Abstract
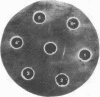
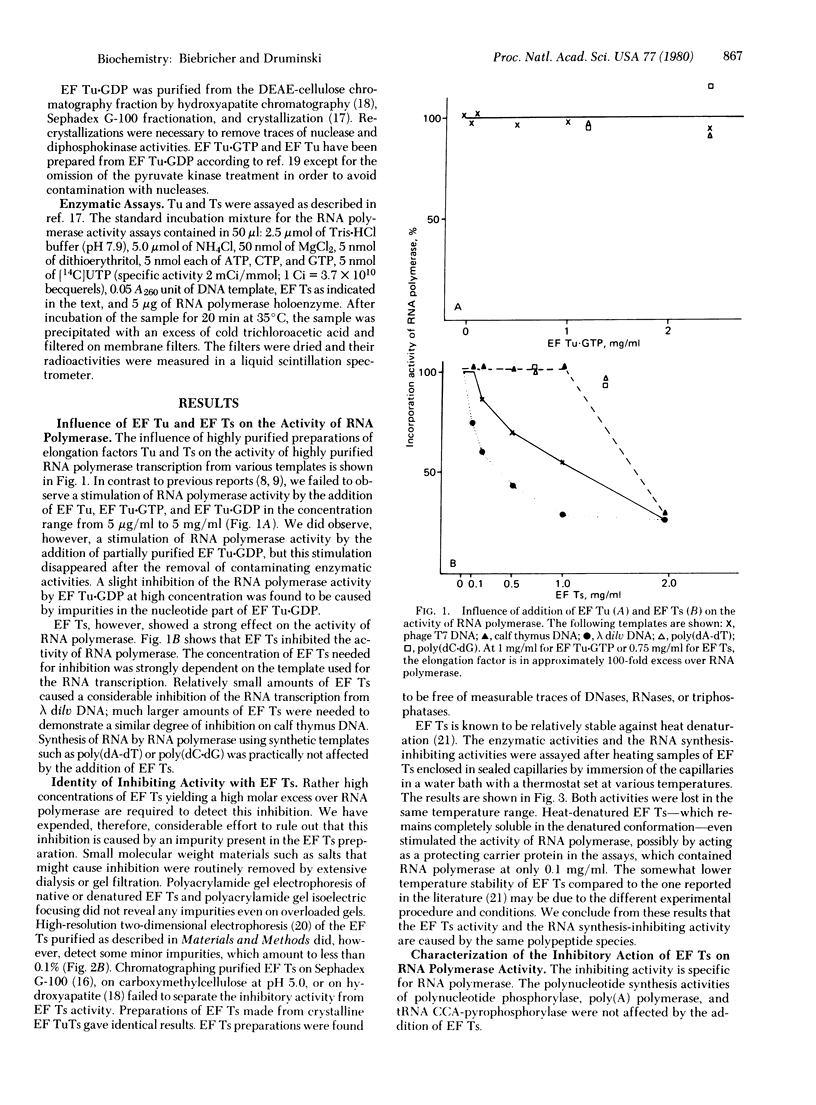
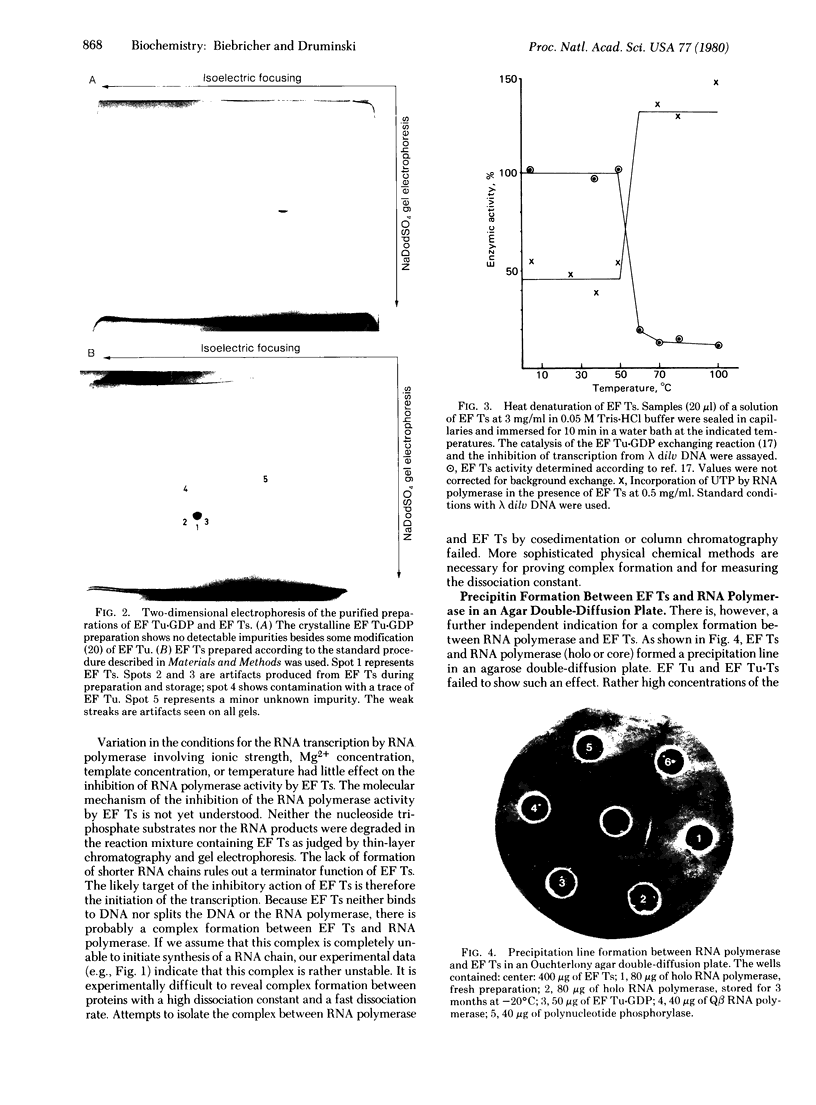
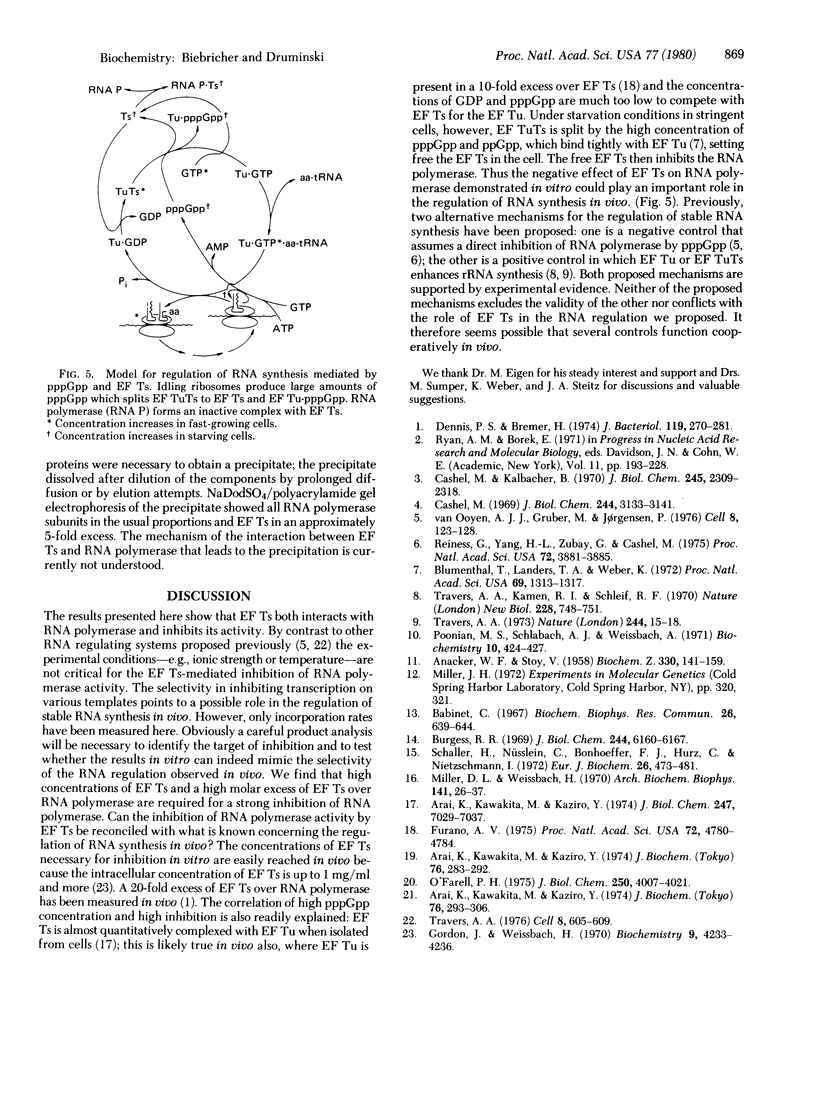
The transcribing activity of DNA-dependent RNA polymerase (nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) from Escherichia coli is inhibited in vitro by addition of preparations of elongation factor Ts purified to homogeneity. The inhibitory activity of elongation factor Ts on the RNA polymerase activity and the enzymatic activity of elongation factor Ts show the same temperature sensitivity. The extent of inhibition is strongly dependent on the template used for transcription. A mechanism for the control of RNA synthesis in vivo based on this inhibition found in vitro is proposed.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANACKER W. F., STOY V. Proteinchromatogrphie an Calciumphosphat. I. Reinigung von Nitrat-reduktase aus Weizenblättern. Biochem Z. 1958;330(2):141–159. [PubMed] [Google Scholar]
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Arai K., Kawakita M., Kaziro Y. Studies on the polypeptide elongation factors from E. coli. IV. Crystalline Tu-GTP, Tu-Gpp(CH2)p, and phenylalanyl-tRNA-Tu-GTP complex. J Biochem. 1974 Aug;76(2):283–292. doi: 10.1093/oxfordjournals.jbchem.a130570. [DOI] [PubMed] [Google Scholar]
- Arai K., Kawakita M., Kaziro Y. Studies on the polypeptide elongation factors from E. coli. V. Properties of various complexes containing EF-Tu and EF-Ts. J Biochem. 1974 Aug;76(2):293–306. doi: 10.1093/oxfordjournals.jbchem.a130571. [DOI] [PubMed] [Google Scholar]
- Babinet C. A new method for the purification of RNA-polymerase. Biochem Biophys Res Commun. 1967 Mar 21;26(6):639–644. doi: 10.1016/s0006-291x(67)80119-0. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B-r. J Bacteriol. 1974 Jul;119(1):270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V. Content of elongation factor Tu in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4780–4784. doi: 10.1073/pnas.72.12.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Weissbach H. Immunochemical distinction between the Escherichia coli polypeptide chain elongation factors Tu and Ts. Biochemistry. 1970 Oct 13;9(21):4233–4236. doi: 10.1021/bi00823a028. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Studies on the purification and properties of factor Tu from E. coli. Arch Biochem Biophys. 1970 Nov;141(1):26–37. doi: 10.1016/0003-9861(70)90102-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Poonian M. S., Schlabach A. J., Weissbach A. Covalent attachment of nucleic acids to agarose for affinity chromatography. Biochemistry. 1971 Feb 2;10(3):424–427. doi: 10.1021/bi00779a011. [DOI] [PubMed] [Google Scholar]
- Schaller H., Nüsslein C., Bonhoeffer F. J., Kurz C., Nietzschmann I. Affinity chromatography of DNA-binding enzymes on single-stranded DNA-agarose columns. Eur J Biochem. 1972 Apr 24;26(4):474–481. doi: 10.1111/j.1432-1033.1972.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Kamen R. I., Schleif R. F. Factor necessary for ribosomal RNA synthesis. Nature. 1970 Nov 21;228(5273):748–751. doi: 10.1038/228748a0. [DOI] [PubMed] [Google Scholar]
- Travers A. Control of ribosomal RNA synthesis in vitro. Nature. 1973 Jul 6;244(5410):15–18. doi: 10.1038/244015a0. [DOI] [PubMed] [Google Scholar]
- Travers A. Multiple modes of ribosomal RNA transcription in vitro. Cell. 1976 Aug;8(4):605–609. doi: 10.1016/0092-8674(76)90228-2. [DOI] [PubMed] [Google Scholar]
- van Ooyen A. J., Gruber M., Jorgensen P. The mechanism of action of ppGpp on rRNA synthesis in vitro. Cell. 1976 May;8(1):123–128. doi: 10.1016/0092-8674(76)90193-8. [DOI] [PubMed] [Google Scholar]