Abstract
This prospective study was to assess interfractional and intrafractional errors and to estimate appropriate margins for planning target volume (PTV) by using daily cone-beam computed tomography (CBCT) guidance in nasopharyngeal carcinoma (NPC). Daily pretreatment and post-treatment CBCT scans were acquired separately after initial patient setup and after the completion of each treatment fraction in 10 patients treated with IMRT. Online corrections were made before treatment if any translational setup error was found. Interfractional and intrafractional errors were recorded in the right–left (RL), superior–inferior (SI) and anterior–posterior (AP) directions. For the translational shifts, interfractional errors >2 mm occurred in 21.7% of measurements in the RL direction, 12.7% in the SI direction and 34.1% in the AP direction, respectively. Online correction resulted in 100% of residual errors ≤2 mm in the RL and SI directions, and 95.5% of residual errors ≤2 mm in the AP direction. No residual errors >3 mm occurred in the three directions. For the rotational shifts, a significant reduction was found in the magnitudes of residual errors compared with those of interfractional errors. A margin of 4.9 mm, 4.0 mm and 6.3 mm was required in the RL, SI and AP directions, respectively, when daily CBCT scans were not performed. With daily CBCT, the margins were reduced to 1.2 mm in all directions. In conclusion, daily CBCT guidance is an effective modality to improve the accuracy of IMRT for NPC. The online correction could result in a 70–81% reduction in margin size.
Keywords: nasopharyngeal carcinoma, intensity-modulated radiotherapy, cone-beam computed tomography, image-guided radiation therapy, setup error
INTRODUCTION
In the past decade, radiation therapy (RT) techniques have evolved rapidly, aiming at delivering a higher tumoricidal dose to improve local and/or regional control, while decreasing side-effects through minimizing irradiation damage to the critical structures. However, variations in patient setup and organ motion are limiting factors to achieving this goal. In general, organ motion during RT delivery in head-and-neck cancer is not significant; setup errors, however, should not be underestimated. Guckenberger et al. [1] found that in patients with head-and-neck cancer, translational errors were ≥2 mm in 13.9% of all measurements for each axis separately, and rotational errors were >2° in 11.1% of all measurements. For patients with elongated target volumes and sharp dose gradients to adjacent organs at risk, both translational and rotational errors resulted in considerably decreased target coverage and highly increased doses to the organs at risk compared with the initial treatment plan.
Recently, several three-dimensional (3D) imaging techniques have been introduced to assess patient setup errors on the basis of the bony anatomy and sufficient soft-tissue contrast. They include kilovoltage (KV) and megavoltage (MV) cone-beam CT (CBCT) [2–5], CT scanner equipped with medical accelerators [6–7] and tomotherapy treatment units [8]. Both KV CBCT and MV CBCT are capable of verifying patient position in 3D, their role in the future may also include dose verification and adaptive planning. With regard to the visualization of soft tissues and undesired extra dose to patients, KV CBCT appears to be superior to MV CBCT [9].
Patients with nasopharyngeal carcinoma (NPC) have critical structures adjacent to the tumor that are more likely to be involved directly with the tumor itself, or be affected by RT, compared with those with other head-and-neck cancers. Impairment of the critical structures may affect their functions, thus ultimately reducing patients' quality of life. Therefore, accurate delivery of radiation doses to the targets and their surrounding normal structures is a prerequisite to maximizing tumor kill while minimizing toxicities. In our department, since May 2009 we have been treating NPC routinely using an Elekta linear accelerator (Synergy, Stockholm, Sweden) with image-guided RT (IGRT) using a mounted KV CBCT scanner. Meanwhile, a prospective study was conducted to assess the interfractional and intrafractional errors in NPC treated with intensity-modulated radiation therapy (IMRT) through daily KV CBCT imaging, and to determine the margin necessary for the clinical target volume (CTV) to planning target volume (PTV) expansion.
MATERIALS AND METHODS
Eligibility criteria
Patients with histologically proven NPC and treated with curative intent were enrolled into this study. Inclusion criteria were as follows: age ≥18 years; Eastern Cooperative Oncology Group (ECOG) Performance Status 0–2; Stages I–IVb according to the 2002 AJCC Staging System. Patients diagnosed with, or treated for other malignances, or treated with non-IMRT techniques were excluded from the study. Written informed consent was obtained for all patients. The study was approved by the Institutional Review Board (IRB) of the People's Hospital of Guangxi Zhuang Autonomous Region (No. 201104).
Immobilization and simulation
All patients were immobilized in a supine position with the head in a neutral position with a tailored thermoplastic mask covering the head, neck and shoulders. Intravenous contrast-enhanced CT using a 2-mm slice from the vertex to the manubriosternal joint was performed for planning. The CT data were imported to the CMS-XiO planning system (CMS Inc., St. Louis, MO, USA).
Target delineation and treatment planning
The target delineation in NPC patients has been described in detail elsewhere [10], and was in accordance with the International Commission on Radiation Units and Measurements Reports 50 and 62, as well as an Institutional Treatment Protocol. In brief, the primary gross volume (GTVnx) and the involved lymph nodes (GTVnd) included all known gross disease as determined by the imaging, clinical and endoscopic findings. CTVnx included the GTVnx plus 5–10-mm margin, and CTVnd included the GTVnd plus 5-mm margins. CTV1 was defined as the entire nasopharynx, parapharyngeal space, pterygopalatine fossa, posterior third of the nasal cavity and maxillary sinuses, inferior sphenoid sinus, posterior ethmoid sinus, skull base and anterior half of the clivus. CTV1 also included the ipsilateral level II for node-negative neck, or extended to the next ipsilateral level for node-positive neck, or included the full length of ipsilateral neck for node-positive in the lower neck. CTV2 was defined as the low-risk node region below the CTV1. Level V was separated by the borderline between the CTV1 and CTV2 (i.e. regions above the borderline were covered by the CTV1 and regions below the borderline were covered by CTV2).
The respective planning target volumes (PTVs) were generated with a 5-mm margin in all directions, except when the corresponding CTV would otherwise overlap with, or was close to, a critical structure (e.g. brain stem, spinal cord or optic nerves), in which case the margin could be as small as 1 mm. Care was also taken to ensure at least a 5-mm gap was present between the PTVs and the skin. The contoured critical structures included the brain stem, chiasm, optic nerves, spinal cord, eyes, lens, cochleas, parotid glands, oral cavity, larynx, mandible and temporomandibular joints. The plans were optimized using a CMS-XiO planning system.
The prescribed radiation dose was 69–70 Gy at 2.12–2.3 Gy per fraction delivered to the PTVnx and PTVnd, and 59.4–60 Gy at 1.8–2.0 Gy per fraction delivered to the PTV1. The PTV2 was treated to 54–55.8 Gy at 1.64–1.8 Gy per fraction. All patients were treated once daily, five fractions weekly.
Setup and image guidance
Daily initial patient setup consisted of aligning in-room lasers with marks on the thermoplastic mask. The pretreatment KV CBCT scan was then acquired on an Elekta Synergy treatment machine with an integrated kilovoltage X-ray source using the following parameters: kVp, 100 kV; nominal milliamperes per frame, 10 mA; nominal milliseconds, 10 ms; kV collimator, s20; kV filter, f0; approximate frames, 361; and total angle, 200. Images were then reconstructed and registered to the planning CT scans based on Elekta's X-ray volume imaging (XVI) technology. Only bony matching was allowed in the present study. The ‘clip box’ typically included the primary target volume, nasopharynx, parapharyngeal space and surrounding bony structures from the base of skull to C6 vertebrae. Manual adjustments were made whenever necessary at the discretion of the radiation therapists.
The initial setup error was recorded for the left–right (LR), superior–inferior (SI) and anterior–posterior (AP) directions, as well as for the three rotational errors (rotations around the x, y and z axes). Online corrections were made by manually shifting the treatment couch if any translational setup error was found. For rotational error >3°, the therapists would re-enter the treatment room to reposition the patient in the mask. A verification CBCT scan was then acquired to verify the correction before the patient underwent the planned treatment. Post-treatment CBCT scan was obtained immediately after each treatment to detect the residual errors during IMRT delivery.
Determination of interfractional and residual errors
The interfractional errors were calculated by using the pretreatment CBCT scan after patient setup, prior to corrections or treatment. The residual errors were determined from data obtained from the post-treatment CBCT scans. Different thresholds from 1 mm to 5 mm with 1-mm increments were used to analysis the error distributions.
Determination of CTV-to-PTV margins
Data obtained for the interfractional and residual errors were used to estimate the ideal CTV-to-PTV margins using van Herk's formula: MPTV = 2.5∑ + 0.7σ, where ∑ is the SD of the mean population shifts and accounts for the systematic errors, and σ is the mean of the population SD and accounts for random errors. ∑ is calculated by use of the SD of the individual mean errors, and σ by the use of the root-mean-square of the individual patient SDs. The equation assumes that minimum dose to CTV is 95% to 90% of patients [11].
RESULTS
Patient characteristics
Between March 2011 and July 2011, a total of 10 patients were enrolled into this study. There were seven men and three women with a mean age of 43 years (range, 28–69 years). Stage distributions according to the 2002 AJCC Staging System were as follows: Stages IIa and IIb, three patients; Stage III, four patients; Stages IVa and IVb, three patients. Concurrent chemotherapy was given to three patients, while induction plus concurrent chemotherapy was given to six patients, and no chemotherapy to one patient. The characteristics of the patient cohort are listed in Table 1.
Table 1.
Patient characteristics
| Variable | Value |
|---|---|
| Gender n | |
| Male | 7 (70.0%) |
| Female | 3 (30.0%) |
| Age (years) | |
| Mean | 43 |
| Range | 28–49 |
| T category n | |
| T2a + T2b | 5 (50.0%) |
| T3 | 3 (30.0%) |
| T4 | 2 (20.0%) |
| N category n | |
| N0 | 2 (20.0%) |
| N1 | 5 (50.0%) |
| N2 | 2 (20.0%) |
| N3a + N3b | 1 (10.0%) |
| AJCC stage group n | |
| IIa + IIb | 3 (30.0%) |
| III | 4 (40.0%) |
| IVa + IVb | 3 (30.0%) |
| Chemotherapy n | |
| None | 1 (10.0%) |
| Induction + concurrent | 6 (60.0%) |
| Concurrent | 3 (30.0%) |
Interfractional and residual errors
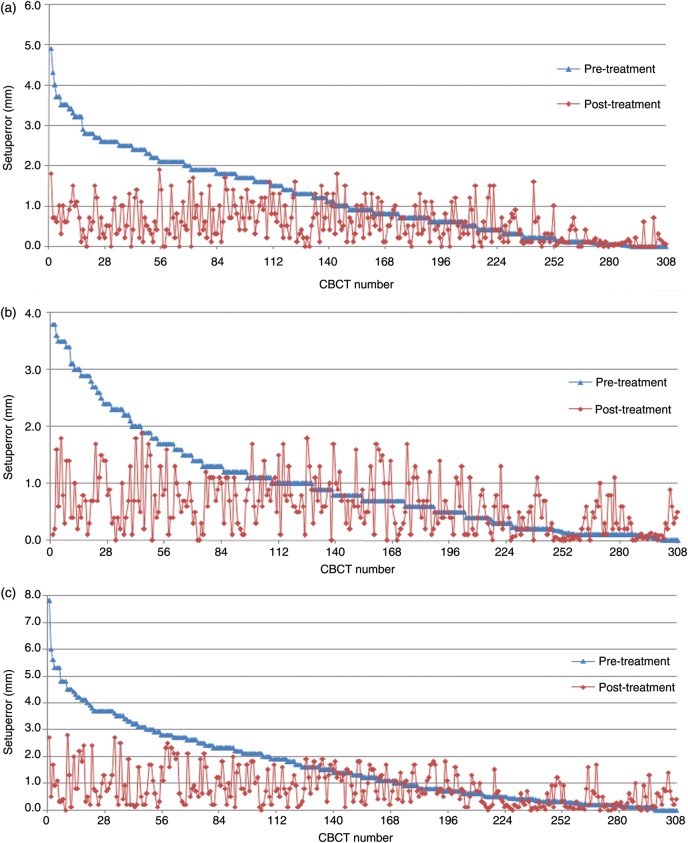
A total of 308 paired pretreatment and post-treatment scans were acquired and analyzed. For the translational shifts, the average interfractional errors were 1.2 ± 1.0 mm, 0.8 ± 1.1 mm and 1.7 ± 1.2 mm for the RL, SI and AP directions, respectively. The corresponding average residual errors were 0.3 ± 0.7 mm, 0.3 ± 0.7 mm and 0.2 ± 1.0 mm. As shown in Table 2, the frequencies of interfractional errors >1 mm in the RL, SI and AP directions were 46.1%, 35.1% and 54.9%, respectively. After the correction, the frequencies of residual errors >1 mm in the respective dimensions were reduced to 17.5%, 19.1% and 30.8%, respectively. The frequencies of interfractional errors >2 mm in the RL, SI and AP directions were 21.7%, 12.7% and 34.1%, respectively. There were no residual errors >2 mm in all dimensions except that in the AP direction 4.5% of measurements had residual errors >2 mm. No residual errors >3 mm occurred in the three directions. Figure 1 illustrates distributions of the 308 paired interfractional and residual errors in the three dimensions measured separately by pretreatment CBCT scans and post-treatment CBCT scans for the 10 patients.
Table 2.
Error distribution in each dimension according to different thresholds
| Shift mm | Interfraction (n) |
Residual error (n) |
||||
|---|---|---|---|---|---|---|
| RL | SI | AP | RL | SI | AP | |
| >1 | 142 (46.1) | 108 (35.1) | 169 (54.9) | 54 (17.5) | 59 (19.1) | 95 (30.8) |
| >2 | 67 (21.7) | 39 (12.7) | 105 (34.1) | 0 (0) | 0 (0) | 14 (4.5) |
| >3 | 16 (5.2) | 11 (3.6) | 48 (15.6) | 0 (0) | 0 (0) | 0 (0) |
| >4 | 2 (0.7) | 0 (0) | 19 (6.2) | 0 (0) | 0 (0) | 0 (0) |
| >5 | 0 (0) | 0 (0) | 6 (1.9) | 0 (0) | 0 (0) | 0 (0) |
Data in parentheses are percentages.
Figure 1.
Distribution of 308 paired interfractional and intrafractional errors in the RL (a), SI (b) and AP (c) directions measured separately by pretreatment and post-treatment CBCT scans
The blue dots denote the interfractional errors in descending order, and the red dots denote the corresponding intrafractional errors. RL = right–left direction; SI = superior–inferior direction; AP = anterior–posterior direction.
We extracted measurements which met a certain criterion from pretreatment CBCT scans to see if post-treatment residual errors were larger than the corresponding pretreatment interfractional errors. There were 166, 200 and 139 pretreatment measurements with errors ≤1 mm in the RL, SI and AP directions, respectively. After online correction, the corresponding residual errors beyond 1 mm still occurred in 10.2% (17/166), 14.5% (29/200) and 15.8% (22/139) of measurements. When interfractional errors were ≤2 mm, 3 mm, 4 mm and 5 mm, no residuals of errors were larger than the corresponding interfractional errors after the online correction.
Rotational shifts were also recorded. The interfractional errors in the RL, SI and AP directions were 1.16 ± 0.90°, 0.98 ± 0.80° and 0.78 ± 0.73°, respectively, as determined by pretreatment CBCT scans. The residual errors in the RL, SI and AP directions were 1.05 ± 0.73°, 0.87 ± 0.69° and 0.71 ± 0.72°, respectively, as determined by post-treatment CBCT scans. When the interfractional errors in each dimension were compared with the respective residual errors, we found a significant reduction in the magnitude of residual errors on the x, y, and z axes with p values of 0.02, 0.03 and 0.04, respectively.
PTV margin determination
By using van Herk's formula, we calculated the ideal PTV margins based on the interfractional and residual errors. As shown in Table 3, both the systematic and random shifts were reduced, from 0.8–1.7 mm and 1.0–1.2 mm prior to IMRT to 0.2–0.3 mm and 0.7–1.0 mm at the completion of IMRT, respectively. To account for the interfractional and residual errors and to ensure that minimum prescription dose to CTV is 95% for 90% of patients, a margin of 4.9 mm, 4.0 mm and 6.3 mm was required in the RL, SI and AP directions, respectively, when daily CBCT scans were not performed. With daily CBCT, the margins were reduced to 1.2 mm in all directions.
Table 3.
CTV-to-PTV expansion
| Variable | Interfraction (mm) |
Residual error (mm) |
||||
|---|---|---|---|---|---|---|
| RL | SI | AP | RL | SI | AP | |
| Systematic shift (∑) | 1.2 | 0.8 | 1.7 | 0.3 | 0.3 | 0.2 |
| Average random shift (σ) | 1.0 | 1.1 | 1.2 | 0.7 | 0.7 | 1.0 |
| PTV margin | 3.7 | 2.8 | 5.1 | 1.2 | 1.2 | 1.2 |
DISCUSSION
CBCT imaging has been used to detect both interfractional setup errors and intrafractional errors during fractionated RT in head and neck cancer. Unlike cancers in other sites like the chest and abdomen, head-and-neck cancer has a smaller intrafraction tumor motion, an important component of intrafractional errors. Thus interfractional setup errors contribute most substantially to overall treatment uncertainties.
In the present study, the frequencies of interfractional errors >1 mm were 46.1% in the RL direction, 35.1% in the SI direction and 54.9% in the AP direction. The frequencies of interfractional errors >2 mm in the RL, SI and AP directions were 21.7%, 12.7% and 34.1%, respectively. However, few interfractional errors exceeded 3 mm, ranging from 3.6% to 15.6%. Errors >5 mm occurred only in the AP direction with a frequency of 1.9%. The results were consistent with those from other researchers. Den et al. [12] evaluated the interfractional and residual errors by using CBCT for 28 patients undergoing IMRT for head-and-neck cancer, and found the pretreatment shifts of ≥3 mm occurred in 11% of setups in the RL, 14% in the SI and 17% in the AP direction, respectively. Only 1.3–3.8% setups were ≥5 mm. This could be largely associated with but not limited to improvement of laser alignment, stability and accuracy of table and gantry, standardized patient setup procedures, and proper utilization of an individualized immobilizing device.
Online correction resulted in 100% of residual errors ≤2 mm in the RL and SI directions, and 95.5% of residual errors ≤2 mm in the AP direction. No residual errors >3 mm occurred in the three directions, as shown in Table 2 and Fig. 1. When interfractional errors were ≤2 mm, 3 mm, 4 mm and 5 mm, no residuals of errors were larger than the corresponding interfractional errors after the online correction. For interfractional shift ≤1 mm, however, the effectiveness of online CBCT correction was limited since some post-treatment measurements still had residual errors exceeding 1 mm, suggesting that the readout of CBCT was not reliable for errors ≤1 mm. Based on our findings, we recommended online CBTV correction should be made when the interfractional error was >2 mm. This could ensure that 100% of residual errors were not >2 mm in the RL and SI directions, and 95.5% of residual errors were not >2 mm in the AP direction.
Dealing with the translations errors based on online CBCT imaging alone is not always enough to overcome geometric uncertainties over the treatment course since the rotational errors may be of clinical relevance. In head-and-neck cancer, even small rotational errors can result in large displacements at the ends of the targets. For a target volume of 10 cm long, a rotational error of 5° can result in a displacement of 4.4 mm at the target ends [1]. This large displacement may ultimately lead to underdosage of the target or overdosage to surrounding normal tissues. In the present study, we recorded both translational and rotational shifts but only the translational shifts were corrected due to limitations of movement of our treatment couch. We found most interfractional rotational shifts were within 1°. The average shifts were generally small with 1.16° in the RL, 0.98° in the SI and 0.78° in the AP directions, respectively. Thus the impact of the rotational shifts on the whole geometric uncertainties could be limited. After the correction, there were significant decreases in the magnitude of residuals compared with the interfractional shifts. This could be explained by the interaction between the translational and rotational shifts.
An IMRT plan is featured with highly conformal dose distribution to the targets and steep dose gradient between the targets and their surrounding structures. A CTV-to-PTV margin is designed to compensate for any variability of day-to-day setup errors and intrafractional residuals. However, the criteria of optimal CTV-to-PTV expansion have not been established. In Radiation Therapy Oncology Group (RTOG) protocol 0615, a minimum 5-mm margin around the CTV in all dimensions is required to create each respective PTV in NPC patients. Wang et al. [13] found a margin of 5.0–6.0 mm was needed to ensure adequate coverage of the CTV if no online correction was applied. This was consistent with our findings. In our study, a margin of 4.9 mm, 4.0 mm and 6.3 mm was required in the RL, SI and AP directions, respectively, when daily CBCT scans were not performed. Based on the concept of PTV margin, a reduction in the margin size could theoretically allow less normal tissue to be involved in the high dose region. Wang et al. [13] showed that the margin was reduced to about 3.0 mm with online CBCT correction, while in our study, the online CBCT resulted in a 70–81% reduction in margin size, with a CTV-to-PTV margin of 1.2 mm in all dimensions. The magnitudes of margin reduction were larger than those from Wang et al. and other researchers [12–13]. The reason for this could be primarily due to smaller residual errors (particularly systematic shifts for residual errors) observed in our patients as shown in Table 2 and 3. However, the findings should be interpreted with caution as we did not include other ingredients of the margin recipe such as variations from target delineation, tumor shrinkage and organ deformation, and rotational shifts. At our institution, a margin of 2–3 mm is routinely used for NPC patients treated with daily CBCT guided-IMRT.
Several reports have addressed the issue of whether a reduction in PTV margin size could transfer into clinical advantages. Schoenfield et al. [14] showed an impressive marginal recurrence rate of only 2% when using a 3-mm PTV margin for head-and-neck cancer. van Asselen et al. [15] found that a reduction in PTV margin from 6 mm to 3 mm resulted in an approximately 20% reduction in normal tissue complication probability for parotid glands. The first study on patterns of failure according to different PTV margins was conducted by Chen et al. [16]. They treated 225 patients with daily volumetric imaging guided-IMRT for head-and-neck cancer and found no differences with respect to 2-year overall survival, local–regional control and distant metastasis-free survival among patients with 5-mm and 3-mm PTV expansion margins. In addition, there were no differences in the marginal failure between the two groups of patients. The study suggested that CTV-to-PTV expansion margins could safely be reduced from 5 mm to 3 mm when daily IGRT was used to guide dose delivery. We could not draw such a conclusion since all patients in our study were treated with a uniform non-reduced PTV margin. Further investigation should focus on the impact of daily image guidance with a reduction PTV margin on clinical outcomes.
In conclusion, daily CBCT guidance is an effective modality to improve the accuracy of IMRT for NPC. A margin of 4.0–6.3 mm is required to ensure adequate coverage of the CTV when daily CBCT corrections are not performed. The online correction could result in a 70–81% reduction in margin size, with a PTV margin of 1.2 mm in all dimensions.
ACKNOWLEDGEMENTS
This work was supported by Guangxi Sci-Tech Office (0235024-10). The authors thank for the following persons for their participations in the acquisition of the CBCT images: Yihang Huang, Xuexiang Yan, Xiaoping Li, Li Liang and Lili Liang.
REFERENCES
- 1.Guckenberger M, Meyer J, Vordermark D, et al. Magnitude and clinical relevance of translational and rotational patient setup errors: a cone-beam CT study. Int J Radiat Oncol Biol Phys. 2006;65:934–42. doi: 10.1016/j.ijrobp.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Pouliot J, Bani-Hashemi A, Chen J, et al. Low-dose megavoltage cone-beam CT for radiation therapy. Int J Radiat Oncol Biol Phys. 2005;61:552–60. doi: 10.1016/j.ijrobp.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Jaffray DA, Siewerdsen JH. Cone-beam computed tomography with a flat-panel imager: Initial performance characterization. Med Phys. 2000;27:1311–23. doi: 10.1118/1.599009. [DOI] [PubMed] [Google Scholar]
- 4.Létourneau D, Wong JW, Oldham M, et al. Cone-beam-CT guided radiation therapy: technical implementation. Radiother Oncol. 2005;75:279–86. doi: 10.1016/j.radonc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Midgley S, Millar RM, Dudson J. A feasibility study for megavoltage cone beam CT using a commercial EPID. Phys Med Biol. 1998;43:155–69. doi: 10.1088/0031-9155/43/1/010. [DOI] [PubMed] [Google Scholar]
- 6.Wong JR, Grimm L, Uematsu M, et al. Image-guided radiotherapy for prostate cancer by CT-linear accelerator combination: Prostate movements and dosimetric considerations. Int J Radiat Oncol Biol Phys. 2005;61:561–9. doi: 10.1016/j.ijrobp.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Dong L, Lee AK, et al. Effect of anatomic motion on proton therapy dose distributions in prostate cancer treatment. Int J Radiat Oncol Biol Phys. 2007;67:620–9. doi: 10.1016/j.ijrobp.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meeks SL, Harmon JF, Jr, Langen KM, et al. Performance characterization of megavoltage computed tomography imaging on a helical tomotherapy unit. Med Phys. 2005;32:2673–81. doi: 10.1118/1.1990289. [DOI] [PubMed] [Google Scholar]
- 9.Broderick M, Menezes G, Leech M, et al. A comparison of kilovoltage and megavoltage cone beam CT in radiotherapy. J Radiother Pract. 2007;6:173–8. [Google Scholar]
- 10.Lu H, Chen J, Huang B, et al. Feasibility and efficacy study of weekly cisplatin with concurrent intensity-modulated radiation therapy for nasopharyngeal carcinoma: preliminary results. Oral Oncol. 2010;46:743–7. doi: 10.1016/j.oraloncology.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 11.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Den RB, Doemer A, Kubicer G, et al. Daily image guidance cone-beam computed tomography for head-and-neck cancer intensity-modulated radiotherapy: a prospective study. Int J Radiat Oncol Biol Phys. 2010;76:1353–9. doi: 10.1016/j.ijrobp.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Bai S, Chen N, et al. The clinical feasibility and effect of online cone beam computed tomography-guided intensity-modulated radiotherapy for nasopharyngeal cancer. Radiat Oncol. 2009;90:221–7. doi: 10.1016/j.radonc.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Schoenfield GO, Amdur RJ, Morris CG, et al. Patterns of failure and toxicity after intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;71:377–85. doi: 10.1016/j.ijrobp.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 15.van Asselen B, Dehnad H, Raaijmakers CP, et al. The dose to the parotid glands with IMRT for oropharyngeal tumors: The effect of reduction of positioning margins. Radiother Oncol. 2002;64:197–204. doi: 10.1016/s0167-8140(02)00152-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen AM, Farwell DG, Luu Q, et al. Evaluation of the planning target volume in the treatment of head and neck cancer with intensity-modulated radiotherapy: What is the appropriate expansion margin in the setting of daily image guidance? Int J Radiat Oncol Biol Phys. 2011;81:943–9. doi: 10.1016/j.ijrobp.2010.07.017. [DOI] [PubMed] [Google Scholar]