Abstract
Hypopharyngeal squamous cell carcinoma (HPSCC) is usually diagnosed at an advanced stage, and early-stage HPSCC is relatively rare. Because of the rarity of early-stage HPSCC, few reports have been published on the efficacy of radiotherapy (RT) in its treatment. We retrospectively reviewed the clinical records of 45 consecutive patients with Stage I and II HPSCC from May 1991 to June 2010. Patient characteristics were as follows: median age, 66 years (range, 44–90 years); male/female, 39/6; and T1/T2, 27/18. The irradiation dose ranged from 60 to 72 Gy (median: 70 Gy). Of the 45 patients, 21 underwent concurrent chemotherapy. With a median follow-up period of 62 months, the 5-year overall survival rate was 81%. Local failure occurred in 5 patients, and the 5-year local control rate was 83%. All local recurrences were successfully salvaged by surgery. The 5-year functional larynx preservation rate was 92%. Acute toxicity was manageable. Grade 3 laryngeal edema and Grade 3 hypothyroidism occurred in 1 patient each. No other late adverse events of Grade 3 or greater were observed. Based on these results, RT seemed to be an effective treatment modality for early HPSCC, with favorable organ preservation and acceptable adverse events. Early detection and accurate management of local recurrence and second malignancy was deemed to be critical.
Keywords: hypopharyngeal carcinoma, radiotherapy, chemotherapy, larynx preservation
INTRODUCTION
Hypopharyngeal squamous cell carcinoma (HPSCC) is usually diagnosed in the advanced stage, and early-stage HPSCC is relatively rare. In recent years, mainly owing to the development of laryngeal and gastrointestinal fiberscopes, HPSCC has tended to be found in an earlier stage. Although optimal treatment for early HPSCC has not been established, treatment options have included surgery and radiotherapy (RT) with or without chemotherapy. RT may be the treatment of choice in terms of functional preservation. However, because of the rarity of early-stage HPSCC, few reports have been published on the efficacy of RT. Nakamura et al. reported the results of chemoradiotherapy for early HPSCC [1]. In that article, chemoradiotherapy was started in the preoperative setting, and patients who achieved complete response after 30 to 40 Gy irradiation underwent further definitive chemoradiation. The authors reported equivalent disease-specific survival rates for early responders and for patients who underwent chemoradiotherapy and surgery. However, the effectiveness of curative chemoradiotherapy for all patient cohorts remains unclear. Nakamura et al. also reported the analysis of questionnaires from 10 institutions regarding early HPSCC treated with curative RT [2]. The authors collected the questionnaires from 115 patients treated between 1990 and 2001. The results indicated the efficacy of RT for early HPSCC. However, deviation of treatment strategy might have existed in the multi-institutional questionnaire study. In our institution, definitive RT was performed as a first line treatment for Stage I and II HPSCC. Salvage surgery was performed for patients with local recurrence or non-responders. In this study, we retrospectively reviewed our single-institution results for definitive RT in Stage I and II HPSCC.
MATERIALS AND METHODS
Patients
From May 1991 to February 2010, 238 patients diagnosed with HPSCC were treated in our Division. Of these, 35 were treated with palliative intent, 2 preoperative, 73 postoperative, and 127 with definitive intent (82 Stage III/IV and 46 Stage I/II) (Table 1). Among the 46 patients with Stage I or II HPSCC, one patient was lost to follow-up after 4 months without any events. In this study, the remaining 45 patients with early (T1–2N0M0) HPSCC who underwent definitive RT were analysed. All patients were followed for at least 12 months or until any events. All patients had histologically proven squamous cell carcinoma. Patient characteristics are summarized in Table 2. There were 39 men and 6 women, with median age of 66 years (range, 44–90 years). Staging work-up included physical examination, laryngoscopy and computed tomography. Esophagogastroduodenoscopy was included as of May 2002, and PET scan was added in November 2006. According to the TNM classification of malignant tumors, 7th Edition [3], there were 27 patients with Stage I (tumor limited to one subsite of the hypopharynx and to ≤2 cm in the greatest dimension), 18 patients with Stage II (tumor that had invaded more than one subsite of the hypopharynx or an adjacent site or measured >2 cm but <4 cm in the greatest dimension, without fixation of the hemilarynx). The primary sites were the pyriform sinus in 35, the posterior pharyngeal wall in 6, and the postcricoid region in 4.
Table 1.
Patient accrual according to treatment strategy and decade of accrual
| 1991–2000 | 2001–10 | Total | |
|---|---|---|---|
| Preoperative | 2 | 0 | 2 |
| Postoperative | 10 | 63 | 73 |
| Palliative | 17 | 18 | 35 |
| Definitive (Stage III–IV) | 15 | 67 | 82 |
| Definitive (Stage I–II) | 13 | 33 | 46a |
aOne patient was lost to follow-up after 4 months without any events and was excluded from this analysis.
Table 2.
Patient characteristics
| Characteristics | No. of patients |
|---|---|
| Total no. patients | 45 |
| Gender | |
| Male | 39 |
| Female | 6 |
| Age | |
| Median (range) | 66 (44–90) |
| Tumor stage (from [3]) | |
| Stage I | 27 |
| Stage II | 18 |
| Tumor differentiation | |
| Well | 5 |
| Moderately | 15 |
| Poorly | 5 |
| Unknown | 20 |
| Subsite | |
| Pyriform sinus | 35 |
| Posterior wall | 6 |
| Postcricoid region | 4 |
Radiotherapy and chemotherapy
All patients underwent RT with radical intent, using 4-MV linear accelerator X-rays. No patient was treated with preoperative intent. A conventional fractionation schedule of 2 Gy/day was used. All patients received prophylactic lymph node irradiation. A prophylactic nodal area (including the retropharyngeal region and supraclavicular nodes) was irradiated up to 40–50 Gy with parallel-opposed lateral fields with a matched anterior lower neck field. The primary lesion was boosted with reduced fields after prophylactic nodal irradiation. The median total irradiated dose was 70 Gy (range: 60 to 72 Gy). Prescriptions for irradiation dose varied in accordance with the treating physician's preference. Concurrent chemotherapy was administered in 21 patients (Table 3). Inclusion criteria for chemotherapy were expanded to T2 disease beginning in the year 2000, and 16 out of 18 patients with T2 disease were treated after 2000; all 16 underwent concurrent chemotherapy. The two patients with T2 disease who were treated before 1999 did not receive chemotherapy. Five out of 27 patients with T1 disease underwent concurrent chemotherapy as per physician's preference. The regimen of chemotherapy is summarized in Table 4. Four patients received adjuvant chemotherapy with TS-1 (Oral fluoropyrimidine consisting of three components: tegafur, a prodrug of 5-FU; 5-chloro-2,4-dihydroxypyridine; and oxonic acid) (80 to 100 mg per body) as a part of feasibility study.
Table 3.
Irradiated dose and chemotherapy
| 1991–2000 (n = 13) | 2001–10 (n = 32) | total | |
|---|---|---|---|
| Irradiated dose | |||
| 60 Gy | 5 | 4 | 9 |
| 66–70 Gy | 7 | 28 | 35 |
| 72 Gy | 1 | 0 | 1 |
| Chemotherapy | |||
| Induction | 0 | 0 | 0 |
| Concurrent | 2 | 19 | 21 |
| Adjuvant | 0 | 4 | 4 |
Table 4.
Chemotherapeutic agents for concurrent chemoradiotherapy
| Chemotherapeutic agents | 1991–2000 | 2001–06 | 2007–10 | total |
|---|---|---|---|---|
| Cisplatin (70–80 mg/m2) | 0 | 4 | 6 | 10 |
| Nedaplatin (70–80 mg/m2) | 0 | 6 | 1 | 7 |
| Cisplatin + 5-FU (Cisplatin, 70 mg/m2 on day 1; 5-FU, 700 mg/m2 on days 1–4) | 1 | 2 | 0 | 3 |
| Low-dose cisplatin (5 mg/m2, daily) | 0 | 1 | 0 | 1 |
5-FU = 5-fluorouracil.
Follow-up
Patients were followed up monthly for the first year after completion of RT, every 3 months for the following 2 years, and then every 6 months until progression or death. Physical examination and laryngoscopy were performed at every visit. Computed tomography was performed 3 to 6 months after completion of RT, and thereafter performed annually. A PET scanner was installed in our Institute in 2005. PET scan was not performed routinely at follow-up examinations except in cases of suspected disease after computed tomography or physical examination. Esophagogastroduodenoscopy was performed at 1 to 2 year intervals, depending on the findings of routine follow-up examinations.
Statistical analysis
Survival was calculated from the date of initiation of RT to the date of any events or date last visited. Patients alive without relapse at the time of analysis were censored at their last follow-up. The progression-free survival (PFS) rate was calculated from the date of initiation of RT to the date of histologically-confirmed local recurrence, date of radiographic diagnosis of distant or nodal metastasis, or date of death from any causes. Local control rates and functional larynx preservation rates were calculated from the date of initiation of RT to the date of histologically-confirmed recurrence or date of surgical removal of larynx. Any death without local recurrence was censored for local recurrence. Any death with functional larynx was censored for functional larynx preservation rate. Survival rates were estimated using the Kaplan–Meier method. Univariate analyses with log-rank tests were performed to identify prognostic factors. Radiation dose, treatment interruption, use of chemotherapy, treatment period, tumor location, tumor stage, histological differentiation, age and gender were evaluated. All P values reported are 2-sided. For all statistical tests, differences were considered significant at the 5% level. Commercially available statistical software (StatView, 5.0; SAS Institute, Cary, NC) was used for analysis. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 [4].
RESULTS
Survivals and larynx preservation
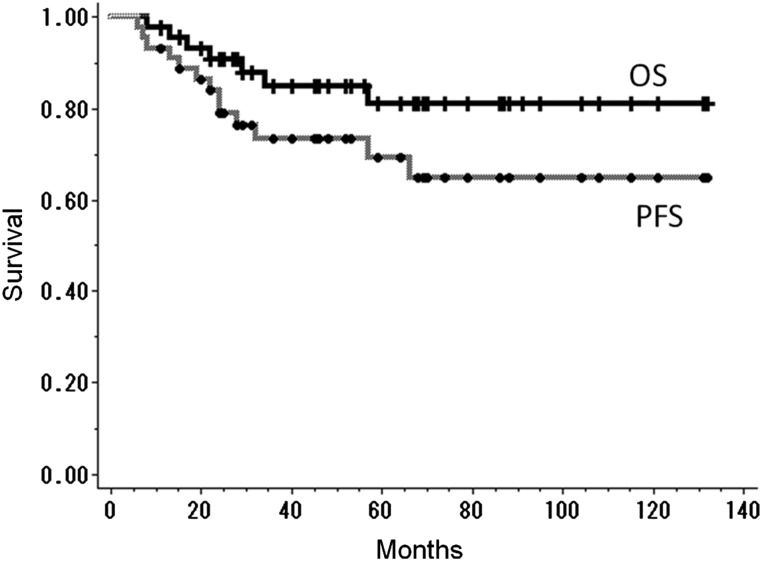
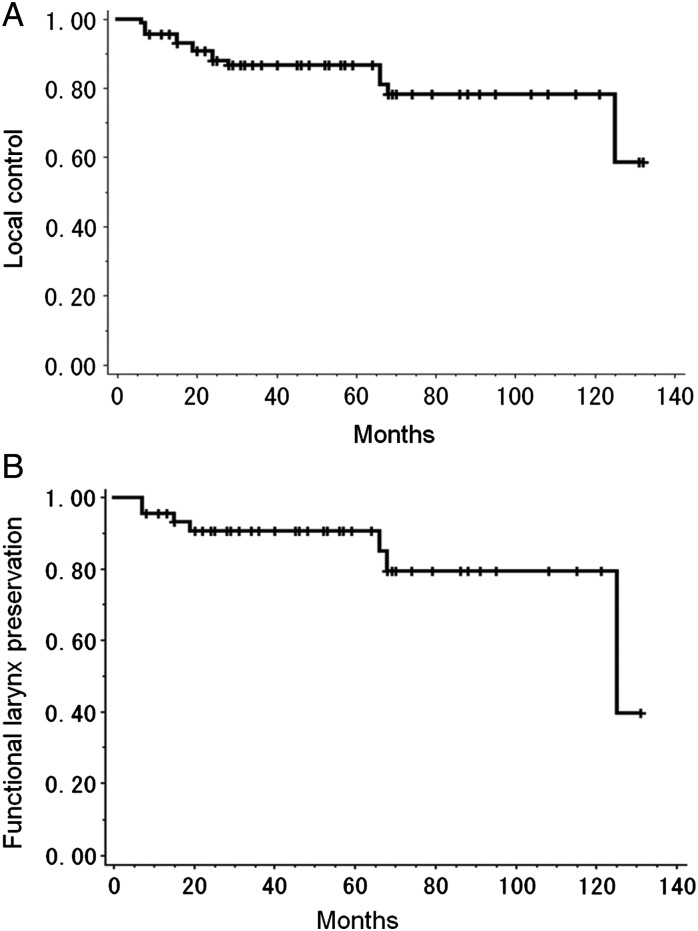
Median follow-up periods for surviving patients and all patients were 62 and 53 months, respectively (range, 12–132 months and 8–132 months, respectively). The 5-year overall survival rate and PFS were 81% and 69%, respectively (Fig. 1). Causes of death were: primary disease (1 patient), other primary cancers (5 patients) and suffocation from aspiration (1 patient). Of the 5 patients who died of other primary cancers, 2 died of synchronous cancer (lung cancer and esophageal cancer), 1 died of recurrent metachronous cancer before treatment of HPSCC (lung cancer) and 2 died of metachronous cancer which arose after completion of the treatment for HPSCC (esophageal cancer and oropharyngeal cancer). Local recurrence occurred in 8 patients, and the 5-year local control rate was 83% (Fig. 2A). All patients with local recurrence were successfully salvaged with surgical resection. Of these 8 patients, 3 were salvaged with laryngeal preservation surgery and 5 were salvaged with laryngectomy. Another 2 patients underwent laryngectomy because of second primary head and neck cancers (cervical esophageal cancer and oropharyngeal cancer). The 5-year functional larynx preservation rate was 92% (Fig. 2B). Three of 7 laryngectomies were performed more than 60 months after initiation of RT (66, 68 and 125 months, respectively). The 6-year functional larynx preservation rate was 79%. No cervical node metastasis was observed. Distant metastasis was observed in 1 patient.
Fig. 1.
Kaplan–Meier curves of overall survival rate (OS) and progression free survival rate (PFS). The 5-year overall survival rate and progression free survival rate for all patients were 81% and 69%, respectively.
Fig. 2.
Kaplan–Meier curves of (A) local control rate and (B) functional larynx preservation rate. The 5-year local control rate and functional larynx preservation rate for all patients were 83% and 92%, respectively.
Metachronous malignancies
Eight patients had previously treated metachronous malignancies before initiation of RT for HPSCC. All of these metachronous malignancies were judged to be cured at the time of initiation of RT.
Seven patients had synchronous malignancies. All of these malignancies were diagnosed at non-metastatic stages and were suitable for curative treatment. Six out of 7 synchronous malignancies were treated with RT concurrently with the HPSCC. One synchronous malignancy (esophageal cancer) was treated with endoscopic mucosal resection after completion of RT for HPSCC.
Fourteen patients developed metachronous malignancies in 17 sites during the follow-up period. Two patients received best supportive care (BSC). Patients in whom the other 15 malignancies were diagnosed without disseminated disease were treated with curative intent. Among 14 patients with metachronous malignancies after RT, 1 died of HPSCC, 2 died of metachronous malignancies, 1 died of suffocation from aspiration and 10 were alive and well at the time of analysis (Table 5).
Table 5.
Synchronous and metachronous malignancies after radiation therapy
| Synchronous malignancies | No. of patients |
|---|---|
| Esophagus | 4 |
| Larynx | 2 |
| Lung | 1 |
| Treatment | |
| RT | 6 |
| Endoscopic treatment | 1 |
| Metachronous malignancies after RT | No. of patients |
| Esophagus | 6 |
| Oropharynx | 3 |
| Lung | 3 |
| Prostate | 2 |
| Breast | 1 |
| Larynx | 1 |
| Hypopharynxa | 1 |
| Treatment | |
| Surgery | 11 |
| Endoscopic treatment | 2 |
| RT | 1 |
| Hormonal therapy | 1 |
| BSC | 2 |
RT = radiation therapy, BSC = best supportive care.
aDe novo carcinoma arising from contralateral pyriform sinus.
Prognostic factors
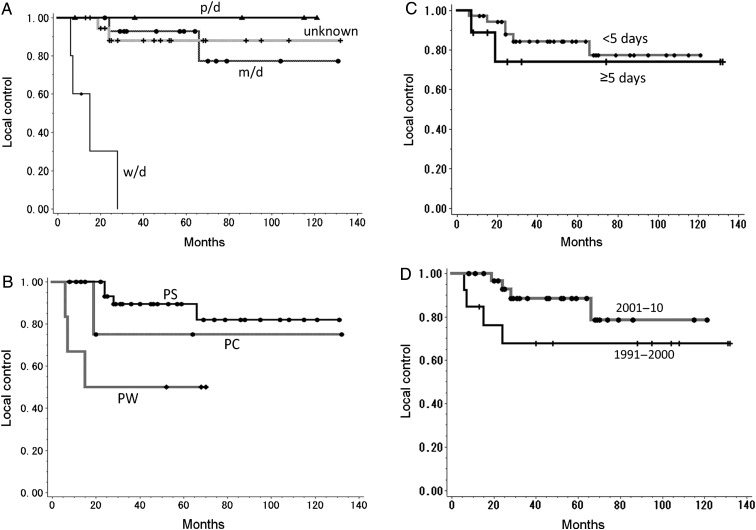
We examined prognostic factors for PFS and local control, including radiation dose, treatment interruption, use of chemotherapy, treatment period, tumor location, tumor stage, histological differentiation, age and sex (Table 6). We found that well-differentiated squamous cell carcinomas (n = 5) were poor prognostic factors for PFS and local control (P < 0.0001). Tumors of the posterior wall (n = 6) were also associated with poor prognosis for local control (P = 0.01) (Fig. 3). The other factors did not show significant impact on PFS or local control.
Table 6.
Prognostic factors
| Variable | 5yr-PFS (%) | P value | 5yr-LC (%) | P value | |
|---|---|---|---|---|---|
| Dose | 60 Gy | 75 | 0.75 | 88 | 0.66 |
| 66–72 Gy | 68 | 81 | |||
| Interruption | < 5 days | 74 | 0.18 | 84 | 0.55 |
| ≥ 5 days | 49 | 74 | |||
| Chemotherapy | yes | 67 | 0.96 | 77 | 0.35 |
| No | 73 | 87 | |||
| Period | 1991–2000 | 62 | 0.41 | 68 | 0.16 |
| 2001–10 | 72 | 89 | |||
| Subsite | PW | 50 | 0.12 | 50 | 0.01 |
| Others | 72 | 72 | |||
| Stage | I | 67 | 0.84 | 80 | 0.93 |
| II | 76 | 86 | |||
| Differentiation | w/d | 0 | < 0.0001 | 0 | < 0.0001 |
| Others | 77 | 91 | |||
| Age | < 70 | 65 | 0.43 | 80 | 0.61 |
| ≥ 70 | 81 | 88 | |||
| Sex | Male | 65 | NAa | 80 | NAa |
| Female | 100 | 100 |
PFS = progression free survival, LC = local control rate, PW = posterior wall, w/d = well-differentiated squamous cell carcinoma, NA = not assessed.
aP value was not assessed because no event occurred in the female arm.
Fig. 3.
Kaplan–Meier curves of local control rate according to (A) histological differentiation, (P < 0.001), (B) tumor location (P = 0.01), (C) treatment interruption (P = 0.55), (D) treatment period (P = 0.16).
p/d = poorly differentiated squamous cell carcinoma, m/d = moderately differentiated squamous cell carcinoma, w/d = well-differentiated squamous cell carcinoma, PS = pyriform sinus, PC = postcricoid region, PW = posterior wall.
Morbidity
No patient required a feeding tube or intravenous hyper-alimentation during and after treatment. In the late period, laryngeal edema of Grade 3 (requiring temporal tracheostomy) was observed in 1 patient, and Grade 3 hypothyroidism (myxedema) was observed in 1 patient. The Grade 3 hypothyroidism was treated with levothyroxine sodium (Thyradin S). No other late toxicity of Grade 3 or greater was documented (Table 7).
Table 7.
Toxicity profiles (grade 3/4 toxicities)
| Concurrent Chemotherapy | yes (n = 21) |
no (n = 24) |
|---|---|---|
| No. of patients (%) | No. of patients (%) | |
| Early | ||
| Renal dysfunction | 0 (0) | 0 (0) |
| Neutropenia | 4 (18) | 0 (0) |
| Anemia | 0 (0) | 1 (4) |
| Thrombocytopenia | 2 (9) | 0 (0) |
| Febrile neutropenia | 1 (4.5) | 0 (0) |
| Late | ||
| Thyroid dysfunction | 0 (0) | 1 (4) |
| Laryngeal edema | 0 (0) | 1 (4) |
Twenty-one patients underwent concurrent chemotherapy. Renal and hematologic toxicities related to chemotherapy were manageable (Table 7). Only 1 patient experienced febrile neutropenia.
DISCUSSION
Although several authors have reported the outcomes of RT for HPSCC, patients with Stage I and II hypopharyngeal cancer were relatively small cohorts in these studies [5–7]. In general, either RT or surgery with or without laryngeal preservation is selected as the initial treatment for T1–2 HPSCC. However, there have been only a few reports focusing on the efficacy of RT for early-stage hypopharyngeal cancer, and the optimal treatment approach remains controversial. RT has been recognized as an effective treatment modality for HPSCC. Mendenhall et al. achieved excellent local control in 80% of patients with T1–2 pyriform sinus carcinoma treated with RT alone [5]. Later, Amdur et al. also reported the results of RT for T1–2 pyriform sinus [6]. They included 101 patients with T1–2 carcinoma of the pyriform sinus and achieved local control rates for T1 and T2 tumors of 90% and 80%, respectively. However, of 101 patients, only 25 patients had Stage I or II disease. Their report showed relatively poor 5-year overall survival rates (57% for Stage I, 61% for Stage II, respectively). It was difficult to determine the long-term efficacy of RT for early-stage HPSCC. Garden et al. reported that the 2-year actuarial local control rate for T1 and T2 tumors after RT alone was 89% and 77%, respectively [8]. These reports included patients with neck node metastasis, and neck nodes were managed with or without neck node dissection. Because these reports contained node-positive patients, the survival period was short. It may be difficult to elucidate the exact long-term benefit of RT for node-negative HPSCC, although these reports suggest the efficacy of curative RT for primary lesions. In our series, definitive RT resulted in a comparable local control rate (83%) in a neck node-negative patient cohort that achieved a relatively longer overall survival rate of 81% with a median follow-up period of 62 months.
In our series, all patients received prophylactic nodal irradiation and no patient experienced cervical node metastasis. Compared to other reported series for early stage HPSCC (Table 8), the nodal control rate in our study seemed to be favorable. There appears to be some potential benefit of prophylactic irradiation for early stage HPSCC. Local recurrence occurred in 8 patients. The incidence of local recurrence was comparable to other studies. All local recurrence was successfully salvaged with surgery. Early detection and adequate management for local recurrence seemed to be critical.
Table 8.
Comparison of prophylactic irradiation, loco-regional control, and salvage surgery
| Series | Treatment period | No. of Pts | median f/u (M) | 5yr-OS (%) | Prophylactic RT yes/no | Prophylactic dose (Gy) | No. of nodal rec. | No. of local rec. | No. of salvage sx for local rec. |
|---|---|---|---|---|---|---|---|---|---|
| Nakamuraa [1] | 1976–2002 | 43 | 52 | 70 | 35/8 (81%) | 30–50 | 3 (7%) | 2 (5%) | 2 (100%) |
| Nakamurab [2] | 1990–2001 | 115 | 47 | 66 | 90/25 (78%) | 36–50 | 14 (12%) | 30 (26%) | 26 (87%) |
| Yoshimura [10] | 1988–2007 | 77 | 33 | 47 | 66/11 (86%) | 20–50 | 11 (14%) | 16 (21%) | 12 (75%) |
| Current study | 1991–2010 | 45 | 53 | 81 | 45/0 (100%) | 40–50 | 0 (0%) | 8 (18%) | 8 (100%) |
aEleven of 43 patients received surgery after 30–40 Gy irradiation.
bQuestionnaire collected from 10 institutions.
Pts = patients, f/u = follow-up period, M = months, OS = overall survival rate, RT = radiation therapy, rec. = recurrence, sx = surgery.
In our series, 5 patients died of second primary cancers. Yoshimura et al. also reported a high incidence of synchronous and metachronous malignancies [9]. In their report, patients with metachronous malignancies had poorer survival outcomes. In our series most metachronous malignancies were diagnosed at non-metastatic stages, and curative treatments were performed. Out of 14 patients with second malignancies, 9 were successfully treated and were alive and well at the time of analysis. We believe close follow-up and accurate management of local failure and metachronous malignancies can provide better outcomes. Careful follow-up and early detection of local recurrences and other malignancies are critical for survival and larynx preservation.
Several authors have reported the additional benefit of chemotherapy for advanced head and neck cancers [10–13]. However, the additional benefit of chemotherapy for early HPSCC remains controversial. Use of concurrent chemotherapy for these tumors differed among the reported articles. While Yoshimura reported that only 16 of 77 patients received concurrent chemotherapy [9], Nakamura reported that 39 of 43 patients received concurrent chemotherapy [1]. Inclusion criteria for concurrent chemotherapy were not documented in these articles. In our series, the treatment strategy included concurrent chemotherapy for T2 disease beginning in the year 2000. Chemotherapeutic agents were varied during the two decades of our study period. Since 2007, cisplatin alone has been the mainstay in our Institute. Out of 18 patients with T2 disease in our series, 16 were treated after 2000. All 16 underwent concurrent chemotherapy. In our results, T2 disease had a local control rate comparable with that of T1 disease. Thus, there may be some potential benefit of chemotherapy for T2 disease. T2 disease has a relatively wide range of tumor sizes (2 to 4 cm) and it may prove that concurrent chemotherapy is beneficial for larger tumors. However, it is still difficult to address the exact benefit of chemotherapy because of small sample sizes and lack of randomized data. Though adverse events related to chemotherapy were manageable, it is important to avoid unnecessary use of chemotherapy in patients who are likely to have tumor control with RT alone.
Well-differentiated squamous cell carcinoma and posterior wall tumors had poor outcomes for local control. Though these patients were a small cohort in our series, the poorer local control in posterior wall tumors was compatible with the report of Yoshimura et al [9]. Exact reasons for the poorer outcome in posterior wall tumors and well-differentiated tumors remain unclear. We might consider a more aggressive treatment strategy for these kinds of high-risk tumors, such as concurrent chemotherapy or volume-reduction surgery prior to RT.
Several authors have reported results of laryngeal preservation surgery for selected patients [14–17]. Though these reports also include node-positive disease, and it would be difficult to compare the long-term efficacy and local control rate with our result, they seemed to obtain comparable local control rates. However, postoperative mortality and morbidity is not negligible. Postoperative death rates of 2 to 10% were reported, and persistent swallowing difficulties and speech impairment were also reported. Radical RT for early-stage HPSCC may have some mortality and morbidity advantage in treatment without reducing local tumor control.
No patients in our series were treated with intensity-modulated RT (IMRT). IMRT is a conformal RT technique that can spare the major salivary glands and may reduce the incidence of long-term radiation-induced xerostomia. All patients with preserved larynxes in our series maintained ability in speech and swallowing. However, lack of saliva affects quality of life (QoL). Recently, the result of a randomized trial comparing conventional RT and parotid-sparing IMRT for head and neck cancers was reported [18]. Parotid-sparing IMRT was found to reduce the incidence of xerostomia and improve QoL. Because of the expected longer survival for early HPCSS, IMRT may be beneficial and should be considered for these patients.
CONCLUSION
In conclusion, RT for early HPSCC is deemed to be a feasible and effective treatment modality with minimal morbidity. In our cohort, 81% 5-year overall survival and 91% functional larynx preservation rates were obtained during a follow-up period of 62 months. Salvage surgery with or without larynx preservation was reserved for recurrent disease. Early detection and adequate management of local recurrence and metachronous malignancies are critical in obtaining longer survival.
ACKNOWLEDGEMENTS
The authors thank Dr Kazuro Sugimura for critical reading of the manuscript.
REFERENCES
- 1.Nakamura K, Shioyama Y, Sasaki T, et al. Chemoradiation therapy with or without salvage surgery for early squamous cell carcinoma of the hypopharynx. Int J Radiat Oncol Biol Phys. 2005;62:680–3. doi: 10.1016/j.ijrobp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura K, Shioyama Y, Kawashima M, et al. Multi-institutional analysis of early squamous cell carcinoma of the hypopharynx treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:1045–50. doi: 10.1016/j.ijrobp.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumors. 7th edn. Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- 4.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v3.0, 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. (18 July 2012, date last accessed). [Google Scholar]
- 5.Mendenhall WM, Parsons JT, Stringer SP, et al. Radiotherapy alone or combined with neck dissection for T1-T2 carcinoma of the pyriform sinus: An alternative to conservation surgery. Int J Radiat Oncol Biol Phys. 1993;27:1017–27. doi: 10.1016/0360-3016(93)90518-z. [DOI] [PubMed] [Google Scholar]
- 6.Amdur RJ, Mendenhall WM, Stringer SP, et al. Organ preservation with radiotherapy for T1-T2 carcinoma of the pyriform sinus. Head Neck. 2001;23:353–62. doi: 10.1002/hed.1044. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto M, Takahashi H, Yao K, et al. Clinical impact of using chemoradiotherapy as a primary treatment for hypopharyngeal cancer. Acta Otolaryngol. 2002;547(Suppl.):11–4. doi: 10.1080/000164802760057491. [DOI] [PubMed] [Google Scholar]
- 8.Garden AS, Morrison WH, Clayman GL, et al. Early squamous cell carcinoma of the hypopharynx: Outcomes of treatment with radiation alone to the primary disease. Head Neck. 1996;18:317–22. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<317::AID-HED2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura R, Kagami Y, Ito Y, et al. Outcomes in patients with early-stage hypopharyngeal cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:1017–23. doi: 10.1016/j.ijrobp.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 10.Wolf GT, Forastiere A, Ang K, et al. Workshop report: Organ preservation strategies in advanced head and neck cancer—current status and future directions. Head Neck. 1999;21:689–93. doi: 10.1002/(sici)1097-0347(199912)21:8<689::aid-hed2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Zelefsky MJ, Kraus DH, Pfister DG, et al. Combined chemotherapy and radiotherapy versus surgery and postoperative radiotherapy for advanced hypopharyngeal cancer. Head Neck. 1996;18:405–11. doi: 10.1002/(SICI)1097-0347(199609/10)18:5<405::AID-HED3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 13.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–8. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Plouin-Gaudon I, Lengelé B, Desuter G, et al. Conservation laryngeal surgery for selected pyriform sinus cancer. Eur J Surg Oncol. 2004;10:1123–30. doi: 10.1016/j.ejso.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Holsinger FC, Motamed M, Garcia D, et al. Resection of selected invasive squamous cell carcinoma of the pyriform sinus by means of the lateral pharyngotomy approach: the partial lateral pharyngectomy. Head Neck. 2006;8:705–11. doi: 10.1002/hed.20375. [DOI] [PubMed] [Google Scholar]
- 16.Chevalier D, Watelet JB, Darras JA, et al. Supraglottic hemilaryngopharyngectomy plus radiation for the treatment of early lateral margin and pyriform sinus carcinoma. Head Neck. 1997;1:1–5. doi: 10.1002/(sici)1097-0347(199701)19:1<1::aid-hed1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 17.Makeieff M, Mercante G, Jouzdani E, et al. Supraglottic hemipharyngolaryngectomy for the treatment of T1 and T2 carcinomas of laryngeal margin and piriform sinus. Head Neck. 2004;8:701–5. doi: 10.1002/hed.20051. [DOI] [PubMed] [Google Scholar]
- 18.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]