared for the detection of NADP+-IDH and GS polypeptides, and 40 μg of protein was loaded per lane for the analysis of Fd-GOGAT.
Analysis of NADP+-IDH in Differentiating Hypocotyl
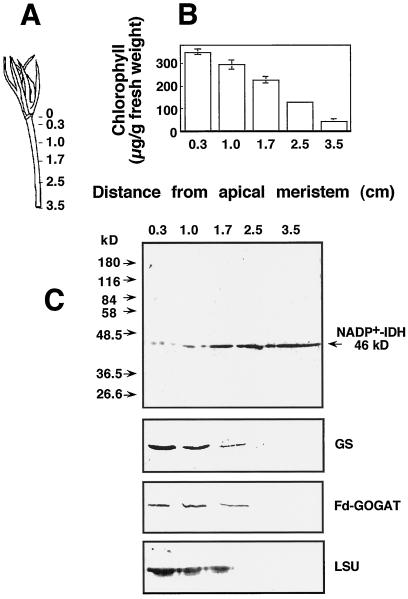
Because of the activity of the apical meristem, a gradient of cell differentiation is created from the top of the hypocotyl to the roots. A high growth rate and cell wall extensibility are features of cells close to the apical meristem (McQueen-Mason, 1996). These characteristics are lost when cells differentiate toward the base of the hypocotyl during development. To investigate whether the expression of NADP+-IDH is associated with the differentiation of this organ, we analyzed the chlorophyll levels and the NADP+-IDH, GS, Fd-GOGAT, and Rubisco contents in serial sections of the hypocotyl from the apical meristem (Fig. 8A). The chlorophyll content was high in the top sections, indicating that chloroplast development takes place in the young cells formed from the meristem and then decreases toward the root in a continuous gradient (Fig. 8B). The GS, Fd-GOGAT, and Rubisco contents matched the pattern of chlorophyll in the organ, abundant in the sections close to the apical meristem and at very low levels in the basal sections (Fig. 8C). In contrast to chlorophyll, GS, Fd-GOGAT, and Rubisco contents, the pattern of NADP+-IDH polypeptide markedly increased from the top to the basal sections of the hypocotyl (Fig. 8C).
Figure 8.
Pattern of NADP+-IDH, GS, Fd-GOGAT, and Rubisco polypeptides in the hypocotyls of pine seedlings. A, Serial dissection of the hypocotyl of a Scots pine seedling with cotyledons 2.5 cm in length. Numbers indicate the distance in centimeters from the apical meristem (the zero value). Total chlorophyll content was determined in the sections (B) and protein extracts were prepared for the analysis of NADP+-IDH, GS, and large subunit of Rubisco (LSU) by western blotting (C). Twenty micrograms of protein was loaded per lane in the gels prepared for the detection of NADP+-IDH and GS polypeptides, 40 μg of protein was loaded per lane for the analysis of Fd-GOGAT, and 5 μg of protein per lane was loaded for the analysis of Rubisco.
DISCUSSION
Characterization of NADP+-IDH Enzyme in Pine Seedlings
For the last 10 years, NADP+-IDH has been considered to be a key step in the generation of 2-oxoglutarate for ammonium assimilation and amino acid biosynthesis in higher plants (Chen and Gadal, 1990a; Gálvez and Gadal, 1995). This hypothesis is supported, among other things, by the fact that NAD+-IDH activity is 10 to 100 times less abundant than NADP+-IDH in all plant organs studied (Chen and Gadal, 1990a). The NADP+-IDH activity in pine seedlings is also notably higher than NAD+-IDH and represents about 90% of the total IDH values detected in any organ. In angiosperms the fractionation of soluble proteins by ion-exchange chromatography has been used to resolve the two main NADP+-IDH isoforms reported in plants, the cytosolic and the chloroplastic isozymes (Chen et al., 1989a; Gálvez et al., 1994). When such an approach was utilized to investigate the isoform pattern in pine cotyledons, only one form was detected, which corresponded to the cytosolic isozyme according to cross-reactions with antibodies raised against tobacco NADP+-IDH isozymes. These data are in accordance with those reported from both photosynthetic and nonphotosynthetic organs in angiosperm species, in which the cytosolic enzyme represents 90% to 100% of the total NADP+-IDH activity (Chen and Gadal, 1990a, 1990b; Gallardo et al., 1995; Nieri et al., 1995).
Purification of the pine enzyme by ammonium sulfate precipitation (45% to 85% saturation), ion-exchange chromatography on a DEAE-celullose column, and affinity chromatography in Matrex Gel Red A led to a homogeneous preparation of the protein with a high recovery (37.6%) and specific activity (451.5 nkat/mg protein). The purification process also revealed that the enzyme is relatively abundant in pine cotyledons, representing about 0.4% of the total soluble protein. This value is intermediate to those reported for nonphotosynthetic (0.8% in pea roots) and photosynthetic organs (0.05% in pea leaves; Chen et al., 1988) and close to that in cultures and tissues with some photosynthetic capacity, such as mixotrophic tobacco cell cultures and tomato fruit pericarp (0.1%–0.2%; Gálvez et al., 1994; Gallardo et al., 1995). The abundance of NADP+-IDH in pine cotyledons may reflect its participation in several physiological processes that take place in the organ, since pine cotyledon is both the main photosynthetic organ and the main storage organ of the seedling. In addition, the synthesis of 2-oxoglutarate and NADPH may be relevant in the organ for the biosynthesis of nitrogen compounds during chloroplast development and for the mobilization of the nitrogen reserves required for development of the first true leaves (needles) of the tree.
The molecular properties of pine NADP+-IDH indicate that the enzyme is a dimeric protein with a subunit size of 46 kD and a native molecular mass of 96 kD. These data are similar to those described for the enzyme in angiosperms: 41 to 48.5 kD for the subunit size and 82 to 152 kD for the holoenzyme (Chen and Gadal, 1990b; Gálvez et al., 1994; Gallardo et al., 1995). Kinetic studies revealed that the pine enzyme exhibits typical Michaelis kinetics for both reaction substrates. The Km values for 2R,3S-isocitrate and NADP+ (80 and 10 μm, respectively) and the Kcat of the enzyme (43 s−1) were also similar to those described or deduced for the cytosolic enzyme in angiosperms (Gálvez et al., 1994; Gallardo et al., 1995; Chen and Gadal, 1990b). The catalytic efficiency of pine NADP+-IDH (Kcat/Km of approximately 105 m−1 s−1 for 2R,3S-isocitrate) is slightly higher than the efficiency that may be deduced for Fd-GOGAT (104 m−1 s−1 for Gln) and cytosolic GS (104 m−1 s−1 for glutamate; Lea et al., 1990). Conversely, the molecular and kinetic data and the fact that the pine protein may be recognized by antibodies raised against a cytosolic enzyme from angiosperms (Chen et al., 1989b; this work) suggest that NADP+-IDH is a protein that was conserved after the evolutive divergence between angiosperm and gymnosperms and that it could play similar physiological roles in both.
NADP+-IDH Expression during Early Development of Pine
The polyclonal antibodies raised against pine NADP+-IDH were used to assess the presence of the enzyme at the protein level in two different physiological contexts that require synthesis of nitrogen compounds, seed germination, and biogenesis of chloroplasts. During germination, protein and lipids of the megagametophyte are mobilized to obtain the material required to support the primary development of the plant. In the megagametophyte this process involves the induction of the enzymes of the glyoxalate pathway, malate synthase and isocitrate lyase, which play a central role in the conversion of lipids into sugars (Muller and Gifford, 1995). Since NADP+-IDH levels are barely detectable in the megagametophyte of water-soaked pine seeds, most of the isocitrate produced is probably used by the glyoxalate cycle and, therefore, a low expression of NADP+-IDH could be essential in the metabolism of the organ. In contrast, water-soaked pine embryos contained NADP+-IDH activity and protein that increased about 4-fold when the postembryonic development was initiated. A similar pattern was also observed for GS polypeptide, suggesting that both proteins may be involved in the supply of precursors for the synthesis of nitrogen compounds associated with the germination process or in the recycling of nitrogen compounds that follows the degradation of seed storage proteins during the very early development of the seedling.
The development of the chloroplasts is another event intimately associated with the synthesis of nitrogenous compounds. In previous works we described the coordinated expression of GS/GOGAT cycle enzymes during plastid differentiation in light- and dark-grown pine seedlings (Cánovas et al., 1991; García-Gutiérrez et al., 1995, 1998). A major difference between pines (conifers) and angiosperms is the content of GS isozymes, since only cytosolic GS is expressed in pine during the seedling stage (Cánovas et al., 1991; Cantón et al., 1993; García-Gutiérrez et al., 1998). The detection of NADP+-IDH, GS, and Fd-GOGAT polypeptides during the development of the chloroplast (cotyledon size of 0.5–1.5 cm) is in agreement with the initial hypothesis of Chen and Gadal (1990a) and suggests that NADP+-IDH is associated with the GS/GOGAT cycle in higher plants. However, when the differentiation of the chloroplast has been completed (cotyledon size of 2.0–2.5 cm), the expression of these proteins differ, suggesting that NADP-IDH may play an additional role not related to the GS/GOGAT cycle.
These differences in protein expression are more evident in the hypocotyl, in which GS and Fd-GOGAT are clearly associated with photosynthetic markers (Rubisco and chlorophyll), whereas NADP+-IDH protein levels increase with the gradient of maturity established along the hypocotyl. In hypocotyls NADP+-IDH could be mainly related to secondary metabolism, since cytosolic NADPH may be used in several biosynthetic reactions. 2-Oxoglutarate is also a cosubstrate of a group of dioxygenases involved in different metabolic pathways, including phytohormone, flavonoid, and alkaloid biosynthesis (Hedden, 1992; Prescott and John, 1996), and in the hydroxylation of Pro residues of Hyp-rich glycoproteins (Showalter and Varner, 1989). It is noteworthy that extensins (Hyp-rich glycoproteins) accumulate in the cell wall of differentiated cells (Showalter and Varner, 1989) and exhibit a similar pattern of expression to NADP+-IDH in the hypocotyl (Bao et al., 1992; Ahn et al., 1996). All of these data suggest that NADP+-IDH could be involved in the supply of 2-oxoglutarate for dioxygenase reactions in differentiated cells and in the decrease of cell wall extensibility associated with differentiation processes.
In conclusion, we have described for the first time, to our knowledge, the purification and characterization of cytosolic NADP+-IDH from a gymnosperm. The catalytic efficiency of the enzyme suggests that the provision of 2-oxoglutarate in pine seedlings may be ensured for the GS/GOGAT cycle even at low levels of NADP+-IDH. The levels of NADP+-IDH during seed germination and chloroplast development in pine are in good agreement with the accepted role of the enzyme in glutamate biosynthesis in processes that demand a net synthesis of nitrogenous compounds (Chen and Gadal, 1990a). Nevertheless, our results also suggest that NADP+-IDH is associated with the differentiation of the hypocotyl and is not related to the GS/GOGAT cycle and photosynthetic metabolism in this organ. Therefore, new roles for NADP+-IDH must be considered to explain the reported expression.
ACKNOWLEDGMENTS
We thank Prof. P. Gadal (Université Paris Sud) for the antisera against tobacco NADP+-IDHs, Dr. A. García-Gutiérrez for his assistance in the preparation of the antiserum against pine NADP+-IDH, R. Crespillo for technical assistance, and Dr. M.G. Claros for the revision and criticism of the manuscript.
Abbreviations:
- GOGAT
glutamate synthase
- GS
Gln synthetase
- IDH
isocitrate dehydrogenase
Footnotes
This work was supported by a grant from the Universidad de Málaga (Ayuda para la Iniciación de Proyectos de Investigación Competitivos) to F.G. and by a grant from the Dirección General de Enseñanza Superior (no. PB95-0482).
LITERATURE CITED
- Ahn JH, Choi Y, Kwon YM, Kim S-G, Choi YD, Lee JS. A novel extensin gene encoding a hydroxyproline-rich glycoprotein requires sucrose for its wound-inducible expression in transgenic plants. Plant Cell. 1996;8:1477–1490. doi: 10.1105/tpc.8.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W, O'Malley DM, Sederoff RR. Wood contains a cell-wall structural protein. Proc Natl Acad Sci USA. 1992;89:6604–6608. doi: 10.1073/pnas.89.14.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray CM. Nitrogen Metabolism in Plants. London: Longman; 1983. [Google Scholar]
- Canino S, Nieri B, Pistelli L, Alpi A, De Bellis L. NADP+-isocitrate dehydrogenase in germinating cucumber cotyledons: purification and characterization of a cytosolic isoenzyme. Physiol Plant. 1996;98:13–19. [Google Scholar]
- Cánovas FM, Cantón FR, Gallardo F, García-Gutiérrez A, De Vicente A. Accumulation of glutamine synthetase during early development of maritime pine (Pinus pinaster) seedlings. Planta. 1991;185:372–378. doi: 10.1007/BF00201059. [DOI] [PubMed] [Google Scholar]
- Cánovas FM, McLarney B, Silverthorne J. Light-independent synthesis of LHCIIb polypeptides and assembly of the major pigmented complexes during initial stages of Pinus palustris seedling development. Photosynth Res. 1993;38:89–97. doi: 10.1007/BF00015065. [DOI] [PubMed] [Google Scholar]
- Cantón FR, García-Gutiérrez A, Crespillo R, Cánovas FM. High-level expression of Pinus sylvestris glutamine synthetase in Escherichia coli. Production of polyclonal antibodies against the recombinant protein and expression in pine seedlings. FEBS Lett. 1996;393:205–210. doi: 10.1016/0014-5793(96)00886-1. [DOI] [PubMed] [Google Scholar]
- Chen RD, Bismuth E, Champigny ML, Gadal P. Chromatographic and immunological evidence that chloroplastic and cytosolic pea (Pisum sativum L.) NADP-isocitrate dehydrogenase are distinct isoenzymes. Planta. 1989a;178:157–163. doi: 10.1007/BF00393190. [DOI] [PubMed] [Google Scholar]
- Chen RD, Bismuth E, Issakidis E, Pacot C, Champigny ML, Gadal P. Etude immunologique comparée de l′isocitrate déshydrogénase à NADP: une protéine cytosolique bien conservée chez les plantes supérieures. CR Acad Sci Ser III Sci Vie. 1989b;308:459–465. [Google Scholar]
- Chen RD, Gadal P. Do the mitochondria provide the 2-oxoglutarate needed for glutamate synthesis in higher plant chloroplasts? Plant Physiol Biochem. 1990a;28:141–145. [Google Scholar]
- Chen RD, Gadal P. Structure, functions and regulation of NAD and NADP-dependent isocitrate dehydrogenases in higher plants and in other organisms. Plant Physiol Biochem. 1990b;28:411–427. [Google Scholar]
- Chen RD, Le Maréchal P, Vidal J, Jacquot JP, Gadal P. Purification and comparative properties of the cytosolic isocitrate dehydrogenases (NADP) from pea (Pisum sativum) roots and green leaves. Eur J Biochem. 1988;175:565–572. doi: 10.1111/j.1432-1033.1988.tb14229.x. [DOI] [PubMed] [Google Scholar]
- Cornu S, Pireaux JC, Gerard J, Dizengremel P. NAD(P)+-dependent isocitrate dehydrogenases in mitochondria purified from Picea abies seedlings. Physiol Plant. 1996;96:312–318. [Google Scholar]
- Fieuw S, Müller-Röber B, Gálvez S, Willmitzer L. Cloning and expression analysis of the cytosolic NADP+-dependent isocitrate dehydrogenase from potato. Implications for nitrogen metabolism. Plant Physiol. 1995;107:905–913. doi: 10.1104/pp.107.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo F, Gálvez S, Gadal P, Cánovas FM. Changes in NADP+-linked isocitrate dehydrogenase during tomato fruit ripening. Characterization of the predominant cytosolic enzyme from green and ripe pericarp. Planta. 1995;196:148–154. [Google Scholar]
- Gálvez S, Bismuth E, Sarda C, Gadal P. Purification and characterization of chloroplastic NADP-isocitrate dehydrogenase from mixotrophic tobacco cells. Plant Physiol. 1994;105:593–600. doi: 10.1104/pp.105.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez S, Gadal P. On the function of the NADP-dependent isocitrate dehydrogenase isoenzymes in living organisms. Plant Sci. 1995;105:1–14. [Google Scholar]
- García-Gutiérrez A, Cantón FR, Gallardo F, Cánovas FM. Immunochemical analysis of chloroplast polypeptides from maritime pine. Phytochemistry. 1993;34:337–341. [Google Scholar]
- García-Gutiérrez A, Cantón FR, Gallardo F, Sánchez-Jiménez F, Cánovas FM. Expression of ferredoxin-dependent glutamate synthase in dark-grown pine seedlings. Plant Mol Biol. 1995;27:115–128. doi: 10.1007/BF00019183. [DOI] [PubMed] [Google Scholar]
- García-Gutiérrez A, Dubois F, Cantón F, Gallardo F, Sangwan R, Cánovas F. Two different modes of early development and nitrogen assimilation in gymnosperm seedlings. Plant J. 1998;13:187–200. [Google Scholar]
- Givan CV. Aminotransferases in higher plants. In: Miflin BJ, editor. The Biochemistry of Plants, Vol 5. London: Academic Press; 1980. pp. 329–357. [Google Scholar]
- Graan T, Ort DR. Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem. 1984;259:14003–14010. [PubMed] [Google Scholar]
- Hanning I, Heldt HW. On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea L.) leaves. Plant Physiol. 1993;103:1147–1154. doi: 10.1104/pp.103.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P. 2-Oxoglutarate-dependent dioxygenases in plants: mechanism and function. Biochem Soc Trans. 1992;20:373–377. doi: 10.1042/bst0200373. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lea PJ, Blackwell RD, Chen F-L, Hecht U. Enzymes of ammonia assimilation. In: Lea PJ, editor. Enzymes of Primary Metabolism, Vol 3. London: Academic Press; 1990. pp. 257–276. [Google Scholar]
- Mariani P, De Carli M, Rascio N, Baldan B, Casadoro G, Gennari G, Bodner M, Larcher W. Synthesis of chlorophyll and photosynthetic competence in etiolated and greening seedlings of Larix decidua as compared with Picea abies. J Plant Physiol. 1990;137:5–14. [Google Scholar]
- McQueen-Mason SJ. Expansins and cell wall expansion. J Exp Bot. 1996;46:1639–1650. [Google Scholar]
- Meixener-Monori B, Kubicek CP, Harrer W, Schreferl G, Rohr M. NADP-specific isocitrate dehydrogenase from the citric acid-accumulating fungus Aspergillus niger. Biochem J. 1986;236:549–557. doi: 10.1042/bj2360549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin BJ, Lea PJ. Ammonia assimilation. In: Miflin B, editor. The Biochemistry of Plants, Vol 5. London: Academic Press; 1980. pp. 169–202. [Google Scholar]
- Morrissey JH. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Muller RT, Gifford DJ. Purification and characterization of the glyoxysomal enzyme malate synthase following seed germination in Pinus taeda. Plant Physiol Biochem. 1995;33:639–648. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nieri B, De Bellis L, Biagi PP, Alpi A. NADP+-isocitrate dehydrogenase in germinating and senescing pumpkin cotyledons. Physiol Plant. 1995;94:351–355. [Google Scholar]
- Omran RG, Dennis DT. Nicotinamide adenine dinucleotide phosphate-specific isocitrate dehydrogenase from a higher plant. Plant Physiol. 1971;47:43–47. doi: 10.1104/pp.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo J (1997) Purificación y caracterización de la isocitrato deshidrogenasa dependiente de NADP+ de pino: análisis cinético-molecular y expresión de la proteína durante el desarrollo. Tesis de Licenciatura, Universidad de Málaga. Málaga, Spain
- Prescott AG, John P. Dioxygenases: molecular structure and role in plant metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:245–272. doi: 10.1146/annurev.arplant.47.1.245. [DOI] [PubMed] [Google Scholar]
- Price NC, Stevens L. Fundamentals of Enzymology. Oxford, UK: Oxford University Press; 1989. [Google Scholar]
- Randall DD, Givan CV. Subcellular location of NADP+-isocitrate dehydrogenase in Pisum sativum leaves. Plant Physiol. 1981;68:70–73. doi: 10.1104/pp.68.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AG, Moller I. NADP-utilizing enzymes in the matrix of plant mitochondria. Plant Physiol. 1990;94:1012–1018. doi: 10.1104/pp.94.3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe W-R, González-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell. 1997;9:783–798. doi: 10.1105/tpc.9.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM, Varner JE. Plant hydroxyproline-rich glycoproteins. In: Marcus A, editor. Molecular Biology, Vol 15. San Diego, CA: Academic Press; 1989. pp. 385–519. [Google Scholar]