Abstract
Parkinson's disease is characterized by a substantial cognitive heterogeneity, which is apparent in different profiles and levels of severity. To date, a distinct clinical profile for patients with a potential risk of developing dementia still has to be identified. We introduce a data-driven approach to detect different cognitive profiles and stages. Comprehensive neuropsychological data sets from a cohort of 121 Parkinson's disease patients with and without dementia were explored by a factor analysis to characterize different cognitive domains. Based on the factor scores that represent individual performance in each domain, hierarchical cluster analyses determined whether subgroups of Parkinson's disease patients show varying cognitive profiles. A six-factor solution accounting for 65.2% of total variance fitted best to our data and revealed high internal consistencies (Cronbach's alpha coefficients >0.6). The cluster analyses suggested two independent patient clusters with different cognitive profiles. They differed only in severity of cognitive impairment and self-reported limitation of activities of daily living function but not in motor performance, disease duration, or dopaminergic medication. Based on a data-driven approach, divers cognitive profiles were identified, which separated early and more advanced stages of cognitive impairment in Parkinson's disease without dementia. Importantly, these profiles were independent of motor progression.
1. Introduction
Beyond the characteristic motor signs, a number of nonmotor symptoms including cognitive aspects are gaining increasing attention in Parkinson's disease (PD). Recent work revealed a substantial heterogeneity of cognitive impairment, which is apparent in both different profiles and different levels of severity ranging from slight and early cognitive changes up to the diagnosis of PD dementia (PDD) [1]. With the demand for an early, individualized, and better therapeutic treatment, the focus is now to identify the patients with a potentially higher risk of dementia [2]. Already a few cognitive alterations may enhance the risk of PDD. However, it needs to be noted that exactly the same cognitive alterations can also be found in PD patients who did not develop dementia [3, 4]. On subtest level, some studies suggest that early executive dysfunction is predictive of the conversion to dementia [2, 3], while others argue for a crucial role of impaired visuospatial and language abilities [4]. Thus, a distinct clinical profile for patients with a potential risk of developing PDD still has to be identified [1].
Most authors define the stage of mild cognitive impairment in PD (PD-MCI) [5] and the involvement of different cognitive domains by a predominately theoretical [6, 7] rather than a data-driven, quantitative approach. Some data-driven studies on different subtypes of PD found a poor test performance in varying neuropsychological tasks, suggesting that these help to diagnose PDD [8, 9]. Recently, a cluster analysis on a small cohort of PD patients without dementia revealed differences in severity of cognitive deterioration but not in cognitive phenotypes [10]. We here propose an approach to identify cognitive profiles based on performance differences within quantitatively determined domains using standardized factor scores. These standard values can be used to compare the mean performance of each single PD patient investigated here to the mean performance of the present, total PD cohort. Our study thus was designed by (i) a data-driven identification of different cognitive domains in a large cohort of both PD patients with and without dementia and (ii) a data-based subdivision of PD patients according to exactly these standardized domain scores to characterize subgroups with divers cognitive profiles and potentially different levels of cognitive impairment.
2. Materials and Methods
2.1. Patients
We investigated 121 patients with idiopathic PD according to the UK Brain Bank criteria [11] admitted to the outpatient clinic of the Department of Neurodegenerative Diseases University of Tuebingen and the Gertrudis Clinic Leun-Biskirchen Germany. All patients received their usual, optimized medication and were able to complete all cognitive tasks (Tables 1 and 2 provide all relevant details).
Table 1.
Demographic, clinical, and neuropsychological characteristics of the two PD groups (PDD patients included) as identified by the first hierarchical cluster analysis.
| Total group of PD and PDD |
Cluster-I PD only* |
Cluster-II PD and PDD* |
P value | |
|---|---|---|---|---|
| Number, (%) | 121 (100.0) | 50 (41.3) | 71 (58.7) | |
| Male gender, n (%) | 81 (66.9) | 33 (66.0) | 48 (67.6) | 0.85 |
| Age at evaluation, years | 68.7 ± 6.9 | 66.1 ± 6.7 | 70.6 ± 6.4 | <0.001 |
| Neurological assessment | ||||
| Disease duration, years | 6.6 ± 5.1 | 5.8 ± 4.7 | 7.1 ± 5.2 | 0.17 |
| UPDRS-III motor score (on state) | 28.3 ± 11.5 | 25.3 ± 11.7 | 30.5 ± 11.0 | 0.01 |
| Hoehn and Yahr stage, n (%) | ||||
| 1 | 12 (9.9) | 6 (12.0) | 6 (8.5) | |
| 1.5 | 5 (4.1) | 3 (6.0) | 2 (2.8) | |
| 2 | 49 (40.5) | 24 (48.0) | 25 (35.2) | 0.06 |
| 2.5 | 32 (26.5) | 14 (28.0) | 18 (25.4) | |
| 3 | 16 (13.2) | 3 (6.0) | 13 (18.3) | |
| 4 | 7 (5.8) | 0 (0) | 7 (9.9) | |
| Medication, daily dose | ||||
| Levodopa dose (mg) | 351.4 ± 304.7 | 330.3 ± 343.6 | 366.3 ± 275.6 | 0.55 |
| Levodopa equivalent dose (mg) | 573.8 ± 417.2 | 585.0 ± 470.2 | 566.0 ± 378.8 | 0.35 |
| Antidepressants, n (%) | 28 (23.1) | 9 (18.0) | 19 (26.8) | 0.12 |
| Neuroleptics, n (%) | 14 (11.6) | 1 (2.0) | 13 (18.3) | 0.11 |
| PD patients with dementia, PDD | 24 (19.8) | 0 (0) | 24 (33.8) | <0.001 |
| MMSE (raw score) | 26.6 ± 2.6 | 28.1 ± 1.5 | 25.5 ± 2.6 | <0.001 |
| Beck's Depression inventory | 8.7 ± 5.7 | 7.1 ± 4.7 | 9.9 ± 6.0 | 0.009 |
| Neuropsychiatric inventory | 4.7 ± 7.3 | 3.5 ± 5.5 | 5.5 ± 8.2 | 0.22 |
| Parkinson's disease Questionnaire-PDQ-39 | 5.4 ± 4.2 | 3.4 ± 3.1 | 6.8 ± 4.3 | 0.001 |
| NAI: NAA-ADL inventory, patients' self-assessment |
48.8 ± 32.4 | 67.2 ± 22.0 | 35.9 ± 32.4 | <0.001 |
| NAI: NAB-ADL inventory, caregivers' assessment |
50.3 ± 31.0 | 67.1 ± 25.1 | 38.4 ± 29.4 | <0.001 |
|
| ||||
| Factor scores |
Standardized values of the total PD cohort (PD norms) |
Mean group performance in relation to the standardized values, that is, below (−) versus above (+) the average of the total PD cohort | ||
|
| ||||
| Factor 1, frontal lobe function | 0 ± 1 | 0.41 ± 0.71 | −0.29 ± 1.07 | 0.005 |
| Factor 2, word-list memory and recall | 0 ± 1 | 0.56 ± 0.90 | −0.39 ± 0.87 | <0.001 |
| Factor 3, attention | 0 ± 1 | −0.58 ± 0.87 | 0.37 ± 0.91 | <0.001 |
| Factor 4, logical memory | 0 ± 1 | −0.68 ± 1.06 | 0.48 ± 0.60 | <0.001 |
| Factor 5, praxis and visual perception | 0 ± 1 | −0.92 ± 0.90 | 0.56 ± 0.76 | <0.001 |
| Factor 6, fluency and naming ability | 0 ± 1 | 0.62 ± 0.84 | −0.44 ± 0.86 | <0.001 |
|
| ||||
| Neuropsychological assessment | Mean group performance in relation to the standardized values provided by the test manuals, that is, below (−) and above (+) the average of healthy control subjects | |||
|
| ||||
| Factor 1: | ||||
| Trail Making Test, part B | 49.8 ± 39.3 | 75.2 ± 29.8 | 32.0 ± 35.3 | <0.001 |
| Tower of London | 39.0 ± 26.7 | 48.5 ± 24.0 | 32.3 ± 26.6 | 0.024 |
| NAI: digit span | 56.3 ± 31.4 | 70.3 ± 28.7 | 46.5 ± 29.6 | <0.001 |
| NAI: figure test | 52.1 ± 27.2 | 62.6 ± 20.1 | 44.7 ± 29.1 | 0.006 |
| Berlin Apraxia Test (raw score) | 35.7 ± 5.5 | 38.7 ± 3.2 | 33.7 ± 5.9 | <0.001 |
| Factor 2: | ||||
| CERAD: word-list memory | 29.9 ± 27.3 | 48.5 ± 16.8 | 16.8 ± 20.3 | <0.001 |
| CERAD: word-list recall | 36.9 ± 30.0 | 54.8 ± 29.1 | 24.2 ± 23.7 | <0.001 |
| CERAD: word-list recognition | 40.7 ± 34.5 | 57.2 ± 30.3 | 20.0 ± 32.7 | <0.001 |
| CERAD: word-list intrusion | 42.3 ± 33.7 | 53.7 ± 28.0 | 34.2 ± 34.0 | 0.005 |
| Factor 3: | ||||
| TAP: phasic alertness | 55.4 ± 29.2 | 44.2 ± 25.7 | 63.3 ± 29.1 | 0.001 |
| TAP: Go-Nogo, median RT | 40.5 ± 33.5 | 60.6 ± 29.1 | 26.4 ± 29.0 | <0.001 |
| Factor 4: | ||||
| WMS-R: logical memory I | 24.4 ± 26.2 | 41.9 ± 26.9 | 12.0 ± 17.0 | <0.001 |
| WMS-R: logical memory II | 25.9 ± 26.2 | 45.0 ± 26.1 | 12.5 ± 16.0 | <0.001 |
| Factor 5: | ||||
| CERAD: praxis | 40.2 ± 35.7 | 63.6 ± 29.9 | 23.7 ± 29.8 | <0.001 |
| CERAD: praxis delay | 35.6 ± 35.8 | 59.0 ± 34.8 | 19.2 ± 26.2 | <0.001 |
| VOSP: object decision | 42.4 ± 30.1 | 56.4 ± 29.6 | 32.5 ± 26.5 | 0.001 |
| Factor 6: | ||||
| CERAD: verbal fluency | 30.0 ± 28.1 | 52.8 ± 27.7 | 24.2 ± 21.8 | <0.001 |
| CERAD: Boston naming test | 46.9 ± 33.2 | 63.1 ± 28.1 | 35.4 ± 31.9 | <0.001 |
| Trail Making Test, part A | 45.8 ± 35.4 | 70.2 ± 28.0 | 28.6 ± 29.6 | <0.001 |
Data are given as mean ± SD; lower standard (that is, percentile rank) scores in neuropsychological tests indicate poorer performance except for the MMSE; UPDRS-III: Unified Parkinson's Disease Rating Scale part III; P values are corrected for age and UPDRS-III motor score; %: Percentage; PD: Parkinson's disease; PDD: Parkinson's disease with dementia; LEDD: levodopa equivalence daily dose according to the following conversion rates: 100 mg Levodopa equalling 125 mg Levodopa sustained release, 1 mg Pergolide, 1 mg Pramipexol, 5 mg Ropinirole, 5 mg Rotigotin, 10 mg Bromocriptine, 10 mg Apomorphine, 1/5 Entacapone, 1.5 mg Cabergoline. Additionally, 5% was added to the total Levodopa dose for every 5 mg of Selegiline or 1 mg of Rasagiline, up to a maximum of 10%; MMSE: Minimental State Examination; NAI: Nuernberger Alters Inventar; RT: reaction time; *Grouping of patients with PDD following the first hierarchical cluster analysis.
Table 2.
Demographic, clinical, and neuropsychological characteristics of the two PD groups (PDD patients excluded) as identified by the second hierarchical cluster analysis.
| PDD only | Cluster-I PD only |
Cluster-II PD only |
P value | |
|---|---|---|---|---|
| Number, (%) | 24 (19.8) | 43 (35.6) | 54 (44.6) | |
| Male gender, n (%) | 18 (75.0) | 28 (65.1) | 35 (64.8) | 0.97 |
| Age at evaluation, years | 74.2 ± 5.9 | 65.7 ± 6.0 | 68.7 ± 6.5 | 0.02 |
| Neurological assessment | ||||
| Disease duration, years | 9.5 ± 5.6 | 5.6 ± 4.3 | 6.1 ± 4.9 | 0.66 |
| UPDRS-III motor score (on state) | 37.5 ± 11.3 | 25.3 ± 11.5 | 26.7 ± 9.6 | 0.52 |
| Hoehn and Yahr stage, n (%) | ||||
| 1 | 0 (0) | 6 (14.0) | 6 (11.1) | |
| 1.5 | 0 (0) | 3 (7.0) | 2 (3.7) | |
| 2 | 7 (29.2) | 21 (48.8) | 21 (38.9) | 0.60 |
| 2.5 | 3 (12.5) | 11 (25.6) | 18 (33.3) | |
| 3 | 8 (33.3) | 2 (4.7) | 6 (11.1) | |
| 4 | 6 (25.0) | 0 (0) | 1 (1.9) | |
| Medication, daily dose | ||||
| Levodopa dose (mg) | 457.2 ± 256.0 | 323.5 ± 352.6 | 326.6 ± 277.2 | 0.57 |
| Levodopa equivalent dose (mg) | 665.7 ± 407.8 | 554.7 ± 435.7 | 548.2 ± 408.3 | 0.82 |
| Antidepressants, n (%) | 7 (29.2) | 8 (18.6) | 13 (24.1) | 0.14 |
| Neuroleptics, n (%) | 7 (29.2) | 1 (2.3) | 6 (11.1) | 0.67 |
| MMSE (raw score) | 23.0 ± 2.7 | 28.1 ± 1.5 | 26.9 ± 1.5 | 0.003 |
| Beck's Depression Inventory | 11.6 ± 6.2 | 7.1 ± 4.8 | 8.8 ± 5.8 | 0.06 |
| Neuropsychiatric inventory | 9.5 ± 10.3 | 3.3 ± 5.1 | 3.3 ± 5.1 | 0.73 |
| Parkinson's disease questionnaire-PDQ-39 | 10.4 ± 4.2 | 3.5 ± 3.1 | 4.9 ± 3.2 | 0.03 |
| NAI: NAA-ADL inventory, patients' self-assessment |
8.8 ± 16.9 | 67.6 ± 22.3 | 51.7 ± 28.9 | 0.002 |
| NAI: NAB-ADL inventory, caregivers' assessment |
11.5 ± 10.3 | 66.8 ± 24.6 | 54.4 ± 27.1 | 0.03 |
|
| ||||
| Factor scores | Mean group performance in relation to the standardized values (0 ± 1), that is, below (−) and above (+) the average of the total PD cohort | |||
|
| ||||
| Factor 1, frontal lobe function | −1.11 ± 0.86 | 0.32 ± 0.71 | 0.23 ± 0.92 | 0.64 |
| Factor 2, word-list memory and recall | −0.76 ± 0.90 | 0.73 ± 0.84 | −0.25 ± 0.77 | <0.001 |
| Factor 3, attention | 0.61 ± 0.98 | −0.56 ± 0.92 | 0.17 ± 0.85 | <0.001 |
| Factor 4, logical memory | 0.51 ± 0.67 | −0.73 ± 1.13 | 0.35 ± 0.62 | <0.001 |
| Factor 5, praxis and visual perception |
0.92 ± 0.70 | −0.67 ± 0.90 | 0.14 ± 0.79 | <0.001 |
| Factor 6, fluency and naming ability | −0.86 ± 0.81 | 0.69 ± 0.81 | −0.17 ± 0.84 | <0.001 |
|
| ||||
| Neuropsychological assessment | Mean group performance in relation to the standardized values provided by the test manuals, that is, below (−) and above (+) the average of healthy control subjects | |||
|
| ||||
| Factor 1: | ||||
| Trail Making Test, part B | 5.3 ± 15.1 | 75.9 ± 30.2 | 48.9 ± 35.0 | 0.001 |
| Tower of London | 14.8 ± 22.0 | 47.7 ± 25.1 | 42.9 ± 23.8 | 0.46 |
| NAI: digit span | 39.0 ± 32.2 | 66.9 ± 29.5 | 55.6 ± 29.4 | 0.04 |
| NAI: figure test | 26.2 ± 29.4 | 62.4 ± 21.3 | 55.3 ± 23.0 | 0.12 |
| Berlin Apraxia Test (raw score) | 29.3 ± 6.6 | 38.6 ± 3.3 | 36.4 ± 3.9 | 0.005 |
| Factor 2: | ||||
| CERAD: word-list memory | 12.7 ± 21.7 | 51.2 ± 25.6 | 21.9 ± 19.4 | <0.001 |
| CERAD: word-list recall | 18.0 ± 24.5 | 60.2 ± 27.4 | 26.7 ± 21.8 | <0.001 |
| CERAD: word-list recognition | 15.5 ± 24.0 | 62.8 ± 27.8 | 34.2 ± 33.3 | <0.001 |
| CERAD: word-list intrusion | 20.8 ± 31.9 | 56.9 ± 29.0 | 40.1 ± 32.8 | 0.008 |
| Factor 3: | ||||
| TAP: phasic alertness | 68.7 ± 32.9 | 43.6 ± 27.1 | 59.0 ± 25.9 | 0.007 |
| TAP: Go-Nogo, median RT | 16.5 ± 28.1 | 60.0 ± 30.1 | 37.3 ± 30.4 | <0.001 |
| Factor 4: | ||||
| WMS-R: logical memory I | 8.5 ± 16.5 | 43.2 ± 27.9 | 16.4 ± 18.8 | <0.001 |
| WMS-R: logical memory II | 7.5 ± 12.2 | 47.3 ± 27.2 | 17.1 ± 17.2 | <0.001 |
| Factor 5: | ||||
| CERAD: praxis | 13.1 ± 27.4 | 63.6 ± 31.3 | 33.7 ± 31.1 | <0.001 |
| CERAD: praxis delay | 10.3 ± 21.5 | 58.9 ± 35.2 | 28.3 ± 30.5 | <0.001 |
| VOSP: object decision | 19.9 ± 19.6 | 56.6 ± 29.2 | 41.1 ± 28.6 | 0.04 |
| Factor 6: | ||||
| CERAD: verbal fluency | 17.6 ± 20.8 | 55.4 ± 27.3 | 28.7 ± 22.3 | <0.001 |
| CERAD: Boston naming test | 21.3 ± 28.8 | 64.4 ± 28.3 | 44.3 ± 30.8 | 0.001 |
| Trail Making Test, part A | 9.0 ± 16.8 | 71.8 ± 27.3 | 41.4 ± 30.6 | <0.001 |
Data are given as mean ± SD; lower standard (i.e. percentile rank) scores in neuropsychological tests indicate poorer performance except for the MMSE; UPDRS-III: Unified Parkinson's Disease Rating Scale part III; P values are corrected for age; %: Percentage; PD: Parkinson's disease; PDD: Parkinson's disease with dementia; LEDD: Levodopa equivalence daily dose according to the following conversion rates: 100 mg Levodopa equalling 125 mg Levodopa sustained release, 1 mg Pergolide, 1 mg Pramipexol, 5 mg Ropinirole, 5 mg Rotigotin, 10 mg Bromocriptine, 10 mg Apomorphine, 1/5 Entacapone, 1.5 mg Cabergoline. Additionally, 5% was added to the total levodopa dose for every 5 mg of Selegiline or 1 mg of Rasagiline, up to a maximum of 10%; MMSE: Minimental State Examination; NAI: Nuernberger Alters Inventar; RT: reaction time.
Inclusion criteria were: age ≥ 50 years, onset of dementia >2 years from PD diagnosis, adequate or corrected hearing/visual abilities, and German as first language. Exclusion criteria were: other neurological diseases affecting the central nervous system, prior surgery for PD, medication interfering with cognition (i.e., hypnotics or tranquilizers), or a minimental state examination [12] score < 18 (testing not feasible). Patients with identified gene mutations and those reporting more than 2 first or second degree relatives with a definitive diagnosis of PD [7] were excluded to avoid monogenetic subgroups in which cognition can be specifically altered [13]. Most patients identified their spouse as caregiver (72.7%); the others indicated an adult child (12.4%), other family members (8.3%), or nonfamily members (6.6%). The study was approved by the local ethical committee. All patients and caregivers gave written informed consent.
2.2. Neuropsychological Assessment
All examinations and expert evaluations were carried out within a week. Each patient underwent a comprehensive test battery according to the recommendations of the Movement Disorder Society (MDS) Task Force [7] comprising the following tests (see also supplemented Table 1 in Supplementary material available online in doi:10.1155/2012/910757): the Tower of London (TL-D, conceptualization) [14], the Trail Making Test parts A and B (TMT-A, TMT-B; psychomotor speed, set shifting) [15], the digit span (DS forward and backward, working memory capacity), and the figure test (FT, nonverbal memory, set maintenance) from the Nuernberger Alters Inventory a battery to assess mild to advanced cognitive impairments (NAI) [16], as well as 8 subtests, that is, word-list memory (WL), including the number of false positive words (WL-I), word-list recall (WL delay), word-list recognition (WL-R, all verbal memory), the Boston naming test (BNT, language), verbal fluency (VF, animal naming, executive function), as well as the copy task (praxis) and its delayed recall (praxis delay, both visuospatial abilities) from the German version of the Consortium to Establish a Registry on Alzheimer's Disease (CERAD) [15]. Further, we applied the logical memory tasks (LogI and LogII) of the Wechsler Memory Scale-Revised (WMS-R, verbal memory) [17], the object decision part (OD) of the Visual Object and Space Perception battery (VOSP, visuospatial abilities) [18], the Berlin Apraxia Test (BAXT, ideomotor apraxia) [19], and two computerized reaction time tasks (Alertness, Go-Nogo; Test of Attentional Performance, TAP) [20], the first providing a specific measure of the ability to respond to a critical stimulus following an auditory cue (phasic alertness). Analyses are based on standard norms (percentile rank scores) of healthy German control subjects as published in the manuals. Data are corrected either for age (NAI, WMS-R, VOSP) or for age and education (CERAD, TAP, TMT, TL-D).
Diagnostic criteria for dementia followed the recommendation of the MDS task force for probable PDD [7]. In detail, our criteria for PDD were (i) scores 1.5 SD (PR < 7) in at least one test below published group norms of healthy control subjects in at least 2 of the following cognitive domains: attention (as measured by Alertness, Go-Nogo), executive function (DS, TL-D, TMT-A, TMT-B, VF), visuospatial function (praxis, praxis delay, OD), memory (LogI, LogII, WL, WL-I, WL delay, WL-R, FT), or language ability (BNT), (ii) cognitive decline with insidious onset and slow progression reported by either the patients or their proxies, and (iii) impairment of nonmotor activities of daily living (ADL) as verified by a structured patient and/or caregiver interview on the perception of cognitively influenced ADL function in the domestic environment.
2.3. Motor Performance, Behavioral Disturbances, and ADL
Clinical assessment included the Hoehn and Yahr stage, the unified Parkinson disease rating scale part III (UPDRS-III) [21] for motor function and the neuropsychiatric inventory (NPI) for behavioral disturbances (e.g., hallucinations) [22]. The Parkinson's Disease Questionnaire (PDQ-39) [23] and the Beck's Depression Inventory (BDI) [24] provided self-rating scales of health related quality of life and mood. Further, we calculated age-corrected standard scores of the patients' ADL function using (i) a self-rating questionnaire (Nuernberger-Alters-Alltagsaktivitaeten-Skala, NAA) and (ii) its corresponding scale for proxies (Nuernberger-Alters-Beobachtungsskala, NAB) [16].
Full drug history includes the total daily dose of levodopa only and the total daily dose of all dopaminomimetics, which was calculated as levodopa equivalent daily dose (LEDD) according to published conversion rates (see legends of Tables 1 and 2, and [25–27]).
2.4. Data Analyses
2.4.1. Identification of Cognitive Domains: Exploratory Factor Analysis (EFA)
First, we performed an EFA on all neuropsychological data to identify cognitive domains in the PD group. The factor matrix was optimized with oblique rotation, because factors were expected to be correlated [28]. Variables with a factor loading >0.5 or <−0.5 were considered as core variables for a given factor [29]. The Kaiser's criterion (eigenvalue > 1) and the corresponding scree plot results were used to determine the number of factors to be retained. Internal consistency was verified by Cronbach's alpha (α) coefficient, which was required to be higher than α > 0.6 for each factor to indicate a sufficient internal consistency structure [6].
2.4.2. Identification of Characteristic Profiles in the PD Cohort: Hierarchical Cluster Analysis
Based on the factor loadings from the EFA, individual factor scores were calculated by the Anderson-Rubin algorithm, which produces factor scores that are uncorrelated and standardized with a mean of zero and a standard deviation of 1. A patient with a factor score of 0 (zero) thus shows average performance compared to the total PD cohort; a positive score indicates performance above, a negative score below the average of the whole patient group investigated here.
With these individual factor scores, we performed two separate hierarchical cluster analyses to identify patient subgroups with different cognitive profiles (Ward's-method). The first analysis was conducted on the total group of PD patients (n = 121), the second, for validation purposes, on all PD patients except those with dementia (n = 97). As PDD patients can be expected to suffer from more severe cognitive, motor, and behavioral impairment, we evaluated this specific influence on our study results by excluding them from the second HCA. Student's t- (age, disease duration, UPDRS-III motor score) or χ²-tests (gender, Hoehn & Yahr stage) were used for between-group comparisons. Analyses of covariance and Mantel-Haenszel statistics accounted for differences in demographics and disease severity. Because of the number of comparisons, the significance levels were set at P = 0.01 to optimize the trade-off between false positive protection (type 1) and sensitivity/power (type 2 error). All analyses were conducted using SPSS 17.0 (SPSS Inc, Chicago, III, USA).
3. Results
3.1. Patient Characteristics
Of the 121 patients, seventeen (14.0%) received L-dopa, 25 (20.7%) dopamine agonists, and one (0.8%) patient amantadine only. Both L-dopa and dopamine agonists were given to 78 (64.5%) patients, of whom 27 additionally received amantadine. Twenty-four patients (19.8%) of the total cohort had PDD; six of them were treated with cholinesterase inhibitors (see Table 1 for further details).
3.2. Cognitive Domains
Table 3 shows the result of the EFA and the internal consistency analysis. The EFA was verified by the Bartlett's test of sphericity (χ 2 = 872.7, df = 171, P < 0.001) and the Kaiser-Meyer-Olkin measures of sampling adequacy (MSA = 0.82). A six-factor solution accounting for 65.2% of total variance explained by the factors fits best to our data (see supplemented Table 1 for information on the rejected five-factor solution). The internal consistency structure of each factor was found to be at least moderately high (0.67 ≤ α ≤ 0.86). Factor 1 consisted of five neuropsychological tasks explaining 33.5% of total variance (α = 0.67). Like the TL-D [14], each test was mainly related to aspects of frontal lobe function. Factor 2 comprised four tasks on word-list memory and recall (α = 0.78, 8.2% of variance explained). Both Factor 3 (6.8% of variance explained, α = −0.85) and Factor 4 (5.9%, α = 0.86) consisted of two neuropsychological tasks on attention and episodic memory (5.7% of variance explained), respectively. Factor 5 consisted of tasks on praxis and visual perception (α = 0.70, 5.7% of variance explained). Factor 6 comprised three tests that are mainly used for assessing fluency and naming ability (5.2% of variance explained, α = 0.70).
Table 3.
Results of the exploratory factor analysis and the consistency analysis on the neuropsychological test results of all 121 patients indicating a six-factor model of cognition in PD.
| Factor interpretation | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 |
|---|---|---|---|---|---|---|
| frontal lobe function |
word-list memory and recall |
attention | logical memory | praxis and visual perception | fluency and naming ability | |
| Tower of London | 0.62 | |||||
| Trail Making Test, part B | 0.64 | |||||
| NAI: digit span | 0.65 | |||||
| NAI: figure test | 0.69 | |||||
| Berlin Apraxia Test (raw score) | 0.66 | |||||
| CERAD: word-list memory | 0.77 | |||||
| CERAD: word-list recall | 0.84 | |||||
| CERAD: word-list recognition | 0.71 | |||||
| CERAD: word-list intrusion | 0.77 | |||||
| TAP: phasic alertness | −0.80 | |||||
| TAP: Go-Nogo, median RT | 0.70 | |||||
| WMS-R: logical memory I | 0.88 | |||||
| WMS-R: logical memory II | 0.87 | |||||
| CERAD: praxis | 0.83 | |||||
| CERAD: praxis delay | 0.83 | |||||
| VOSP: object decision | 0.62 | |||||
| CERAD: verbal fluency | 0.84 | |||||
| CERAD: Boston naming test | 0.77 | |||||
| Trail Making Test, part A | 0.66 | |||||
|
| ||||||
| Variance explained (%) | 33.51 | 8.15 | 6.78 | 5.90 | 5.70 | 5.19 |
| Cronbach's alpha coefficient | 0.67 | 0.78 | −0.85 | 0.86 | 0.70 | 0.70 |
Analyses are based on standard norms (i.e percentile rank scores, PR: indicating the patient's relative position in the norm group with a range between 0 and 100) of healthy German control subjects as published in the manuals; data are corrected either for age (NAI, WMS-R, VOSP) or for age and education (CERAD, TAP, TMT, TL-D). Only for the BAXT raw data were used; CERAD: Consortium to Establish a Registry For Alzheimer's Disease, German version; WMS-R: Wechsler Memory Scale-Revised; NAI: Nuernberger Alters Inventory; VOSP: Visual Object and Space Perception battery; TAP: Test of Attentional Performance; RT: Reaction Time.
3.3. Cognitive Profiles in PD
Table 1 refers to the hierarchical cluster analysis on the total group of PD patients (n = 121, incl. 24 PDD), Table 2 to the subsequent hierarchical cluster analysis on all PD patients except those with dementia (n = 97). Both analyses revealed two different, independent clusters regarding the degree of cognitive impairment within the domains defined by the EFA (see Figure 1). Our first hierarchical cluster analysis assigned all 24 patients with PDD to Cluster-II, that is, to the group with poorer neuropsychological test performances (see Table 1, PD and PDD). In contrast, all Cluster-I patients showed less cognitive impairment and, crucially had no dementia (“PD only”). The second hierarchical cluster analysis, carried out for validation purposes, replicated the grouping in 92.8% of all PD patients without dementia (n = 97, Table 2). Most important, no patient initially assigned to the more severely impaired Cluster-II, was regrouped in Cluster-I.
Figure 1.
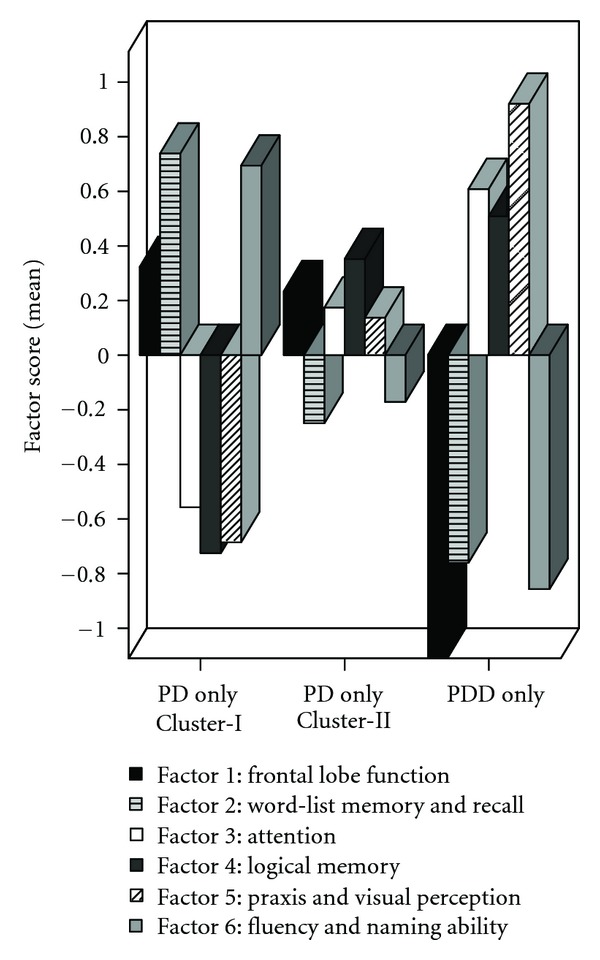
Mean group performance (mean factor scores) of PD patients without dementia clustered into two distinct groups (PD only, n = 43, Cluster-I versus PD only, n = 54, Cluster-II) as well as of the group of PD patients with dementia (PDD only, n = 24).
Our approach of identifying characteristic profiles within the PD cohort (i.e., an identification of those patients with poorer individual performances in the specific tests of the corresponding EFA domain compared to all other PD patients investigated here) revealed a clear-cut division of the six cognitive domains into two subgroups (all P values < 0.005). PD patients with lower factor scores of Factor 3 (attention P < 0.001), Factor 4 (logical memory P < 0.001), and Factor 5 (praxis and visual perception, P < 0.001) were grouped in Cluster-I. In contrast, Cluster-II patients showed lower factor scores in neuropsychological tasks assigned to Factor 1 (frontal lobe function, P < 0.005), Factor 2 (word-list memory and recall, P < 0.001), and Factor 6 (fluency and naming ability, P < 0.001). The analysis without PDD patients revealed comparable results except for frontal lobe functions (Figure 1).
3.4. Subgroup Comparison of Clinical Parameters in Patients with “PD Only”
To identify a cognitive profile in patients who had not developed dementia at the time of examination, we compared the clinical parameters of the two Clusters without PDD (Table 2). Cluster-II tended to be older (P < 0.02). As age is a risk factor for PDD [30], all P values were corrected for it.
No differences were found for motor disability, disease duration, and psychiatric symptoms. Compared to published group norms that are standardized on healthy control subjects (not to the present factor scores), Cluster-II patients showed overall lower performances in most neuropsychological tests. However, comparison on subgroup level without PDD failed significance, for example, for most executive tasks (see Table 2), suggesting that particularly PDD patients had led to significant differences in the first hierarchical cluster analysis because of marked difficulties in this domain.
Regarding the impact of ADL dysfunction on PD, it is notable that members of Cluster-II (without PDD) rated themselves as more impaired (P = 0.002). These self-impressions tended to be confirmed by their caregivers as well as by their reduced quality of life reports (PDQ-39, P = 0.03).
4. Discussion
We introduce a data-driven approach to identify different profiles and stages of cognitive impairment in PD. First, we determined cognitive domains based on a comprehensive neuropsychological test battery, and second we identified subgroups that differ with respect to their standardized individual performance in these domains. Whereas the first part has already been addressed to some extent [8–10, 31], the second part provides a first attempt to identify PD patients with a potentially higher PDD risk using PD related rather than healthy control norms. This method allows the differentiation within the group of those PD patients, who show a severe impairment in almost all cognitive tasks and who thus might have a potential risk of developing dementia. While the standard procedure (i.e., using healthy control standard norms) turned out insufficient to differentiate within this overall severely impaired patient group, our approach of using factor scores revealed varying cognitive profiles that differ with respect to both, severity and most affected cognitive functions.
4.1. Factor Analysis
Focusing on both, internal consistency structure and adequate factor correlations, we found that a six-factor solution fitted best to our data. Our results are in accordance with the theoretical assumption of the MDS Task Force [7] and recent work on the factorial structure of cognition in PD [8, 9] that differentiate between executive, long term memory, and retrieval ability as well as language and visual function. In contrast to others [8], we identified six instead of three domains. The rotation algorithm, the greater sample size, and our larger number of neuropsychological tasks may account for this difference. It is well known that the PD phenotype can vary largely. Thus, our more homogenous cohort without potential genetic variants of PD or other confounding factors could also have influenced the results.
In line with previous observations [8], verbal fluency performance was more closely related to psychomotor speed and language tasks than to other frontal lobe assessments (please compare Factor 1). Thus, it may be concluded that the verbal fluency task addresses a different cognitive aspect than the other frontal lobe instruments used here. This interpretation is further supported by the finding that semantic fluency impairment reflects structural grey matter changes in regions that are known to be involved in language networks [32]. Moreover, this task has been found to correlate with disease severity and motor assessment, which may explain an association to psychomotor speed performance in patients with PD [33, 34].
Another interesting finding is that the BAXT, an inventory for the assessment of ideomotor apraxia, was closely related to other frontal lobe tests in our PD cohort. Actually, patients with frontal lobe dysfunction may also show signs of ideomotor apraxia [35]. PD patients are known to suffer from an action-sequence planning deficit [36] that can at least partly explain the clinical signs of apraxia in PD. Recently, a strong association of finger dexterity with praxis function but not with the Parkinson's symptoms has been described [37]. This finding indicates that impaired finger dexterity in PD probably has an apraxic component, which is clinically more apparent in later disease stages. It thus seems that ideomotor deficits may rather contribute to an incorrect selection of action sequences than to a dysfunction in action semantics as suggested for patients with parietal lobe involvement. Indeed, symptoms of apraxia have been reported repeatedly in PD, although they are not as frequent and evident as in other neurodegenerative disorders [38, 39]. It was not the scope of this study to clarify which mechanism causes impairment in different cognitive domains or even in ideomotor apraxia. Nevertheless, our results suggest that apraxia could be a variant of the dysexecutive syndrome in PD. It might be interesting to address this hypothesis in future research.
The two logical memory subtests of the WMS-R did not reveal high loadings on Factor 2 (list learning and recall). In contrast to the CERAD memory tests, the verbal recall of the logical memory tasks may be more demanding with respect to working memory or metamemory, because it requires memory self-monitoring [40]. Thus, one may argue that the corresponding test performance is more dependent on frontal lobe functions [41]. Additionally, impaired logical memory abilities are known to be related to a decline in dopaminergic activity in the basal ganglia in both, healthy persons and PD patients [42–45]. This finding also supports the assumption that logical memory assessments address aspects of frontal lobe and working memory function and additionally may mirror dopaminergic dysfunction.
4.2. Cluster Analysis
4.2.1. Cognitive Profile of Clusters
Based on the individual factor scores, both analyses revealed two independent groups with a subdivided, domain structure regarding the most affected cognitive functions. Crucially, the groups clustered even more closely without PDD patients (see Figure 1), arguing for a validation of the present grouping by our second analysis.
Cluster-II patients without dementia reported more ADL dysfunctions beyond their objectively more advanced cognitive decline. Interestingly, the CamPaIGN study showed that the PDD diagnosis at followup was linked to poorer semantic fluency at baseline and reduced visuoconstruction [4]. Others found that cognitive progression is strongly associated with memory and visuoconstructive skills [46, 47]. Likewise, all these functions were still more impaired in our overall more affected Cluster-II, even without PDD.
Currently, we can only speculate that Cluster-I and Cluster-II patients suffer from different pathologies. Dopaminergic loss modulates cognition (e.g., attention and psychomotor speed) especially in the early stages [48]. Interestingly, Cluster-I patients showed reduced attention as indicated by the corresponding factor scores. In contrast, advanced PD affects a broad range of cognitive abilities [49] as confirmed by the present Cluster-II. Since this cannot be fully attributed to dopaminergic loss [50], the extent of Lewy body pathology [51], an imbalance of other neurotransmitter systems, a primarily cholinergic deficit [52, 53], or Alzheimer's histopathology [54] should be considered.
4.2.2. Implications for the Characterization of Cognitive Impairment in PD
At first sight there seems to be a contradiction between the cognitive profiles revealed by the two different analyses, that is, by either standard or factor scores. The comparison to the commonly used standard scores showed, as would be expected, that the Cluster-II patients are more impaired in almost all neuropsychological tests. The factor score analysis, however, identified a different cognitive profile in this formerly homogenous patient group (see Figure 1). Crucially, one needs to keep in mind that both, the standardized factor score and the standard score refer to the patients' individual test performance. The major difference is that the results are compared to different standardized group norms. The factor score represents the performance of one individual person in relation to the average performance of the total PD cohort. In contrast, the standard score specifies the individual test performance by normative data from healthy controls comparable with respect to age and education. Most important for the present study is that only the combination of these two sources revealed that patients of Cluster-I are predominately affected in attention performance, visual spatial abilities, and logical memory but not in the other cognitive domains. In contrast, patients of Cluster-II suffer additionally to the impairment of those of Cluster-I from a more extensive impairment in memory, frontal lobe function, fluency, and naming ability. Our alternative approach of analyzing the data driven standardized factor scores (instead of standardized percentile rank scores) offers the opportunity for a more precise differentiation within the PD group. Actually, the presence of two cognitive profiles within the group of PD patients without dementia could only be detected by the use of factor scores and not by the commonly applied standard scores.
At present, the PD-MCI concept [7, 55] is defined theoretically by the severity of dysfunction in one or more cognitive domains. However, its predictive value has not yet been proven [56]. One main difficulty is the heterogeneity of affected cognitive domains and severity of cognitive dysfunction in PD which is supported by many previous [1] as well as our present data. To date, it remains open which of the various cut-off values are most predictive of PDD and which neuropsychological tasks might be the most promising to identify PD patients at risk for dementia according to the MCI concept.
Following our preliminary results of a clinical sample, we argue that a global neuropsychological (domain) score based on standardized assessments and compared to population based PD norms (e.g., factor scores) may help reflect the level of cognitive impairment and its progression more appropriately than various or even single cut-off scores from healthy control subjects. Such PD norms may offer the possibility to specify for each single PD patient whether the deficits occur to a greater or lesser extent compared to other PD patients, resulting in a more sensitive characterization of both kind and severity of cognitive impairment. Such PD norms should be derived from representative PD samples that undergo a well-defined, standardized neuropsychological test battery that is widely used and accepted, for example, following MDS Task Force recommendations [7].
5. Limitations
It needs to be considered that the generalizability of our results is limited by the small sample size. Still, although the cohort is not population based, our PDD patients present the well-known phenotype, that is, they were older and had a longer disease duration (please see [2, 57]).
Further, we are aware of the methodological limitations of explorative factor analyses. Nevertheless, our data driven approach provides a useful alternative to generate even more specific hypotheses on the resulting factor structure, which have to be verified by future research using, for example, confirmatory models. Such studies may offer a promising perspective to evaluate PD patients' cognitive progression or conversion to dementia over time.
6. Conclusions
Our data-driven approach suggests at least two different subtypes of cognitive impairment in PD, which are rather independent of motor function, disease duration, and PD medication but do have an impact on activities of daily living. Moreover, our data driven approach confirms the cognitive domains suggested by the consensus guidelines.
Supplementary Material
Supplementary Table 1: Results of the exploratory factor analysis and consistency analysis (Cronbach's alpha coefficients) of a five-factor model of cognition in Parkinson's Diesease patients indicating highest internal consistency for the presented six-factor solution.
Conflict of Interests
The authors declare that there is no conflict of interests.
Disclosure
Dr. I. Liepelt-Scarfone has received a travel grant from the Movement Disorders Society and a grant from the Dr. Werner Jackstaedt foundation. Dr. S. Gräber reported no disclosures. Dr. M. Fruhmann Berger reported no disclosures. Mrs. Feseker reported no disclosures. Mrs. G. Baysal reported no disclosures. Dr. J. Godau has received honoraria for lectures from UCB and Novartis and travel grants from Novartis and the Movement Disorders Society. Dr. A. Gaenslen reported no disclosures. Dr. I. Csoti received financial contributions from Novartis Pharma AG for the organisation of CME workshops, as well as honoraria for taking part in an expert meeting and advisory boards. She has received honoraria for presentations from Boehringer Ingelheim, TEVA Pharma GmbH, Lundbeck, Desitin, Orion Pharma, and UCB. Dr. H. Huber reported no disclosures. Dr. K. Srulijes reported no disclosures. Dr. K. Brockmann has received honoraria for lectures from GlaxoSmithKline and travel grants from GlaxoSmithKline and the Movement Disorders Society. Dr. D. Berg has served as advisory board member for Novartis, UCBSchwarzPharma, GSK, TEVA, Merz Pharmaceuticals GmbH, received research grants from the Michael J. Fox Foundation, BmBF, Janssen Pharmaceutica, TEVA Pharma GmbH, dPV (German Parkinson's disease association), Solvay and the University of Tuebingen and speakers honoraria from the following companies: Novartis, UCBSchwarzPharma, GSK, TEVA, Lundbeck, Merck, and Boehringer.
Acknowledgments
This study was supported by the Dr. Werner Jackstaedt foundation, with a grant to the first author for the investigation of characteristics of MCI in PD (Grant no. S134-10.032). Special thanks to Josephine Christ and Deborah Prakash for their help in assessing the patients.
References
- 1.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. The Lancet Neurology. 2010;9(12):1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 2.Azuma T, Cruz RF, Bayles KA, Tomoeda CK, Montgomery EB. A longitudinal study of neuropsychological change in individuals with Parkinson's disease. International Journal of Geriatric Psychiatry. 2003;18(12):1115–1120. doi: 10.1002/gps.1022. [DOI] [PubMed] [Google Scholar]
- 3.Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson's disease: a community-based, 4-year longitudinal study. Journal of Geriatric Psychiatry and Neurology. 2005;18(3):149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- 4.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muslimović D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 7.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the Movement Disorder Society Task Force. Movement Disorders. 2007;22(16):2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 8.Vingerhoets G, Verleden S, Santens P, Miatton M, De Reuck J. Predictors of cognitive impairment in advanced Parkinson's disease. Journal of Neurology Neurosurgery and Psychiatry. 2003;74(6):793–796. doi: 10.1136/jnnp.74.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verleden S, Vingerhoets G, Santens P. Heterogeneity of cognitive dysfunction in Parkinson's disease: a cohort study. European Neurology. 2007;58(1):34–40. doi: 10.1159/000102164. [DOI] [PubMed] [Google Scholar]
- 10.McKinlay A, Grace RC, Dalrymple-Alford JC, Roger D. Cognitive characteristics associated with mild cognitive impairment in parkinson's disease. Dementia and Geriatric Cognitive Disorders. 2009;28(2):121–129. doi: 10.1159/000235247. [DOI] [PubMed] [Google Scholar]
- 11.Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. Journal of Neurology Neurosurgery and Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. ‘Mini mental state’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Brockmann K, Srulijes K, Hauser AK, et al. GBA associated PD presents with non-motor characteristics. Neurology. 2011;77:276–280. doi: 10.1212/WNL.0b013e318225ab77. [DOI] [PubMed] [Google Scholar]
- 14.Tucha O, Lange KW. The Tower of London—German Version. Göttingen, Germany: Hogrefe; 2004. [Google Scholar]
- 15.Memory Clinic NPZ. The Consorium to Establish a Registry of Alzheimer's Disease CERAD-Plus. Basel, Switzerland: Basel Memory Clinic-NPZ; 2005. [Google Scholar]
- 16.Oswald WD, Fleischmann UM. Nürnberger-Alters-Inventar (NAI) Vol. 4. Göttingen, Germany: Hogrefe; 1999. [Google Scholar]
- 17.Haerting C, et al. Wechsler Gedächtnistest-Revidierte Fassung Deutsche Adaptation der revidierten Fassung der Wechlser Memory Scale. Bern, Switzerland: Huber; 2000. [Google Scholar]
- 18.Warrington EK, James M. VOSP—Testbatterie für visuelle Objekt- und Raumwahrnehmung. Bury St. Edmunds, UK: Thames Valley Test Company; 1992. [Google Scholar]
- 19.Liepelt I, Trenner MU, Freund S, Engel U, Lueschow A, Platz T. Der Berliner-Apraxie-Test für ideomotorische und ideatorische Apraxie: Bestimmung der Itemkennwerte. Zeitschrift für Neuropsychologie. 2007;18:193–206. [Google Scholar]
- 20.Zimmermann P, Fimm B. Testbatterie zur Aufmerksamkeitsprüfung (Version 1. 7) Herzogenrath, Germany: Psytests; 2002. [Google Scholar]
- 21.Fahn S, Elton RL, UPDRS Program Members . Unified Parkinsons disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinsons Disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 22.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's disease questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age and Ageing. 1997;26(5):353–357. doi: 10.1093/ageing/26.5.353. [DOI] [PubMed] [Google Scholar]
- 24.Hautzinger M, et al. Becks-Depressions-Inventar (BDI) Vol. 2. Bern, Switzerland: Huber; 1995. [Google Scholar]
- 25.Stoffers D, Bosboom JLW, Deijen JB, Wolters EC, Berendse HW, Stam CJ. Slowing of oscillatory brain activity is a stable characteristic of Parkinson's disease without dementia. Brain. 2007;130(7):1847–1860. doi: 10.1093/brain/awm034. [DOI] [PubMed] [Google Scholar]
- 26.Esselink RAJ, De Bie RMA, De Haan RJ, et al. Unilateral pallidotomy versus bilateral subthalamic nucleus stimulation in PD: a randomized trial. Neurology. 2004;62(2):201–207. doi: 10.1212/01.wnl.0000103235.12621.c3. [DOI] [PubMed] [Google Scholar]
- 27.Grosset K, Needleman F, Macphee G, Grosset D. Switching from ergot to nonergot dopamine agonists in Parkinson's disease: a clinical series and five-drug dose conversion table. Movement Disorders. 2004;19(11):1370–1374. doi: 10.1002/mds.20210. [DOI] [PubMed] [Google Scholar]
- 28.Higginson CI, King DS, Levine D, Wheelock VL, Khamphay NO, Sigvardt KA. The relationship between executive function and verbal memory in Parkinson's disease. Brain and Cognition. 2003;52(3):343–352. doi: 10.1016/s0278-2626(03)00180-5. [DOI] [PubMed] [Google Scholar]
- 29.Costello AB, Osborne JW. Best practise in exploratory factor analysis: four Recommendations for getting the most from your analysis. Practical Assessment, Research & Evaluation. 2005;10:173–178. [Google Scholar]
- 30.Aarsland D, Kvaløy JT, Andersen K, et al. The effect of age of onset of PD on risk of dementia. Journal of Neurology. 2007;254(1):38–45. doi: 10.1007/s00415-006-0234-8. [DOI] [PubMed] [Google Scholar]
- 31.Bronnick K, Ehrt U, Emre M, et al. Attentional deficits affect activities of daily living in dementia-associated with Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77(10):1136–1142. doi: 10.1136/jnnp.2006.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira JB, Junqué C, Marti MJ, Ramirez-Ruiz B, Bartrés-Faz D, Tolosa E. Structural brain correlates of verbal fluency in Parkinson's disease. NeuroReport. 2009;20(8):741–744. doi: 10.1097/WNR.0b013e328329370b. [DOI] [PubMed] [Google Scholar]
- 33.Riepe MW, Kassubek J, Tracik F, Ebersbach G. Screening for cognitive impairment in Parkinson's disease—which marker relates to disease severity? Journal of Neural Transmission. 2006;113(10):1463–1468. doi: 10.1007/s00702-006-0433-6. [DOI] [PubMed] [Google Scholar]
- 34.Paschali A, Messinis L, Kargiotis O, et al. SPECT neuroimaging and neuropsychological functions in different stages of Parkinson's disease. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(6):1128–1140. doi: 10.1007/s00259-010-1381-9. [DOI] [PubMed] [Google Scholar]
- 35.Buxbaum LJ, Schwartz MF, Montgomery MW. Ideational apraxia and naturalistic action. Cognitive Neuropsychology. 1998;15:617–643. doi: 10.1080/026432998381032. [DOI] [PubMed] [Google Scholar]
- 36.Qureshi M, Williamson JB, Heilman KM. Ideational apraxia in Parkinson disease. Cognitive and Behavioral Neurology. 2011;24(3):122–127. doi: 10.1097/WNN.0b013e3182343692. [DOI] [PubMed] [Google Scholar]
- 37.Vanbellingen T, Kersten B, Bellion M, et al. Impaired finger dexterity in Parkinson's disease is associated with praxis function. Brain and Cognition. 2011;77(1):48–52. doi: 10.1016/j.bandc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Uluduz D, Ertürk O, Kenangil G, et al. Apraxia in Parkinson's disease and multiple system atrophy. European Journal of Neurology. 2010;17(3):413–418. doi: 10.1111/j.1468-1331.2009.02905.x. [DOI] [PubMed] [Google Scholar]
- 39.Zadikoff C, Lang AE. Apraxia in movement disorders. Brain. 2005;128(7):1480–1497. doi: 10.1093/brain/awh560. [DOI] [PubMed] [Google Scholar]
- 40.Gabrieli JDE. Memory systems analyses of mnemonic disorders in aging and age-related diseases. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(24):13534–13540. doi: 10.1073/pnas.93.24.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: a review. Neuropsychology Review. 2005;15(3):105–130. doi: 10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- 42.Müller U, Wächter T, Barthel H, Reuter M, Von Cramon DY. Striatal [123I]β-CIT SPECT and prefrontal cognitive functions in Parkinson's disease. Journal of Neural Transmission. 2000;107(3):303–319. doi: 10.1007/s007020050025. [DOI] [PubMed] [Google Scholar]
- 43.Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. American Journal of Psychiatry. 1998;155(3):344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 44.Lewis SJG, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson's disease. Neuropsychologia. 2005;43(6):823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Reeves SJ, Grasby PM, Howard RJ, Bantick RA, Asselin MC, Mehta MA. A positron emission tomography (PET) investigation of the role of striatal dopamine (D2) receptor availability in spatial cognition. NeuroImage. 2005;28(1):216–226. doi: 10.1016/j.neuroimage.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 46.Muslimović D, Schmand B, Speelman JD, De Haan RJ. Course of cognitive decline in Parkinson's disease: a meta-analysis. Journal of the International Neuropsychological Society. 2007;13(6):920–932. doi: 10.1017/S1355617707071160. [DOI] [PubMed] [Google Scholar]
- 47.Reid WGJ, Hely MA, Morris JGL, Loy C, Halliday GM. Dementia in Parkinson's disease: a 20-year neuropsychological study (Sydney Multicentre Study) Journal of Neurology, Neurosurgery and Psychiatry. 2011;82(9):1033–1037. doi: 10.1136/jnnp.2010.232678. [DOI] [PubMed] [Google Scholar]
- 48.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neuroscience and Biobehavioral Reviews. 2006;30(1):1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 49.Green J, McDonald WM, Vitek JL, et al. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59(9):1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- 50.Bosboom JLW, Stoffers D, Wolters EC. Cognitive dysfunction and dementia in Parkinson's disease. Journal of Neural Transmission. 2004;111(10-11):1303–1315. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- 51.Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: as prospective, community-based study. Annals of Neurology. 2005;58(5):773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- 52.Hilker R, Thomas AV, Klein JC, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65(11):1716–1722. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- 53.Bohnen NI, Kaufer DI, Hendrickson R, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson's disease and parkinsonian dementia. Journal of Neurology. 2006;253(2):242–247. doi: 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- 54.Mastaglia FL, Johnsen RD, Byrnes ML, Kakulas BA. Prevalence of amyloid-β deposition in the cerebral cortex in Parkinson's disease. Movement Disorders. 2003;18(1):81–86. doi: 10.1002/mds.10295. [DOI] [PubMed] [Google Scholar]
- 55.Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: movement Disorder Society Task Force guidelines. Movement Disorders. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubois B. Is PD-MCI a useful concept? Movement Disorders. 2007;22(9):1215–1216. doi: 10.1002/mds.21566. [DOI] [PubMed] [Google Scholar]
- 57.Aarsland D, Andersen K, Larsen JP, et al. The rate of cognitive decline in Parkinson disease. Archives of Neurology. 2004;61(12):1906–1911. doi: 10.1001/archneur.61.12.1906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Results of the exploratory factor analysis and consistency analysis (Cronbach's alpha coefficients) of a five-factor model of cognition in Parkinson's Diesease patients indicating highest internal consistency for the presented six-factor solution.


