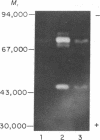
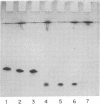
Abstract
Islets of Langerhans isolated from rat pancreas contain and secrete plasminogen activator (PA). Production of PA is increased up to 15-fold by culture in the presence of high concentrations of glucose, and the dose-response curves for the effect of glucose on secretion of PA and on insulin are superimposable. Alloxan, a diabetogenic agent that is selectively cytotoxic for beta cells, abolishes the PA response to glucose. Various agents and hormones that affect beta-cell function affect the secretion of PA in a manner parallel to their modulation of insulin secretion. These observations suggest that PA is produced by beta cells and that enzyme synthesis and secretion are regulated in concert with those of the hormone. The potential role of PA and of plasmin in the physiology of the islets is considered. In particular, because plasmin cleaves proinsulin to a product that is electrophoretically indistinguishable from insulin, it is suggested that the PA/plasmin system may play a part in the conversion of proinsulin to the active hormone.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Recovery of surface membrane in anterior pituitary cells. Variations in traffic detected with anionic and cationic ferritin. J Cell Biol. 1978 Jun;77(3):R35–R42. doi: 10.1083/jcb.77.3.r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- Hellman B., Lernmark A. Evidence for an inhibitor of insulin release in the pancreatic islets. Diabetologia. 1969 Feb;5(1):22–24. doi: 10.1007/BF01212214. [DOI] [PubMed] [Google Scholar]
- Hermann L. S., Deckert T. The effect of epinephrine and isoproterenol on insulin secretion and glucose utilization in isolated islets of Langerhans from mice. Acta Endocrinol (Copenh) 1977 Jan;84(1):105–114. doi: 10.1530/acta.0.0840105. [DOI] [PubMed] [Google Scholar]
- Kalhan S. C., Adam P. A. Inhibitory effect of prednisone on insulin secretion in man: model for duplication of blood glucose concentration. J Clin Endocrinol Metab. 1975 Sep;41(3):600–610. doi: 10.1210/jcem-41-3-600. [DOI] [PubMed] [Google Scholar]
- Kemmler W., Peterson J. D., Steiner D. F. Studies on the conversion of proinsulin to insulin. I. Conversion in vitro with trypsin and carboxypeptidase B. J Biol Chem. 1971 Nov 25;246(22):6786–6791. [PubMed] [Google Scholar]
- Koerker D. J., Ruch W., Chideckel E., Palmer J., Goodner C. J., Ensinck J., Gale C. C. Somatostatin: hypothalamic inhibitor of the endocrine pancreas. Science. 1974 Apr 26;184(4135):482–484. doi: 10.1126/science.184.4135.482. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Young D. A., Fink C. J. Studies on insulin secretion in vitro from isolated islets of the rat pancreas. Endocrinology. 1968 Dec;83(6):1155–1161. doi: 10.1210/endo-83-6-1155. [DOI] [PubMed] [Google Scholar]
- Lernmark A., Chan S. J., Choy R., Nathans A., Carroll R., Tager H. S., Rubenstein A. H., Swift H. H., Steiner D. F. Biosynthesis of insulin and glucagon: a view of the current state of the art. Ciba Found Symp. 1976;41:7–30. doi: 10.1002/9780470720233.ch2. [DOI] [PubMed] [Google Scholar]
- Mihalyi E., Weinberg R. M., Towne D. W., Friedman M. E. Proteolytic fragmentation of fibrinogen. I. Comparison of the fragmentation of human and bovine fibrinogen by trypsin or plasmin. Biochemistry. 1976 Nov 30;15(24):5372–5381. doi: 10.1021/bi00669a025. [DOI] [PubMed] [Google Scholar]
- Orci L., Perrelet A., Gorden P. Less-understood aspects of the morphology of insulin secretion and binding. Recent Prog Horm Res. 1978;34:95–121. doi: 10.1016/b978-0-12-571134-0.50007-7. [DOI] [PubMed] [Google Scholar]
- Pace C. S., Matschinsky F. M., Lacy P. E., Conant S. Electrophysiological evidence for the autoregulation of beta-cell secretion by insulin. Biochim Biophys Acta. 1977 Apr 27;497(2):408–414. doi: 10.1016/0304-4165(77)90198-2. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis and secretion. Fed Proc. 1975 Jun;34(7):1549–1555. [PubMed] [Google Scholar]
- Ratnoff O. D., Naff G. B. The conversion of C'IS to C'1 esterase by plasmin and trypsin. J Exp Med. 1967 Feb 1;125(2):337–358. doi: 10.1084/jem.125.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Gorden P., Pastan I. "Big insulin": a new component of plasma insulin detected by immunoassay. Proc Natl Acad Sci U S A. 1968 Sep;61(1):138–145. doi: 10.1073/pnas.61.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando H., Borg J., Steiner D. F. Studies on the secretion of newly synthesized proinsulin and insulin from isolated rat islets of Langerhans. J Clin Invest. 1972 Jun;51(6):1476–1485. doi: 10.1172/JCI106944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando H., Grodsky G. M. Dynamic synthesis and release of insulin and proinsulin from perifused islets. Diabetes. 1973 May;22(5):354–360. doi: 10.2337/diab.22.5.354. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Beers W. H. Studies on the role of plasminogen activator in ovulation. In vitro response of granulosa cells to gonadotropins, cyclic nucleotides, and prostaglandins. J Biol Chem. 1976 Sep 25;251(18):5694–5702. [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Hamilton J., Reich E. Macrophage plasminogen activator: induction by concanavalin A and phorbol myristate acetate. Cell. 1977 Jul;11(3):695–705. doi: 10.1016/0092-8674(77)90086-1. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Hamilton J., Reich E. Macrophage plasminogen activator: modulation of enzyme production by anti-inflammatory steroids, mitotic inhibitors, and cyclic nucleotides. Cell. 1976 Jun;8(2):271–281. doi: 10.1016/0092-8674(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size and charge distinctions between endogenous human plasma gastrin in peripheral blood and heptadecapeptide gastrins. Gastroenterology. 1970 May;58(5):609–615. [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun. 1971 Jul 16;44(2):439–445. doi: 10.1016/0006-291x(71)90620-6. [DOI] [PubMed] [Google Scholar]
- Zühlke H., Steiner D. F., Lernmark A., Lipsey C. Carboxypeptidase B-like and trypsin-like activities in isolated rat pancreatic islets. Ciba Found Symp. 1976;41:183–195. doi: 10.1002/9780470720233.ch10. [DOI] [PubMed] [Google Scholar]