Abstract
Kaiso, a p120 catenin-binding protein, is expressed in the cytoplasmic and nuclear compartments of cells; however, the biological consequences and clinical implications of a shift between these compartments have yet to be established. Herein, we report an enrichment of nuclear Kaiso expression in cells of primary and metastatic prostate tumors relative to the normal prostate epithelium. Nuclear expression of Kaiso correlates with Gleason score (P < 0.001) and tumor grade (P < 0.001). There is higher nuclear expression of Kaiso in primary tumor/normal matched samples and in primary tumors from African American men (P < 0.0001). We further found that epidermal growth factor (EGF) receptor up-regulates Kaiso at the RNA and protein levels in prostate cancer cell lines, but more interestingly causes a shift of cytoplasmic Kaiso to the nucleus that is reversed by the EGF receptor–specific kinase inhibitor, PD153035. In both DU-145 and PC-3 prostate cancer cell lines, Kaiso inhibition (short hairpin RNA-Kaiso) decreased cell migration and invasion even in the presence of EGF. Further, Kaiso directly binds to the E-cadherin promoter, and inhibition of Kaiso in PC-3 cells results in increased E-cadherin expression, as well as re-establishment of cell–cell contacts. In addition, Kaiso-depleted cells show more epithelial morphology and a reversal of the mesenchymal markers N-cadherin and fibronectin. Our findings establish a defined oncogenic role of Kaiso in promoting the progression of prostate cancer.
Prostate cancer is the most commonly diagnosed malignancy in men, with African American men experiencing a rate 60% higher than white patients. At the time of diagnosis, approximately 50% of men have clinically advanced disease.1 The molecular mechanisms associated with tumor development are the result of genetic and epigenetic changes that promote tumor cell growth. DNA methylation, the most common epigenetic change, results from changes in cytosine methylation, typically at cytosine-guanine dinucleotides (CpG), or from changes in DNA-associated proteins. Recently, it was proposed that methylation alone is insufficient to silence transcription2 and that recognition of methylated DNA by two classes of proteins, those with a methyl-CpG binding domain and those with C2H2 zinc fingers, such as Kaiso, are required for transcriptional inactivation. Methyl-CpG binding proteins recognize 5-methylcytosine and act as intermediates between the transcriptional machinery and methylated DNA. Of the methyl-binding proteins, Kaiso has a 10-fold higher affinity and represses transcription in part by recruiting the N-CoR corepressor.3
Kaiso, first identified as a p120 catenin (ctn)-binding protein,4 is a member of the broad complex, tramtrak, bric-a-brac/pox virus, and zinc finger superfamily. Structurally, Kaiso contains a carboxyl-terminal region with three zinc finger motifs of the C2H2 type and recognizes clusters of methylated CpG dinucleotides as well as sequence-specific Kaiso binding sites.4 Several lines of evidence from both in vitro and in vivo models suggest a number of tumor suppressor genes, frequently silenced by hypermethylation, such as HIC1, MTA2, E-cadherin (CDH1), and matrilysin (MMP7) have been linked to Kaiso transcriptional regulation.5,6 A clinical role for Kaiso also has been proposed. Immunohistochemical analysis in various tissue types shows that Kaiso is expressed in both the cytoplasmic and/or nuclear compartments. Studies in lung cancer show that Kaiso expression correlates with clinicopathologic characteristics of malignant tumors. However, functional studies using Kaiso antibody or the shRNA-–depletion strategy resulted in enhanced proliferation and invasiveness.6 Surprisingly, an opposite role for Kaiso was implicated in colorectal studies, in which down-regulation of Kaiso expression resulted in decreased proliferation and an overall antitumor effect.2 Because Kaiso expression has been determined in multiple tumor types, additional studies are required to provide a detailed description of Kaiso expression and function in individual epithelial tumors.
Growth factors such as epidermal growth factor (EGF) have been well established to promote cell motility, invasion, and metastasis in multiple tumors. This promotes genetic and epigenetic changes that lead to a down-regulation of E-cadherin, via receptor tyrosine kinase signaling, and/or promoter hypermethylation. As a result, epithelial cells undergo an epithelial-to-mesenchymal transition, in which cells are directed to assume a less-differentiated phenotype with the acquired ability for metastasis. Previously, we showed that E-cadherin can be re-expressed through direct pharmacologic abrogation of EGF receptor (EGFR) signaling7 or within the metastatic tumor microenvironment,8 resulting in less invasive and more cohesive cells. However, whether Kaiso has a functional role and specific involvement in EGFR-associated epithelial-to-mesenchymal transition has not been explored. Herein, we show that Kaiso is highly expressed in primary prostate tumors and lymph node metastases, with significant increases for African Americans compared with white patients in high-grade tumors. Furthermore, a cytoplasmic-to-nuclear shift occurs in tumors and this correlates with increasing tumor grade and Gleason score. We further identified that EGF induces nuclear localization of Kaiso, which subsequently is associated with an increase in cell migration and invasion and suppression of tumor suppressor E-cadherin expression. These results show that Kaiso functions in an oncogenic manner, in which aberrant localization in the nucleus is essential for prostate cells to acquire the ability to metastasize.
Materials and Methods
Cell Culture, Antibodies, and Reagents
Human prostate cancer cell lines LNCaP, DU-145, and PC-3 were obtained from the ATCC and routinely were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Gibco, Paisley, Scotland) and antibiotics in a humidified atmosphere of 5% CO2 in air. In these conditions the duplication period of the cells is 36 hours.
DU-145 EGFR-overexpressing cells [wild type (WT) DU-145] were generated by transfecting DU-145 cells with retroviral-containing EGFR constructs.7 Primary antibodies were obtained as follows: Kaiso 6F clone (Abcam, Boston, MA); anti-Kaiso clone 6F (Upstate Biotechnology, Billerica, MA); anti-Kaiso 12H (Santa Cruz, CA); p120ctn, E-cadherin, and N-cadherin (BD Biosciences, OR). Mouse secondary antibodies, Alexa 488, 594, and 625, were obtained from Invitrogen (OR). Human EGF was obtained from BD Biosciences (KY). The EGFR-specific tyrosine kinase inhibitor, PD153035, was purchased from CalBiochem (CA). Other reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Immunohistochemistry
The prostate cancer tissue microarrays were obtained from US Biomax (Rockville, MD) or from the Anatomical Pathology Division at the University of Alabama at Birmingham. The use of tissue was approved by the Institutional Review Board of both Tuskegee University and the University of Alabama at Birmingham. Immunohistochemistry was performed using the anti-Kaiso clone 6F (Upstate Biotechnology) and 12H clone (Santa Cruz) as previously described. Duplicate microarrays were stained for evaluation by immunohistochemistry.8 Briefly, cells were blindly scored by two pathologists with similar results. Individual specimens were scored separately for membranous, cytoplasmic, and nuclear staining for Kaiso and classified with respect to the intensity of immunostaining, with the percentage of cells determined at each staining intensity from 0 to +4.9 To permit numeric analysis, the proportion of cells at each intensity can be multiplied by that intensity. Statistical analyses were performed using the Pearson χ2 test to analyze the relationships between cytoplasmic and nuclear expression of Kaiso and clinicopathologic factors.
Immunoblotting
Cells were grown to 80% confluency in six-well plates. Lysates were prepared from cultured cells in a solution containing 50 mmol/L Tris, pH 7.5; 120 mmol/L NaCl; 0.5% Nonidet p-40; 40 μmol/L phenylmethylsulfonyl fluoride; 50 μg/mL leupeptin; and 50 μg/mL aprotinin (all from Sigma-Aldrich). Cells were allowed to lyse for 1 hour on ice. The lysed cells were centrifuged, and the resulting supernatants were extracted and quantitated by use of a Bradford assay. Lysates (30 μg of protein) were separated by 8% SDS PAGE, immunoblotted, and analyzed by chemiluminescence (Amersham Biosciences, NJ). Densitometry was performed using NIH ImageJ software version 1.46 (Bethesda, MD).
Immunoblotting of Subcellular Fractions
Subcellular fractionation of cells was performed as previously described.10 Cytosolic and nuclear fractions, and the DU-145, DU-145 WT, and PC-3 cells were resuspended in a hypotonic buffer [10 mmol/L Tris (pH 7.5), 1.5 mmol/L MgCl2, 10 mmol/L KCl, 1 mmol/L dithiothreitol, pepstatin, leupeptin] and homogenized using a glass douncer. The cells were centrifuged at 13,000 × g and the supernatant was collected (cytosolic fraction). The nuclei were resuspended in a high-salt buffer [20 mmol/L HEPES (pH 7.9), 25% glycerol, MgCl2, 1.2 mol/L KCl, 0.2 mmol/L EDTA, 0.2 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L dithiothreitol] and rotated at 4°C. Lysates then were separated by 7.5% SDS PAGE, immunoblotted, and analyzed by chemiluminescence (Amersham Biosciences).
Immunofluorescence Microscopy
Cells (3 × 105) were grown to 80% confluency on glass coverslips. Cells then were fixed with methanol alone or 4% paraformaldehyde, permeabilized with 100 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 10 mmol/L EGTA, 1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride, and 50 μg/mL aprotinin (all from Sigma-Aldrich) and subsequently blocked with 5% bovine serum albumin for 1 hour at room temperature. Identical results were obtained with both methods. Samples were incubated with indicated primary antibodies diluted in blocking buffer at 4°C overnight. Fluorescein isothiocyanate–conjugated secondary antibody (BD Biosciences) was added. Cells then were treated with DAPI for nuclear staining and analyzed with a disk scanning unit confocal microscope (Olympus, Pittsburgh, PA). To determine the relative intensities, the total area of cytoplasmic and nuclear regions of each image was measured as well as the threshold intensity for each channel using Metamorph Imaging Software version 7.5 (Molecular Devices, Inc, Sunnyvale, CA). Differences between intensities then were determined by Excel (Microsoft, Redmond, WA). Bar graphs represent n = 3 images sectioned and individually analyzed for total area. All quantitative data were normalized to appropriate control images.
Quantitative RT-PCR
RNA was extracted from prostate cancer cells using TRIzol (Invitrogen). cDNA was prepared using Superscript III First Strand cDNA Synthesis kits (Invitrogen) and detected by Kaiso-specific TaqMan (Invitrogen). The housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HRPT1; Applied Biosystems, Carlsbad, CA) was used as an endogenous control for all RNA samples. RNA analyses were performed in triplicate, and fold change was calculated using the ΔΔCt value method.
RNA Interference
To generate stable short hairpin RNA (shRNA) Kaiso cells, the HuSH 29-mer for Kaiso was provided in the pRFP-C-RS plasmid driven by the U6-RNA promoter. Plasmid DNA pRFP-C-RS, containing puromycin-resistant gene, expressing Kaiso-specific shRNA, and scrambled shRNA were transfected into DU-145 or PC-3 cells using Lipofectamine 2000 (Invitrogen). The medium was replaced by T medium containing 2 μg/mL puromycin for selection of antibiotic-resistant colonies over a period of 3 weeks. The puromycin-resistant cells were further selected by use of red fluorescence protein as a marker to enrich for cells expressing shRNA. sh-Kaiso cells were plated at clonal densities, and more than 20 clones were chosen to determine the degree of knockdown. Clones with the lowest Kaiso levels were retained for further analysis.
Cell Migration
Migration of cells was assessed by their ability to move into an acellular area; this was accomplished with a two-dimensional wound-healing assay, as previously described.11 With cells at 70% to 80% confluence, a denuded area was generated in the middle of each well with a rubber policeman. The cells then were exposed to EGF (0 or 10 ng/mL) and incubated for 24 hours in dialyzed media. The rate of migration was determined and quantified in Metamorph Imaging Software. All measurements were normalized to values for controls.
Invasion Assay
Cell invasiveness was determined by the capacity of cells to migrate across a layer of extracellular matrix, matrigel, in a Boyden chamber. Briefly, 20,000 cells were plated in the matrigel-containing chamber in serum-free medium containing 1% bovine serum albumin for 24 hours; this then was replaced with a serum-free medium for an additional 24 hours. The number of cells that invaded through the matrix over a 48 hour-period was determined by counting cells that stained with crystal violet on the bottom of the filter. All experiments were performed in triplicate.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation experiments were performed with the use of the ChIP-IT Kit (Active Motif, Carlsbad, CA). In brief, PC3 cells were fixed in 1% formaldehyde at room temperature for 10 minutes; the fixation reaction was stopped by adding a 1:10 volume of 10× glycine to the tube at room temperature for 5 minutes. The cell pellets were resuspended and incubated for 30 minutes in ice-cold lysis buffer with phenylmethylsulfonyl fluoride and proteinase inhibitor cocktail. The nuclear pellets were resuspended in shearing buffer, and chromatin was sheared to an average size of 200 to 1500 bp by sonication at 25% power for 10 pulses of 20 seconds each, with a 30-second rest on ice between each pulse. Chromatin (10 μL) was saved for input DNA control. Sheared chromatin was incubated in chromatin immunoprecipitation buffer with 25 μL of protein G magnetic beads and anti-Kaiso antibody (Abcam), mouse RNA Polymerase II (Active Motif), and rabbit IgG antibody (Active Motif) as a negative control on a rolling shaker at 4°C for 4 hours. The immunoprecipitated chromatin was purified from the chromatin-antibody mixture by several washing steps, and the chromatin-immunoprecipitated DNA was eluted in 50 μL of elution buffer AM2 (Active Motif). Cross-links were reversed by adding 50 μL of reverse cross-link buffer. After removing proteins by digestion with proteinase K, the purified DNA was used as templates for PCR analysis. The primers used were designed to amplify a 422-bp methylated fragment of the E-cadherin promoter (−1163 to −1585): 5′-AGGAGGCTGATAGAGGAGAACC-3′ and 5′-GATTGAGACCATCCTGGCTAAC-3′.
Statistical Analysis
For all experiments, statistics were performed with Microsoft Excel or Prism software version 5.0 (GraphPad, La Jolla, CA). An independent Student's t-test was used to determine statistical differences between experimental and control values. Median scores were obtained from a subset of patients to statistically evaluate Kaiso expression. Tissue correlations were performed with Matlab (Mathworks, Inc, Natick, MI). P values <0.05 were considered statistically significant.
Results
Kaiso Expression and Subcellular Localization
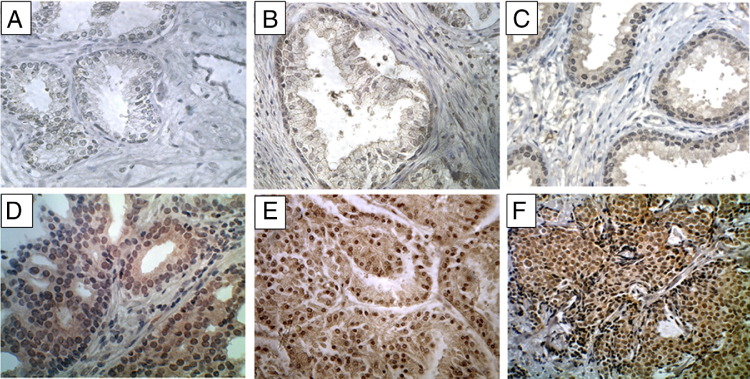
To evaluate the expression and localization of Kaiso during prostate cancer progression, immunohistochemistry was used to evaluate samples from 172 patients, consisting of normal tissue (9 patients), benign prostatic hyperplasia (14 patients), adjacent normal tissue (17 patients), primary tumors (142 patients), and metastases (4 patients). There was low expression of the Kaiso protein in luminal cells of noncancerous samples (Figure 1A); expression was predominantly in the membrane or cytoplasm (Figure 1, B and C). There was, however, nuclear expression of Kaiso in the basal cells of adjacent normal tissue (Figure 1B). Scoring for nuclear Kaiso staining intensities 0 to 4 (as mentioned in Materials and Methods) between normal, low Gleason, and high Gleason are summarized in Table 1.
Figure 1.
Abnormal nuclear expression of Kaiso in prostate cancer specimens. Representative data from immunohistochemical studies of 172 prostate cancer specimens are shown. A: Kaiso levels in a normal, healthy prostate with low staining seen in glandular epithelia. B: Kaiso expression in normal epithelia from adjacent PCa tumors showed discernible cytoplasmic staining in epithelia, and nuclear positivity in the basal cells. C: Kaiso expression in benign prostatic hyperplasia showed cytoplasmic positivity with low nuclear positivity. D: Kaiso expression in low grade 1 tumors showed a general up-regulation of Kaiso expression with cytoplasmic and nuclear positivity. E and F: High grade 4 and lymph node metastasis showed uniform intense nuclear expression of Kaiso. Original magnification: ×400.
Table 1.
Nuclear Kaiso Expression in Normal, Low Gleason, High Gleason, and Lymph Node Metastases Prostate Carcinomas
| Kaiso | Normal (n = 26)⁎ | Low Gleason (n = 52) | High Gleason (n = 50) | Mets |
|---|---|---|---|---|
| No score (0) | 9 | |||
| Weak (0–1) | 10 | 18 | 4 | |
| Moderate (2–3) | 7⁎ | 28 | 10 | |
| Strong (3–4) | 6 | 36 | 4 |
Adjacent normal.
In contrast to previous reports, Kaiso expression was observed within the nucleus, with weak to moderate expression in tumors with low Gleason scores (Figure 1D), and strong intense expression in tumors with high Gleason scores and in metastases (Figure 1, E and F). To determine the clinical significance of subcellular localization of Kaiso expression, we performed χ2 analysis. Median values of scoring intensities were used to separate the low and high tumor grades and Gleason scores. Nuclear expression of Kaiso was found to correlate significantly with tumor grade of ≥2 (P < 0.001) and Gleason score of ≥7 (P < 0.001) (Table 2). Cytoplasmic expression was observed in tumor samples; however, correlations with clinicopathologic features were not found to be significant. Increased nuclear expression occurred in a stage-specific manner, with the largest differential expression between metastatic tumors and normal samples; however, differences between primary tumors and normal samples were significant as well (Figure 2A). Further characterization of nuclear Kaiso expression in high grade tumors of similar-age African American men (n = 22) and white (n = 18) primary tumors showed that African American patients expressed higher mean values of Kaiso (P < 0.0001) independent of grade and age (Figure 2B). Further Kaiso nuclear expression significantly correlated with race (P = 0.0032) (see Supplemental Table S1 at http://ajp.amjpathol.org).
Table 2.
Correlation of Kaiso Subcellular Localization with Clinical Features
| Characteristics | All patients | Cytoplasmic Kaiso expression |
P⁎ | Nuclear Kaiso expression |
P⁎ | ||
|---|---|---|---|---|---|---|---|
| ≤0.53 (median) | >0.53 | ≤2.45 (median) | >2.45 | ||||
| Total | 142 | 70 | 72 | 73 | 69 | ||
| Age | |||||||
| ≤69.5 (median) | 71 | 27 | 44 | 0.0072 | 35 | 36 | 0.6145 |
| >69.5 | 71 | 43 | 28 | 38 | 33 | ||
| Grade | |||||||
| ≤2 | 70 | 39 | 31 | 0.1066 | 57 | 13 | <0.001 |
| >2 | 69 | 29 | 40 | 13 | 56 | ||
| Gleason score | |||||||
| ≤7 | 52 | 20 | 32 | 0.6394 | 29 | 23 | <0.001 |
| >7 | 50 | 17 | 33 | 10 | 40 | ||
| PSA, ng/mL | 15 | 4 | 11 | 0.1587 | 3 | 12 | 0.058 |
| ≤17, median | 15 | 5 | 10 | 8 | 7 | ||
P value for the correlation of the mean expression with clinical feature. P values were obtained with the χ2 test. Statistics were not performed on samples without clinical information. Median scores were obtained for each subset.
Figure 2.
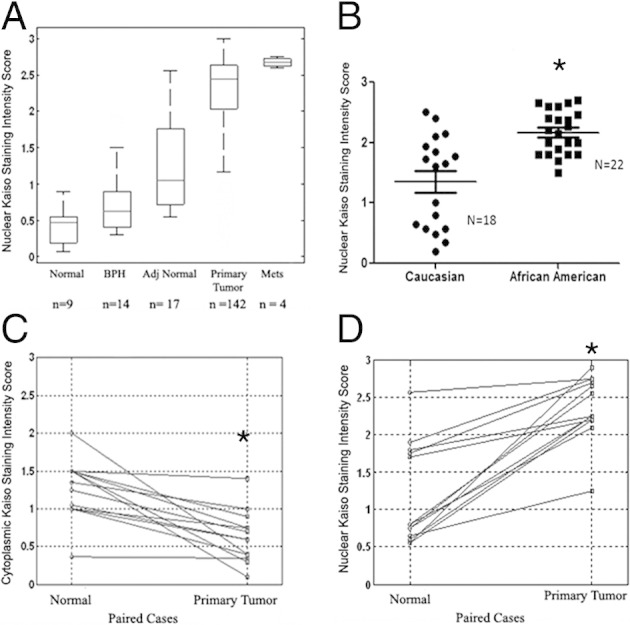
Quantitative analysis of nuclear Kaiso in prostate tumor progression. A: Nuclear expression of Kaiso was analyzed and presented in a box plot. Nuclear Kaiso levels increase monotonically from normal, benign prostatic hyperplasia (BPH), adjacent normal, primary tumor, and metastasis, with all four P values less than 0.05. (P = 0.016, normal and BPH; P = 0.01, BPH and adjacent normal; P < 0.0001, adjacent normal and malignant; and P < 0.0001, malignant and metastasis). B: Points represent nuclear Kaiso staining intensity of individual African American and white patients of similar age (ages, 67 to 80) and grade 3; bars represent the median value for the sample set. *P < 0.0001. Cytoplasmic Kaiso expression (C) and nuclear Kaiso expression (D) in paired (surrounding nontumor) normal and primary tumor tissues from n = 13 prostate cancer patients. *P < 0.0001.
To determine whether there is a shift in Kaiso localization in prostate tumors, matched normal and tumor samples were evaluated (n = 13). Cytoplasmic expression was decreased significantly in paired primary tumors compared with normal samples (P < 0.0001) (Figure 2C); however, there were significant increases in nuclear expression within the same patients (P < 0.0001) (Figure 2D). This analysis supports the idea that there is a progressive enhancement of abnormal Kaiso expression during prostate cancer progression and that the extent of abnormal expression correlates with progression.
Expression and Subcellular Localization of Kaiso in Prostate Cancer Cell Lines
Because there have been no reports of Kaiso expression in prostate cancer cell lines, its expression and localization were evaluated in LNCaP, DU-145, and PC-3 cells, and in a DU-145 subline (DU-145WT) that was genetically engineered to overexpress EGFR. DU-145 WT cells escape EGFR down-regulation and show enhanced invasiveness in vitro13 and in vivo.14 Quantitative RT-PCR showed that Kaiso levels were increased at the mRNA in DU-145 WT and PC-3 cells compared with LNCaP and DU-145 cells (see Supplemental Figure S1A at http://ajp.amjpathol.org). Confocal images show that Kaiso is located in both the cytoplasmic and nuclear compartments in all cell lines. However, the more aggressive DU-145WT and PC-3 cells showed increased presence of nuclear expression compared with LNCaP and DU-145 cells (see Supplemental Figure S1B at http://ajp.amjpathol.org), which was verified after quantification of fluorescent intensity in each compartment (see Supplemental Figure S1C at http://ajp.amjpathol.org). The influence of EGFR expression on Kaiso localization was shown further by the fact that subcellular fractions of DU-145 WT cells show increased nuclear expression, whereas DU-145 cells show low amounts of nuclear levels, which correlated with the confocal images (see Supplemental Figure S1D at http://ajp.amjpathol.org). Thus, it appears that the subcellular localization of Kaiso is associated with EGFR expression.
Activation of EGFR Signaling Results in Increased Kaiso Expression and Nuclear Localization
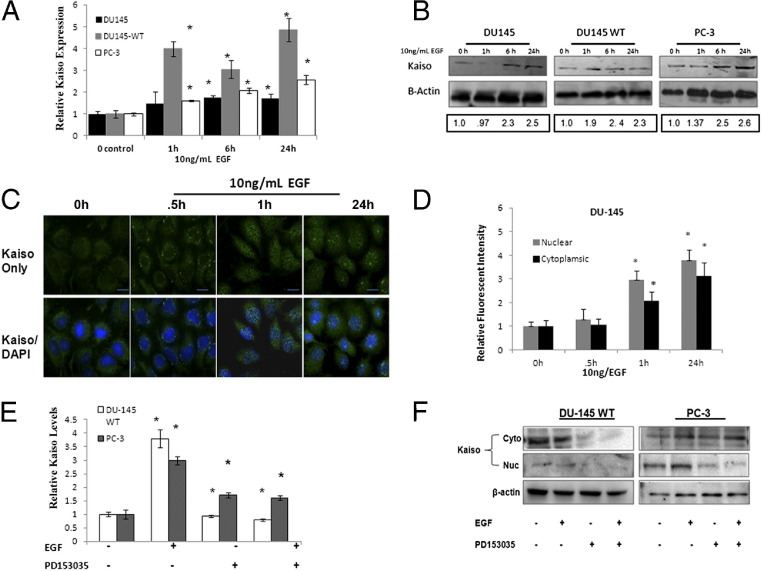
Various lines of evidence suggest the involvement of EGFR signaling in prostate cancer.15,16 To identify EGFR as an upstream regulator of Kaiso, 10 ng/mL EGF, a concentration showing the most significant fold increase (data not shown), was used in a time-dependent assay over 24 hours. DU-145, DU-145 WT, and PC-3 lines showed incremental increases in Kaiso expression at the RNA level; DU145WT cells showed the greatest increase (fourfold) as early as 1 hour after EGF stimulation (Figure 3A). Increases in Kaiso expression also were observed at the protein level, as determined by immunoblots, with significant increases observed as early as 6 hours and sustained over 24 hours of exposure to EGF (Figure 3B).
Figure 3.
EGF induces Kaiso expression and cytoplasmic-to-nuclear localization in prostate cancer cell lines. A: DU-145, DU-145 WT, and PC-3 prostate cancer cell lines were treated with 10 ng/mL of EGF for 0, 1, 6, and 24 hours and assayed for mRNA Kaiso levels by quantitative RT-PCR with Kaiso-specific TaqMan primers and hypoxanthine-guanine phosphoribosyltransferase (HPRT1) as the loading control. Data are normalized to control; n = 4 ± SE (*P < 0.05). B: Kaiso protein levels in whole-cell lysates by immunoblot using anti-Kaiso antibody and anti–β-actin antibody as loading control. Images shown are representative of three individual experiments. Densitometry was performed on individual time intervals and compared with control. C: Kaiso subcellular localization (green) was determined by immunofluorescence. Note the colocalization of Kaiso in nucleus (green) with nuclear stain DAPI (blue) after EGF treatment in DU-145 cells. Images shown are representative of three individual experiments. Scale bars: 25 μmol/L. D: Bar-graph quantification of Kaiso intensity in the individual cytoplasmic and nuclear compartments of DU-145 cells treated with 10 ng/mL of EGF compared with untreated control; shown is the mean ± SD of three individual experiments. Data are normalized to 0 hours control. *P < 0.05. E: Kaiso mRNA levels were determined by quantitative RT-PCR, in the presence or absence of 10 ng/mL EGF or EGFR-specific kinase inhibitor, PD153035, in DU-145 WT and PC-3 cells using Kaiso-specific TaqMan primers and HPRT1 as the loading control. Data were normalized to control; n = 4 ± SE. *P < 0.05. F: Kaiso protein levels were determined by immunoblot in the cytoplasm and the nucleus in the presence or absence of 10 ng/mL EGF or EGFR-specific kinase inhibitor, PD153035, in DU-145 WT and PC-3 cells. β-actin served as loading control. Images shown are representative of three individual experiments. h, hours.
Because we observed that increases in Kaiso expression are associated with a subcellular localization pattern in our patient cohort, we further determined if EGFR-induced increases in Kaiso expression coincided with a subcellular localization in our cell culture model. After only 0.5 hours, EGF caused perinuclear accumulation of previously dispersed Kaiso in DU-145 cells, with visible nuclear accumulation at 1 hour. After 24 hours, significant increases in both cytoplasmic and nuclear expression were observed, although nuclear expression was the most significant (Figure 3, C and D). DU-145WT and PC-3 cells, which endogenously express high levels of nuclear Kaiso, showed similar trends as DU-145 cells. Both cytoplasmic and nuclear Kaiso expression increased on exposure to EGF, however, nuclear expression remained significantly higher throughout the exposure time periods (see Supplemental Figure S2, A and B, at http://ajp.amjpathol.org). To more clearly define the role of EGFR activation on increases in Kaiso expression and localization, we used an EGFR-specific kinase inhibitor, PD153035 (500 nmol/L), in the presence or absence of EGF. PD153035 significantly reduced mRNA Kaiso expression levels even after EGF pretreatment (Figure 3E). After subcellular fractionation and subsequent immunoblot of both DU-145 WT and PC-3 cells, we also observed that PD153035 significantly reduced expression of Kaiso in the cytoplasmic and nuclear compartments (Figure 3F; see also Supplemental Figure S2C at http://ajp.amjpathol.org), which is a reversal of the expression pattern observed in endogenously expressing and EGF-treated DU-145 WT and PC-3 cells. Thus, EGFR signaling positively affects Kaiso expression and subcellular localization.
Promotion by Kaiso of EGFR-Induced Prostate Cancer Cell Migration and Invasion
To further define the function of Kaiso in prostate cancer cells, DU-145 and PC-3 cells were stably transduced with a plasmid vector containing the sh-Kaiso silencing sequence (Figure 4A). Both sh-Kaiso DU-145 and sh-Kaiso PC-3 clones showed delayed migration, even in the presence of EGF stimulation, as measured by wound-healing assays (Figure 4, B and C). These results show that Kaiso is a mediator of EGFR-induced migration of prostate cancer cells. For cancer cells to invade surrounding tissue, the cells must degrade the underlying basement membrane. To determine the function of Kaiso in invasion by prostate cancer cells, sh-Kaiso PC-3 and sh-DU-145 were seeded onto a filter coated with Matrigel and compared with cells exposed to the vector only. Suppression of endogenous Kaiso expression resulted in inhibition of cell invasion, resulting in a reduction in the ability of the cells to invade through Matrigel (Figure 4D).
Figure 4.
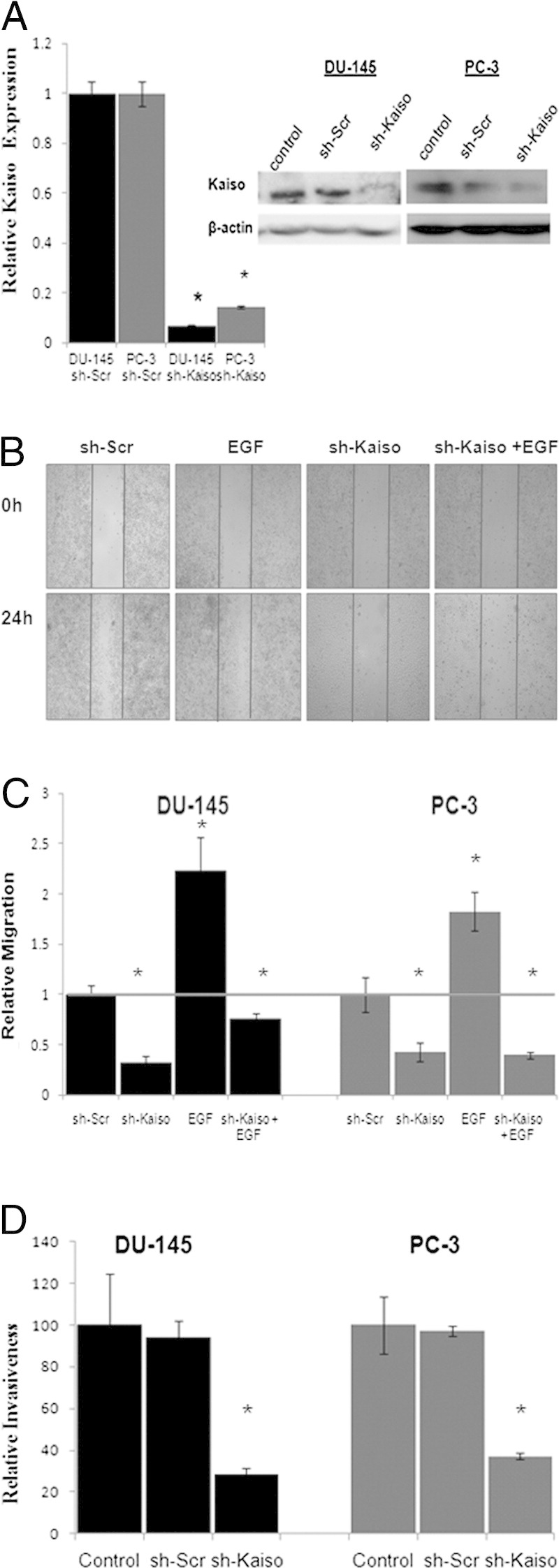
Kaiso is required for EGF-induced cell migration and invasion. A: shRNA Kaiso down-regulated Kaiso levels at mRNA as determined by quantitative RT-PCR with Kaiso-specific TaqMan primers and HPRT1 as the loading control or protein levels by immunoblot. B: β-actin served as loading control, shRNA DU-145 or PC-3 cells were wounded and treated with EGF for 24 hours. Vertical bars indicate the starting area migration on day 0. Photographs were taken at ×100 magnification and DU-145 images serve as representative images of both DU-145 and PC-3 cell lines. C: Quantification of area migrated in the presence or absence of EGF stimulation in shRNA DU-145 or PC-3 cells compared with scrambled control si-Scr show that Kaiso depletion significantly decreased cell migration. Data were normalized to control (gray bar). D: shRNA DU-145 and PC-3 cells were plated on Matrigel-coated filters and the invasive cells were fixed, stained with crystal violet, and counted. shRNA Kaiso cells show decreased invasiveness compared with sh-Scr and controls. All data presented are the mean of three independent experiments ± SE. *P < 0.05.
Repression of E-Cadherin by Kaiso in Prostate Cancer Cells
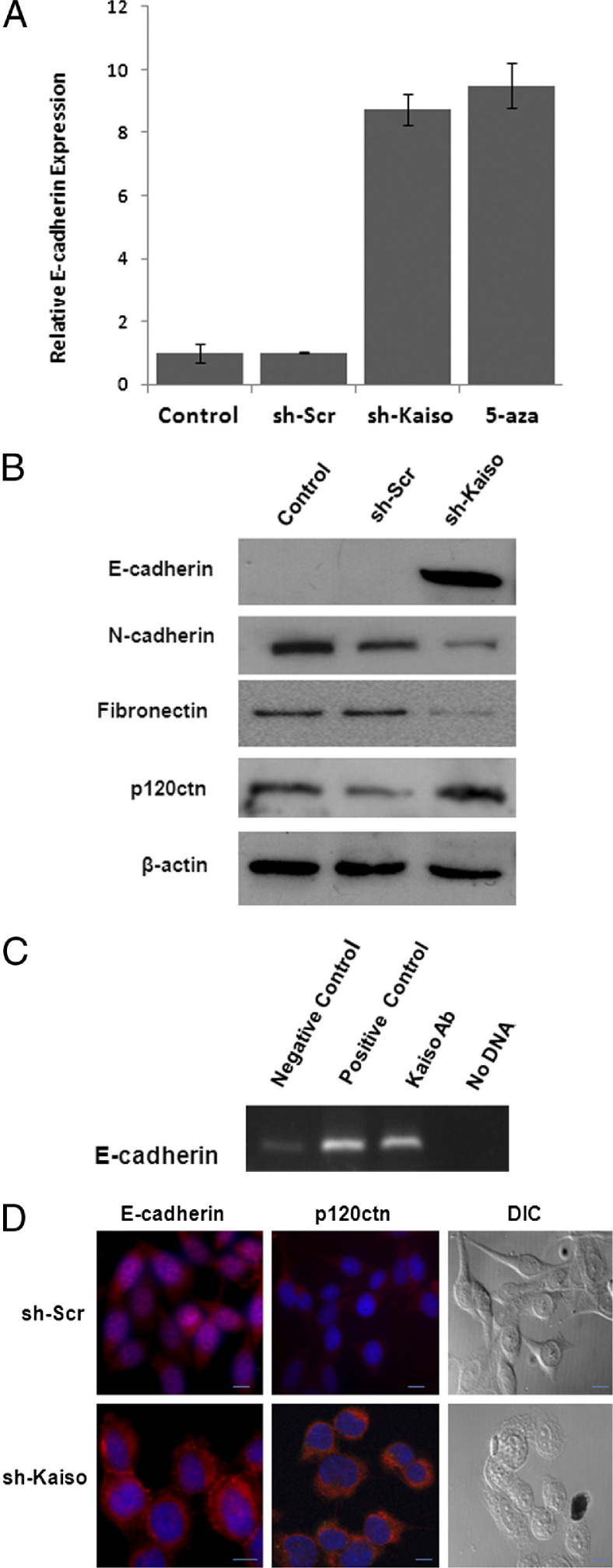
In various cancer types, increased cell migration and invasion has been attributed to growth factor–induced loss of E-cadherin or to hypermethylation of the E-cadherin promoter.17,18 Because Kaiso has high affinity for methylated dinucleotide sequences and is regulated by EGFR (Figure 3A), we next sought to determine whether suppression of Kaiso restored E-cadherin expression in sh-Kaiso PC-3 cells. Control PC-3 cells and vector-only cells showed no E-cadherin expression, as previously reported.18 sh-Kaiso PC-3 cells, however, show eightfold increased expression of E-cadherin mRNA, as measured by quantitative RT-PCR. Furthermore, the level of re-expression was comparable with that of PC-3 cells exposed to the demethylating agent 5-aza-2′-deoxycytidine (Figure 5A). There was also an increase in epithelial markers E-cadherin and p120ctn expression, and a decrease in mesenchymal markers N-cadherin and fibronectin expression at the protein level, as determined by immunoblots (Figure 5B). To determine whether Kaiso directly binds to E-cadherin, we performed a chromatin immunoprecipitation assay. Immunoprecipitated DNA was incubated with anti-Kaiso antibody, anti-RNA pol II (positive control), or IgG antibody (negative control), and subjected to PCR with specific primers designed to amplify the Kaiso (mCGmCG) binding sites in the E-cadherin promoter region. Our results showed that the Kaiso antibody (not negative control IgG antibody) enriched a mCGmCG fragment within the E-cadherin promoter (Figure 5C). These results show that Kaiso can bind directly to methylated regions in the E-cadherin promoter in PC-3 cells.
Figure 5.
Kaiso regulates E-cadherin expression. A: E-cadherin mRNA levels were determined by quantitative RT-PCR in sh-Kaiso PC-3 cells, sh-Scr (vector only), and control cell treated with demethylating agent, 5-aza-2′-deoxycytidine (5-aza) using E-cadherin TaqMan-specific primer with HPRT1 as the loading control. Data were normalized to control (n = 4) ± SE B: sh-Kaiso PC-3 cells, sh-Scr (vector only), and control lysates were immunoblotted with an anti–E-cadherin, anti–N-cadherin antibody, antifibronectin antibody, and anti-p120ctn antibody. β-actin served as loading control. Shown is one of two representative blot series. C: Chromatin sample from PC-3 cells was subjected to chromatin immunoprecipitation by mouse IgG (lane 1) and specific antibodies against RNA pol II (lane 2) and Kaiso (6F/6F8, ChIP grade, lane 3). Mouse IgG was used as a negative control. Lane 4 was no DNA negative control. Chromatin immunoprecipitation products were analyzed by real-time PCR specific E-cadherin primer set (−1290 to −1570) to amplify the methylated region. D: E-cadherin and p120ctn localization was determined by immunofluorescence in sh-Kaiso or sh-Scr PC-3 cells using anti–E-cadherin (red) and DAPI nuclear stain (blue). Arrows indicate E-cadherin staining at cell–cell junctions. Differential interference contrast (DIC) images show an altered cellular morphology in sh-Kaiso PC-3 compared with sh-Scr PC-3 cells (bar, 25 μmol/L).
It is well recognized that membrane expression of E-cadherin regulates cell polarity and increases cell–cell cohesiveness, limiting the migratory ability of tumor cells.10,19 Therefore, we performed immunofluorescence for E-cadherin and p120ctn in sh-Kaiso PC-3 cells compared with vector only sh-Scr PC-3 cells. sh-Kaiso cells showed E-cadherin at cell–cell contacts as well as increased p120ctn, which is rate limiting for E-cadherin stability,20,21 at the cellular membrane (Figure 5D). Furthermore, sh-Kaiso PC-3 cells also showed more of an epithelial morphology compared with the mesenchymal morphology shown by sh-Scr PC-3 cells (Figure 5C). Collectively, these results suggest that EGFR-regulated expression and subcellular re-localization of Kaiso promotes methylation-related gene silencing of E-cadherin.
Discussion
Kaiso previously was observed to be localized predominately in the cytoplasm of various tumor types, including prostate cancer.22 Herein, we observed positive nuclear expression of Kaiso in aggressive tumors from prostate cancer patients. This expression profile was reproducible, using two commercially available antibodies to both the 6F8 and 12H epitopes. Semiquantitative analysis of Kaiso expression shows that nuclear expression significantly correlates with increasing tumor grade and Gleason score. A correlation with nuclear expression and PSA levels also was observed, although it was not found to be significant, possibly owing to the limited number of patients with documented PSA information. Although a large number of patients showed nuclear positivity, we indeed observed cytoplasmic expression within our patient cohort. Low levels of expression were observed in the cytoplasm in normal and benign patients. Critical analysis of a subpopulation of normal/tumor paired samples from 13 individual patients showed that although Kaiso expression was cytoplasmic in normal tissues, the corresponding malignant samples showed a shift to the nuclear compartment. Furthermore, colorectal tumors from the Muc2−/− in vivo mouse model also showed significant increases in Kaiso expression, as well as positive nuclear expression compared with normal matched control.23 These findings further emphasize a role for nuclear Kaiso in tumorigenesis.
Various cell types have displayed subcellular localization of Kaiso in culture. A report by Soubry et al22 showed that micronenvironmental differences, such as two-dimensional versus three-dimensional Matrigel culture conditions or cell density, influences both subcellular localization and expression. Dense three-dimensional cultures of nontumorigenic MCF-10A cells over a multiday period, showed a cytoplasmic re-localization and eventual loss of Kaiso expression as cultures become dense, which also is associated with E-cadherin present at the membrane. HT29 or SW48 colon cancer cells, which display nuclear Kaiso, did not show a difference in Kaiso localization, even under hypoxic conditions. However, no single factor has been associated with localization of Kaiso in the cytoplasmic and nuclear compartments to date.22,24,25 EGF, abundantly secreted in the primary tumor microenvironment,26,27 is up-regulated during hypoxia,28 and is a robust promoter of cell migration, invasion, and metastasis.14,29 Commonly used DU-145 and PC-3 cells, and a DU-145 WT cell line, in which we overexpress EGFR,14,30 reveal that the more aggressive DU-145 WT and PC-3 cells show increased Kaiso expression and nuclear localization, whereas LNCaP and DU-145 cells show lower levels and an increased ratio of cytoplasmic-to-nuclear Kaiso. Furthermore, this is independent of culture density. This would suggest that Kaiso subcellular localization, at least in part, is influenced by Kaiso levels within the cell. There are two lines of evidence in support of this hypothesis. First, we observed that EGF stimulation results in an increased expression and a cytoplasmic-to-nuclear expression shift in DU-145 cells. However, in the reverse experiment in DU-145 WT and PC-3 cells, blocking EGFR signaling resulted in a decrease in overall Kaiso levels in both the cytoplasmic and nuclear compartments. Second, Madin-Darby canine kidney epithelial cells and MCF-7 breast cancer cells, which both display Kaiso predominately in the cytoplasm, display immediate nuclear expression after overexpression with Kaiso cDNA in both cell lines.31 The fact that activation or attenuation of EGFR signaling, as opposed to cell density alone, modulated Kaiso expression and localization is likely owing to reinforced autocrine signaling in the highly aggressive carcinoma cell lines compared with noncarcinoma and/or early stage carcinoma cell lines, which do not possess this feature. Although we did not determine whether Kaiso is phosphorylated in response to EGF, it appears that Kaiso is similar to other cancer-related transcription factors (ERK and ZEB1) that normally reside in the cytoplasm until they are signaled to translocate to the nucleus. These findings establish that expression and subcellular localization of Kaiso is at least partially influenced by EGFR.
We have found in prostate carcinoma lines that inhibition of the autocrine EGFR loop (and likely the EGFR-induced hepatocyte growth factor/c-met autocrine loop32), either by direct disruption of the signaling loop or by secondary site signaling trans-attenuation, results in E-cadherin re-expression and a re-localization of both p120ctn and E-cadherin to cell–cell contacts.18,33,34 E-cadherin expression is down-regulated by two mechanisms: posttranslational modification35 or hypermethylation of promoter.36 In addition to E-cadherin, the proposed Kaiso target gene SA100A4 (mts-1) typically is silenced by methylation in epithelial tumors,5,37 and has been implicated in EGFR-mediated cell migration as well.18,38,39 Our results showed that shRNA-targeted depletion of Kaiso in both DU-145 and PC-3 cells showed decreased cell migration and invasion, even in the presence of EGF stimulation. Furthermore, sh-Kaiso PC-3 cells, which have a partially methylated E-cadherin promoter,40 re-express E-cadherin at RNA and protein levels similar to those caused by exposure to the demethylating agent 5-aza-2′-deoxycytidine. Similar to findings in NIH3T3 cells,5 we did observe that Kaiso directly binds to CpG-rich regions in the E-cadherin promoter. As a result of E-cadherin re-expression, sh-Kaiso cells also displayed morphologic changes, which coincided with decreases in the mesenchymal markers N-cadherin and fibronectin. Together these markers are of clinical importance in determining epithelial versus mesenchymal cells in various tumor types including prostate cancer.41 We did not observe any changes in S100A4 or MMP expression in this model (data not shown), although both have been implicated in EGFR signaling. This is surprising, given that both SA100A4 and MMP-7 have been linked to Kaiso through methylation or consensus sequence binding.12,37 However, collectively, our findings suggest that Kaiso is a promoter of cell migration through loss of cell–cell cohesiveness.
These observations provide a plausible explanation for a number of events during the progression to aggressiveness in epithelial tumors. For example, E-cadherin promoter is hypermethylated in most early stage lobular breast tumors; however, expression of E-cadherin protein is retained. It is only in late-stage tumors, which coincide with our observed nuclear Kaiso shift, that a lack of E-cadherin protein expression coincides with promoter hypermethylation.42 More specifically, immunohistochemical staining displayed loss of p120ctn and E-cadherin expression at the leading edge of squamous cell carcinomas, which coincides with nuclear Kaiso positivity.22 Although a Kaiso-p120ctn complex has been observed in the nucleus of other cell types,15,24,43 similar to squamous cell carcinomas, nuclear p120ctn has not been observed in prostate tumors.44,45 We did not observe nuclear p120ctn in any of the prostate cancer cell lines, even after EGF treatment (data not shown). Thus, the p120ctn–Kaiso relationship in the nucleus, at least in prostate cancer, does not appear to contribute to aggressiveness.
The observation that Kaiso was increased in African American patients was intriguing. Several reports have shown that EGFR is overexpressed in African American prostate cancer patients.46 In addition, SOS1, which is a regulator of EGFR expression and downstream signaling, also is increased in African American prostate cancer patients as well.47 Because our findings show that Kaiso expression is positivity-influenced by EGFR activation, it is possible that overexpression of EGFR contributes to increased Kaiso levels in this patient population. Although this currently remains speculative, it does begin to provide more insight into why African American prostate cancer patients show more aggressive disease progression than do white patients in epidemiologic studies.48,49 More work should be performed to further define this relationship, specifically in African American prostate cancer patients.
In summary, Kaiso cytoplasmic-to-nuclear localization correlates with many features of prostate cancer progression, including race. Epithelial-to-mesenchymal transition has been identified as a common mechanism underlying therapeutic resistance and has been linked to poor prognosis in many types of cancer, including prostate cancer.50 The fact that we found that Kaiso is regulated through EGFR activity provides additional mechanistic insight into the signaling pathway that apparently contributes to aggressive prostate cancer. Because a large number of tumor/metastasis suppressor genes are silenced as a result of methylation, Kaiso could be a central regulator of many key events that contribute to tumorigenesis and aggressiveness. Targeting of growth factor receptors has shown minimal therapeutic effects for prostate cancers. Nevertheless, targeting of downstream mediators of metastasis, such as Kaiso, could be a rational approach for developing a new target for directed therapies.
Acknowledgment
We thank the members of the Yates laboratory for their comments and discussions.
Footnotes
Supported by the Department of Defense Prostate Cancer Research Program (PC073977 to C.Y.), the NIH/Research Centers for Minority Research (RCMI) (G12 RR03059-21A1 to C.Y.), a pilot project of the NIH/National Cancer Institute (U54 CA118623 to C.Y.), and a Veterans Affairs (VA) Merit Award.
J.J. and H.W. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.08.008.
Supplementary data
References
- 1.Stangelberger A., Waldert M., Djavan B. Prostate cancer in elderly men. Rev Urol. 2008;10:111–119. [PMC free article] [PubMed] [Google Scholar]
- 2.Lopes E.C., Valls E., Figueroa M.E., Mazur A., Meng F.G., Chiosis G., Laird P.W., Schreiber-Agus N., Greally J.M., Prokhortchouk E., Melnick A. Kaiso contributes to DNA methylation-dependent silencing of tumor suppressor genes in colon cancer cell lines. Cancer Res. 2008;68:7258–7263. doi: 10.1158/0008-5472.CAN-08-0344. [DOI] [PubMed] [Google Scholar]
- 3.Yoon H.G., Chan D.W., Reynolds A.B., Qin J., Wong J. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein Kaiso. Mol Cell. 2003;12:723–734. doi: 10.1016/j.molcel.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Daniel J.M., Reynolds A.B. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prokhortchouk A., Hendrich B., Jorgensen H., Ruzov A., Wilm M., Georgiev G., Bird A., Prokhortchouk E. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes Dev. 2001;15:1613–1618. doi: 10.1101/gad.198501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai S.D., Wang Y., Miao Y., Zhao Y., Zhang Y., Jiang G.Y., Zhang P.X., Yang Z.Q., Wang E.H. Cytoplasmic Kaiso is associated with poor prognosis in non-small cell lung cancer. BMC Cancer. 2009;9:178. doi: 10.1186/1471-2407-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells A., Welsh J.B., Lazar C.S., Wiley H.S., Gill G.N., Rosenfeld M.G. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- 8.Manne U., Myers R.B., Srivastava S., Grizzle W.E. Re: loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 1997;89:585–586. doi: 10.1093/jnci/89.8.585. [DOI] [PubMed] [Google Scholar]
- 9.Grizzle W., Myers R., Manne U., Stockard C., Harkins L., Srivastava S. Factors Affecting Immunohistochemical Evaluation of Biomarker Expression in Neoplasia. In: Walaszek M.Ha.Z., editor. Humana Press, Inc; New Jersey: 1998. pp. 161–179. [Google Scholar]
- 10.Graham T.R., Zhau H.E., Odero-Marah V.A., Osunkoya A.O., Kimbro K.S., Tighiouart M., Liu T., Simons J.W., O'Regan R.M. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68:2479–2488. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- 11.Yates C.C., Whaley D., Kulasekeran P., Hancock W.W., Lu B., Bodnar R., Newsome J., Hebda P.A., Wells A. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol. 2007;171:484–495. doi: 10.2353/ajpath.2007.061092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel J.M., Spring C.M., Crawford H.C., Reynolds A.B., Baig A. The p120(ctn)-binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res. 2002;30:2911–2919. doi: 10.1093/nar/gkf398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie H., Turner T., Wang M.H., Singh R.K., Siegal G.P., Wells A. In vitro invasiveness of DU-145 human prostate carcinoma cells is modulated by EGF receptor-mediated signals. Clin Exp Metastasis. 1995;13:407–419. doi: 10.1007/BF00118180. [DOI] [PubMed] [Google Scholar]
- 14.Turner T., Chen P., Goodly L.J., Wells A. EGF receptor signaling enhances in vivo invasiveness of DU-145 human prostate carcinoma cells. Clin Exp Metastasis. 1996;14:409–418. doi: 10.1007/BF00123400. [DOI] [PubMed] [Google Scholar]
- 15.Gan Y., Shi C., Inge L., Hibner M., Balducci J., Huang Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene. 2010;29:4947–4958. doi: 10.1038/onc.2010.240. [DOI] [PubMed] [Google Scholar]
- 16.Traish A.M., Morgentaler A. Epidermal growth factor receptor expression escapes androgen regulation in prostate cancer: a potential molecular switch for tumour growth. Br J Cancer. 2009;101:1949–1956. doi: 10.1038/sj.bjc.6605376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L.C., Zhao H., Nakajima K., Oh B.R., Ribeiro Filho L.A., Carroll P., Dahiya R. Methylation of the E-cadherin gene promoter correlates with progression of prostate cancer. J Urol. 2001;166:705–709. [PubMed] [Google Scholar]
- 18.Yates C.C., Shepard C.R., Stolz D.B., Wells A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br J Cancer. 2007;96:1246–1252. doi: 10.1038/sj.bjc.6603700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chunthapong J., Seftor E.A., Khalkhali-Ellis Z., Seftor R.E., Amir S., Lubaroff D.M., Heidger P.M., Jr, Hendrix M.J. Dual roles of E-cadherin in prostate cancer invasion. J Cell Biochem. 2004;91:649–661. doi: 10.1002/jcb.20032. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds A.B., Carnahan R.H. Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer. Semin Cell Dev Biol. 2004;15:657–663. doi: 10.1016/j.semcdb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Miao Y., Liu N., Zhang Y., Liu Y., Yu J.H., Dai S.D., Xu H.T., Wang E.H. p120ctn isoform 1 expression significantly correlates with abnormal expression of E-cadherin and poor survival of lung cancer patients. Med Oncol. 2010;27:880–886. doi: 10.1007/s12032-009-9300-2. [DOI] [PubMed] [Google Scholar]
- 22.Soubry A., van Hengel J., Parthoens E., Colpaert C., Van Marck E., Waltregny D., Reynolds A.B., van Roy F. Expression and nuclear location of the transcriptional repressor Kaiso is regulated by the tumor microenvironment. Cancer Res. 2005;65:2224–2233. doi: 10.1158/0008-5472.CAN-04-2020. [DOI] [PubMed] [Google Scholar]
- 23.Prokhortchouk A., Sansom O., Selfridge J., Caballero I.M., Salozhin S., Aithozhina D., Cerchietti L., Meng F.G., Augenlicht L.H., Mariadason J.M., Hendrich B., Melnick A., Prokhortchouk E., Clarke A., Bird A. Kaiso-deficient mice show resistance to intestinal cancer. Mol Cell Biol. 2006;26:199–208. doi: 10.1128/MCB.26.1.199-208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly K.F., Spring C.M., Otchere A.A., Daniel J.M. NLS-dependent nuclear localization of p120ctn is necessary to relieve Kaiso-mediated transcriptional repression. J Cell Sci. 2004;117:2675–2686. doi: 10.1242/jcs.01101. [DOI] [PubMed] [Google Scholar]
- 25.Park J.I., Kim S.W., Lyons J.P., JI H., Nguyen T.T., Cho K., Barton M.C., Deroo T., Vleminckx K., Moon R.T., McCrea P.D. Kaiso/p120-catenin and TCF/beta-catenin complexes coordinately regulate canonical Wnt gene targets. Dev Cell. 2005;8:843–854. doi: 10.1016/j.devcel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Amalinei C. [Reciprocal epithelio-stromal interactions in normal and neoplastic prostate]: Romanian. Rev Med Chir Soc Med Nat Iasi. 2006;110:391–398. [PubMed] [Google Scholar]
- 27.Lin J., Freeman M.R. Transactivation of ErbB1 and ErbB2 receptors by angiotensin II in normal human prostate stromal cells. Prostate. 2003;54:1–7. doi: 10.1002/pros.10160. [DOI] [PubMed] [Google Scholar]
- 28.Fechner G., Muller G., Schmidt D., Garbe S., Hauser S., Vaupel P., Muller S.C. Evaluation of hypoxia-mediated growth factors in a novel bladder cancer animal model. Anticancer Res. 2007;27:4225–4231. [PubMed] [Google Scholar]
- 29.Angelucci A., Gravina G.L., Rucci N., Millimaggi D., Festuccia C., Muzi P., Teti A., Vicentini C., Bologna M. Suppression of EGF-R signaling reduces the incidence of prostate cancer metastasis in nude mice. Endocr Relat Cancer. 2006;13:197–210. doi: 10.1677/erc.1.01100. [DOI] [PubMed] [Google Scholar]
- 30.Yates C., Wells A., Turner T. Luteinising hormone-releasing hormone analogue reverses the cell adhesion profile of EGFR overexpressing DU-145 human prostate carcinoma subline. Br J Cancer. 2005;92:366–375. doi: 10.1038/sj.bjc.6602350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel J.M., Ireton R.C., Reynolds A.B. Monoclonal antibodies to Kaiso: a novel transcription factor and p120ctn-binding protein. Hybridoma. 2001;20:159–166. doi: 10.1089/027245701750293484. [DOI] [PubMed] [Google Scholar]
- 32.Mamoune A., Kassis J., Kharait S., Kloeker S., Manos E., Jones D.A., Wells A. DU145 human prostate carcinoma invasiveness is modulated by urokinase receptor (uPAR) downstream of epidermal growth factor receptor (EGFR) signaling. Exp Cell Res. 2004;299:91–100. doi: 10.1016/j.yexcr.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Wells A., Yates C., Shepard C.R. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis. 2008;25:621–628. doi: 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yates C., Shepard C.R., Papworth G., Dash A., Beer Stolz D., Tannenbaum S., Griffith L., Wells A. Novel three-dimensional organotypic liver bioreactor to directly visualize early events in metastatic progression. Adv Cancer Res. 2007;97:225–246. doi: 10.1016/S0065-230X(06)97010-9. [DOI] [PubMed] [Google Scholar]
- 35.Jawhari A.U., Farthing M.J., Pignatelli M. The E-cadherin/epidermal growth factor receptor interaction: a hypothesis of reciprocal and reversible control of intercellular adhesion and cell proliferation. J Pathol. 1999;187:155–157. doi: 10.1002/(SICI)1096-9896(199901)187:2<155::AID-PATH193>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 36.Graff J.R., Herman J.G., Lapidus R.G., Chopra H., Xu R., Jarrard D.F., Isaacs W.B., Pitha P.M., Davidson N.E., Baylin S.B. E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res. 1995;55:5195–5199. [PubMed] [Google Scholar]
- 37.Ogden S.R., Wroblewski L.E., Weydig C., Romero-Gallo J., O'Brien D.P., Israel D.A., Krishna U.S., Fingleton B., Reynolds A.B., Wessler S., Peek R.M., Jr p120 and Kaiso regulate Helicobacter pylori-induced expression of matrix metalloproteinase-7. Mol Biol Cell. 2008;19:4110–4121. doi: 10.1091/mbc.E08-03-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mimori K., Yamashita K., Ohta M., Yoshinaga K., Ishikawa K., Ishii H., Utsunomiya T., Barnard G.F., Inoue H., Mori M. Coexpression of matrix metalloproteinase-7 (MMP-7) and epidermal growth factor (EGF) receptor in colorectal cancer: an EGF receptor tyrosine kinase inhibitor is effective against MMP-7-expressing cancer cells. Clin Cancer Res. 2004;10:8243–8249. doi: 10.1158/1078-0432.CCR-04-0849. [DOI] [PubMed] [Google Scholar]
- 39.Klingelhofer J., Moller H.D., Sumer E.U., Berg C.H., Poulsen M., Kiryushko D., Soroka V., Ambartsumian N., Grigorian M., Lukanidin E.M. Epidermal growth factor receptor ligands as new extracellular targets for the metastasis-promoting S100A4 protein. FEBS J. 2009;276:5936–5948. doi: 10.1111/j.1742-4658.2009.07274.x. [DOI] [PubMed] [Google Scholar]
- 40.Reinhold W.C., Reimers M.A., Maunakea A.K., Kim S., Lababidi S., Scherf U., Shankavaram U.T., Ziegler M.S., Stewart C., Kouros-Mehr H., Cui H., Dolginow D., Scudiero D.A., Pommier Y.G., Munroe D.J., Feinberg A.P., Weinstein J.N. Detailed DNA methylation profiles of the E-cadherin promoter in the NCI-60 cancer cells. Mol Cancer Ther. 2007;6:391–403. doi: 10.1158/1535-7163.MCT-06-0609. [DOI] [PubMed] [Google Scholar]
- 41.Gravdal K., Halvorsen O.J., Haukaas S.A., Akslen L.A. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 42.Zou D., Yoon H.S., Perez D., Weeks R.J., Guilford P., Humar B. Epigenetic silencing in non-neoplastic epithelia identifies E-cadherin (CDH1) as a target for chemoprevention of lobular neoplasia. J Pathol. 2009;218:265–272. doi: 10.1002/path.2541. [DOI] [PubMed] [Google Scholar]
- 43.Kelly K.F., Otchere A.A., Graham M., Daniel J.M. Nuclear import of the BTB/POZ transcriptional regulator Kaiso. J Cell Sci. 2004;117:6143–6152. doi: 10.1242/jcs.01541. [DOI] [PubMed] [Google Scholar]
- 44.Lu Q., Dobbs L.J., Gregory C.W., Lanford G.W., Revelo M.P., Shappell S., Chen Y.H. Increased expression of delta-catenin/neural plakophilin-related armadillo protein is associated with the down-regulation and redistribution of E-cadherin and p120ctn in human prostate cancer. Hum Pathol. 2005;36:1037–1048. doi: 10.1016/j.humpath.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Kallakury B.V., Sheehan C.E., Winn-Deen E., Oliver J., Fisher H.A., Kaufman R.P., Jr, Ross J.S. Decreased expression of catenins (alpha and beta), p120 CTN, and E-cadherin cell adhesion proteins and E-cadherin gene promoter methylation in prostatic adenocarcinomas. Cancer. 2001;92:2786–2795. doi: 10.1002/1097-0142(20011201)92:11<2786::aid-cncr10128>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 46.Shuch B., Mikhail M., Satagopan J., Lee P., Yee H., Chang C., Cordon-Cardo C., Taneja S.S., Osman I. Racial disparity of epidermal growth factor receptor expression in prostate cancer. J Clin Oncol. 2004;22:4725–4729. doi: 10.1200/JCO.2004.06.134. [DOI] [PubMed] [Google Scholar]
- 47.Timofeeva O.A., Zhang X., Ressom H.W., Varghese R.S., Kallakury B.V., Wang K., Ji Y., Cheema A., Jung M., Brown M.L., Rhim J.S., Dritschilo A. Enhanced expression of SOS1 is detected in prostate cancer epithelial cells from African-American men. Int J Oncol. 2009;35:751–760. [PMC free article] [PubMed] [Google Scholar]
- 48.Evans S., Metcalfe C., Ibrahim F., Persad R., Ben-Shlomo Y. Investigating black-white differences in prostate cancer prognosis: a systematic review and meta-analysis. Int J Cancer. 2008;123:430–435. doi: 10.1002/ijc.23500. [DOI] [PubMed] [Google Scholar]
- 49.Berger A.D., Satagopan J., Lee P., Taneja S.S., Osman I. Differences in clinicopathologic features of prostate cancer between black and white patients treated in the 1990s and 2000s. Urology. 2006;67:120–124. doi: 10.1016/j.urology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Nauseef J.T., Henry M.D. Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle? Nat Rev Urol. 2011;8:428–439. doi: 10.1038/nrurol.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.