Abstract
We have previously reported that radon inhalation activates anti-oxidative functions and inhibits carbon tetrachloride (CCl4)-induced hepatopathy. It has also been reported that antioxidant vitamins can inhibit CCl4-induced hepatopathy. In the current study, we examined the comparative efficacy of treatment with radon, ascorbic acid and α-tocopherol on CCl4-induced hepatopathy. Mice were subjected to intraperitoneal injection of CCl4 after inhaling approximately 1000 or 2000 Bq/m3 radon for 24 h, or immediately after intraperitoneal injection of ascorbic acid (100, 300, or 500 mg/kg bodyweight) or α-tocopherol (100, 300, or 500 mg/kg bodyweight). We estimated the inhibitory effects on CCl4-induced hepatopathy based on hepatic function-associated parameters, oxidative damage-associated parameters and histological changes. The results revealed that the therapeutic effects of radon inhalation were almost equivalent to treatment with ascorbic acid at a dose of 500 mg/kg or α-tocopherol at a dose of 300 mg/kg. The activities of superoxide dismutase, catalase, and glutathione peroxidase in the liver were significantly higher in mice exposed to radon than in mice treated with CCl4 alone. These findings suggest that radon inhalation has an anti-oxidative effect against CCl4-induced hepatopathy similar to the anti-oxidative effects of ascorbic acid or α-tocopherol due to the induction of anti-oxidative functions.
Keywords: Radon, ascorbic acid, α-tocopherol, antioxidant, hepatopathy
INTRODUCTION
Carbon tetrachloride (CCl4) is a well-established hepatotoxin, producing free radicals during its metabolism in the liver [1–2]. Overproduction of these radicals initiates lipid peroxidation of polyunsaturated fatty acids in the cell membrane and eventually leads to cell death by necrosis. Ascorbic acid (AA; vitamin C), a well-known antioxidant, is a water-soluble vitamin that can protect against damage induced by reactive oxygen species (ROS) or free radicals. It has been reported that AA protects against cellular damage from lipid peroxide-derived 2-alkenals [3]. A recent study suggested that AA treatment reduces CCl4-induced oxidative damage in the liver, and that AA has a highly protective effect against hepatotoxicity and oxidative stress caused by CCl4 [4]. α-Tocopherol (vitamin E) is another antioxidant vitamin and is lipid-soluble. A previous report indicated that vitamin E protects against CCl4-induced chronic liver damage [5].
Low-dose irradiation induces various stimulus effects, such as activation of anti-oxidative factors [6–11]. Low-dose X- or γ-irradiation activates anti-oxidative functions in some organs [12–13]. For example, low-dose X-irradiation inhibits brain edema induced by cold injury [12] and paw edema induced by ischemia-reperfusion injury [13]. These findings indicate that low-dose irradiation has anti-oxidative effects and inhibits oxidative damage in certain organs.
Therapy involving radon gas volatilized from radon-enriched water is performed for various diseases at Misasa Medical Center, Okayama University Hospital. Most conditions treated with radon therapy are lifestyle-related diseases such as arteriosclerosis, osteoarthritis [14] and bronchial asthma [15]. In addition, a large number of patients are treated in various countries with traditional spa therapy (Japan [14–16], central Europe [17]). To assess the effects of radon, we have developed a radon-exposure system for small animals; using this system, we demonstrated that radon inhalation has anti-inflammatory effects and inhibits carrageenan-induced inflammatory paw edema [18]. We also demonstrated that radon inhalation inhibits the oxidative damage associated with CCl4-induced hepatopathy in mice, indicating that radon inhalation has anti-oxidative effects [19]. It is highly possible that radon inhalation acts by preventing or reducing ROS-related injuries. These findings also suggest that radon inhalation has anti-oxidative effects similar to X-irradiation.
Recently, in a search for new indications for radon therapy, we reported the responsiveness of superoxide dismutase (SOD) in mouse organs to radon [20]. In that study, we examined the changes in SOD activity in many mouse organs, including plasma, brain, lung, thymus, heart, liver, stomach, pancreas, kidney and small intestine. The results suggest that radon inhalation increases SOD activities in most organs; however, to date there have been no quantitative reports on the anti-oxidative effects of radon so the optimal conditions for radon therapy remain undetermined. The purpose of this study was to compare the anti-oxidative effects of radon and antioxidant vitamins such as AA and α-tocopherol. To assess the anti-oxidative effects of radon, we used a CCl4 -induced liver injury model. The following biochemical and histological parameters were examined to assess the anti-oxidative effects of radon: SOD activity, catalase activity, glutathione peroxidase (GPx) activity, total glutathione content (t-GSH), lipid peroxide levels and triglyceride levels (TG) in the liver; glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) activity, TG and total cholesterol (T-CHO) levels in the serum; and histological examination of liver tissue.
MATERIALS AND METHODS
Animals
Female ICR mice (aged 8 weeks, body weight approximately 28 g) were obtained from Charles River Laboratories Japan Inc. (Yokohama, Japan). Ethics approval for all protocols and experiments was obtained from the Animal Experimental Committee of Okayama University. Mice inhaled radon at a concentration of 1000 or 2000 Bq/m3 for 24 h. The radon concentration in the mouse cage was measured using a radon monitor (CMR-510; femto-Tech Inc., OH, USA). Mice had free access to food and water during radon inhalation and sham treatment. Then, 4 ml/kg bodyweight of CCl4 (5% in olive oil; Wako Pure Chemical Industries, Ltd., Osaka, Japan) was injected into the peritoneum of the mice immediately after radon inhalation or immediately after intraperitoneal (i.p.) injection of l(+)-ascorbic acid (100, 300 or 500 mg/kg weight; Kanto Chemical Co. Inc., Tokyo, Japan) or dl-α-tocopherol (100, 300 or 500 mg/kg weight; Nacalai Tesque Inc., Kyoto, Japan). Twenty-four hours after CCl4 administration, blood was drawn from the heart for serum analysis, and livers were quickly excised to analyze the levels of SOD, catalase, t-GSH, GPx, TG and lipid peroxide. Serum was separated by centrifugation at 3000 × g for 5 min for the assay of GOT and GPT activity, and the TG and T-CHO levels. These samples were preserved at –80°C until the biochemical assay. Liver tissue samples were fixed in 10% neutral-buffered formalin for histological examinations.
Biochemical assays
The serum activity of GOT and GPT, serum levels of TG and T-CHO, and the levels of TG in liver were measured using TA-LN, TG-EN and T-CHO kainosu, respectively (Kainosu Co., Ltd., Tokyo, Japan) according to the manufacturer's recommendations.
Mouse livers were homogenized on ice in 10 mM phosphate-buffered saline (PBS; pH 7.4). The homogenates were centrifuged at 12 000 × g for 45 min at 4°C and the supernatants were used to assay the activity of SOD and catalase.
SOD activity was measured by the nitroblue tetrazolium (NBT) reduction method [21] using the Wako-SOD test (Wako Pure Chemical Industry, Co., Ltd., Osaka, Japan) according to the manufacturer's recommendations. Briefly, the extent of inhibition of the reduction in NBT was measured at 560 nm using a spectrophotometer. One unit of enzyme activity was defined as 50% inhibition of NBT reduction.
Catalase activity was measured as the rate of hydrogen peroxide (H2O2) reduction at 37°C at 240 nm wavelength [22]. The assay mixture consisted of 50 µl 1 M Tris-HCl buffer containing 5 mM ethylenediaminetetraacetic acid (pH 7.4), 900 µl 10 mM H2O2, 30 µl deionized water, and 20 µl liver supernatant. Activity was calculated using a molar extinction coefficient of 7.1 × 10−3 M−1cm−1.
Total glutathione content was measured using the Bioxytech GSH-420 assay kit (Oxis Health Products, Inc., Portland, OR, USA) according to the manufacturer's recommendations. This assay is based on the formation of a chromophoric thione, whose absorbance at 420 nm is directly proportional to the total glutathione concentration. Briefly, liver samples were suspended in 10 mM PBS (pH 7.4), mixed with ice-cold 7.5% trichloroacetic acid solution and homogenized. The homogenates were centrifuged at 3000 × g for 10 min. The supernatants were used for the assay.
Lipid peroxide levels were assayed using the Bioxytech LPO-586 assay kit (Oxis Health Products, Inc.) according to the manufacturer's recommendations. The lipid peroxide assay is based on the reaction between a chromogenic reagent, N-methyl-2-phenylidole, and malondialdehyde and 4-hydroxyalkenals at 45°C. Data are derived from the optical density of the colored products at 586 nm. Briefly, liver samples were homogenized in 10 mM PBS (pH 7.4) on ice. Prior to homogenization, 10 µL of 0.5 M butylated hydroxytoluene in acetonitrile was added per 1 mL buffer-tissue mixture. After homogenization, the homogenate was centrifuged at 15 000 × g for 10 min at 4°C, and the supernatant was used for the assay.
GPx activity was measured using the BIOXYTECH GPx-340 assay kit (Oxis Health Products, Inc.) according to the manufacturer's recommendations. Briefly, liver samples were homogenized in 1 M Tris–HCl buffer (pH 7.4) containing 5 mM ethylenediaminetetraacetic acid and 1 mM dithiothreitol. Homogenates were centrifuged at 10 000 × g for 20 min at 4°C. The supernatants were used for the assay. The reduction of nicotinamide adenine dinucleotide phosphate (NADPH) to nicotinamide adenine dinucleotide phosphate (NADP+) is accompanied by a decrease in absorbance at 340 nm based on which GPx enzyme activity is calculated. The molar extinction coefficient for NADPH is 6220 M−1 cm−1 at 340 nm. To assay GPx levels, the supernatant is added to a solution containing glutathione, glutathione reductase (GR) and NADPH. The enzyme reaction is initiated by adding the substrate, tert-butyl hydroperoxide, and absorbance at 340 nm is recorded for 3 min.
The protein content in each sample was measured by the Bradford method, using the Protein Quantification Kit-Rapid (Dojindo Molecular Technologies, Inc., Kumamoto, Japan), as described previously [23].
Histological examination
Liver samples were fixed in 10% formalin, processed through a graded ethanol series and finally xylene, and embedded in paraffin. Six-micrometer-thick tissue sections were prepared and stained with hematoxylin-eosin (HE).
Inhibition score
We defined the inhibition rate (%) of GOT, GPT, TG, T-CHO, lipid peroxide level and histology by the following formula:
 |
To compare the inhibitory effects, the baseline clinical score was determined according to the inhibition rate. Briefly, no inhibitory effect (<10%) was scored as 0; inhibition rate of 10% to 40% from baseline was scored as 1; 40% to 70% was scored as 2; and >70% was scored as 3.
Statistical analyses
Data are presented as the mean ± standard error of the mean (SEM). Each experimental group consisted of samples from 5–8 animals. The statistical significance of differences was determined by Student's t-test for comparisons between the control group the and CCl4-administered group. Dunnett's test was used for multiple comparisons.
RESULTS
Changes in the radon concentration
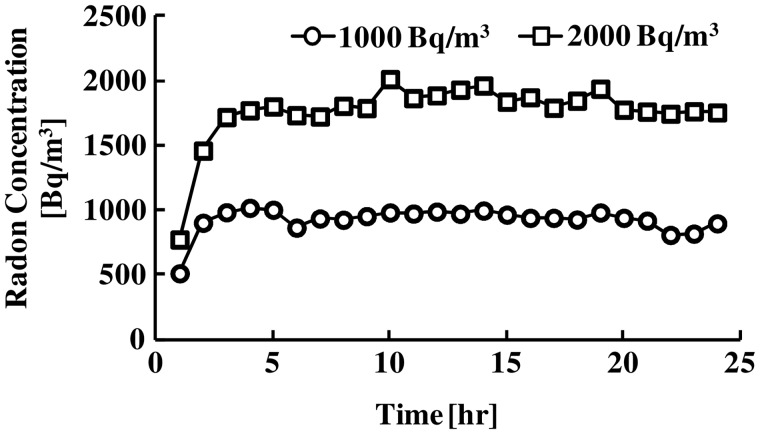
Radon concentrations in the mouse cages are shown in Fig. 1. The mean concentrations of treatment radon were approximately 1000 Bq/m3 and 2000 Bq/m3, respectively (Fig. 1).
Fig. 1.
Changes in radon concentration in mouse cages over the period of radon inhalation using a radon-exposure system.
Effects of AA, α-tocopherol or radon on hepatic function following CCl4 administration
The effects of CCl4 administration on hepatic function in mice pretreated with AA, α-tocopherol or radon were examined. In mice injected with CCl4 in the absence of AA, α-tocopherol or radon pretreatment, the activities of GOT and GPT in serum were significantly higher (P < 0.001) and the TG level in serum was significantly lower (P < 0.001) than in control animals. However, GOT activities in the serum of AA- (300 or 500 mg/kg weight, P < 0.05 and P < 0.01, respectively), α-tocopherol- (300 or 500 mg/kg weight, P < 0.05 and P < 0.001, respectively) and radon- (2000 Bq/m3, P < 0.05) treated mice, and GPT activities in the serum of AA- (300 or 500 mg/kg weight, P < 0.05), α-tocopherol- (500 mg/kg weight, P < 0.01) and radon- (2000 Bq/m3, P < 0.05) treated mice were significantly lower than those of CCl4-administered mice. The activities of GOT and GPT in the serum of other groups tended to be lower than those of CCl4-administered mice, but these differences were not statistically significant. In the absence of AA, α-tocopherol or radon pretreatment, the T-CHO levels in the serum of CCl4-administered mice were lower than in control animals, but those differences were not statistically significant. T-CHO levels in the serum of α-tocopherol- (300 mg/kg weight) treated mice were significantly higher (P < 0.05) than in CCl4-administered mice; however, there were no significant differences in TG levels between CCl4-administered mice and mice treated with AA, α-tocopherol or radon (Fig. 2).
Fig. 2.
Effects of AA, α-tocopherol and radon on hepatic function-associated parameters in the serum of CCl4-administered mice. Each value is the mean ± SEM. The number of mice per experimental point is 5–8. *P < 0.05, **P < 0.01, ***P < 0.001 vs CCl4 in the absence of AA, α-tocopherol or radon, ###P < 0.001 vs Control.
Effects of AA, α-tocopherol or radon on TG level in liver following CCl4 administration
The effects of CCl4 administration on TG accumulation in the liver of mice pretreated with AA, α-tocopherol or radon were examined. In mice injected with CCl4 in the absence of AA, α-tocopherol or radon pretreatment, TG levels in the liver were significantly higher (P < 0.001) than in control animals; however, TG levels in the liver of α-tocopherol- (300 or 500 mg/kg weight, P < 0.001) or radon- (2000 Bq/m3, P < 0.05) treated mice were significantly lower than in CCl4-administered mice. TG levels in the liver of other groups tended to be lower than those in the CCl4-administered group, but those differences were not statistically significant (Fig. 3).
Fig. 3.
Effects of AA, α-tocopherol and radon on TG levels in the liver of CCl4-administered mice. Each value is the mean ± SEM. The number of mice per experimental point is 7–8. *P < 0.05, ***P < 0.001 vs CCl4 in the absence of AA, α-tocopherol or radon, ###P < 0.001 vs Control.
Effects of AA, α-tocopherol or radon on hepatocytes
CCl4 administration resulted in centrilobular necrosis of the liver. Necrosis in the CCl4 group tended to be greater than in groups pretreated with AA, α-tocopherol or radon, but these differences were not statistically significant. In contrast, no centrilobular necrosis was observed in control animals (Fig. 4).
Fig. 4.
Effects of AA, α-tocopherol and radon on CCl4-induced hepatopathy in mice. Mouse livers were examined histologically. A Control; B CCl4 administration; C 300 mg/kg weight of AA treatment + CCl4 administration; D 300 mg/kg weight of α-tocopherol treatment + CCl4 administration; E 2000 Bq/m3 of radon inhalation + CCl4 administration. Scale bar represents 250 µm. All samples were stained with HE. The area of cell necrosis surrounding the central vein (cv), but not portal vein (pv), in the CCl4-administered mice was measured; F Area of damage was less in mice pretreated with AA, α-tocopherol or radon relative to those treated with only CCl4. No centrilobular necrosis was observed in control animals (ND = not detectable). Each value is the mean ± SEM. The number of mice per experimental point was 5–8.
Effects of AA, α-tocopherol and radon on lipid peroxide levels in liver following CCl4 administration
The effects of CCl4 administration on lipid peroxide levels in the liver of mice pretreated with AA, α-tocopherol and radon were examined. In mice injected with CCl4 in the absence of AA, α-tocopherol, or radon pretreatment, lipid peroxide levels in the liver were significantly higher (P < 0.05) than in control animals. However, the lipid peroxide levels in the liver of AA- (500 mg/kg weight, P < 0.01) or α-tocopherol- (300 or 500 mg/kg weight, P < 0.05 and P < 0.01, respectively) treated mice were significantly lower than in CCl4-administered mice. The lipid peroxide levels in the liver of other groups tended to be lower than in the CCl4-administered group, but these differences were not statistically significant (Fig. 5).
Fig. 5.
Effects of AA, α-tocopherol and radon on lipid peroxide levels in the liver of CCl4-administered mice. Each value is the mean ± SEM. The number of mice per experimental point is 6–8. *P < 0.05, **P < 0.01 vs CCl4 in the absence of AA, α-tocopherol or radon, #P < 0.05 vs Control.
Inhibition ratio of CCl4-induced hepatopathy
We scored the inhibition rate of CCl4-induced hepatopathy as shown in Materials and Methods. The score of AA-treated mice at a dose of 100, 300 and 500 mg/kg bodyweight was 0.9 ± 0.1, 1.3 ± 0.4 and 1.4 ± 0.3, respectively. The score of α-tocopherol-treated mice at a dose of 100, 300 and 500 mg/kg bodyweight was 0.7 ± 0.3, 1.4 ± 0.5 and 1.6 ± 0.4, respectively.
In addition, the score of mice which had inhaled radon at a concentration of 1000 or 2000 Bq/m3 was 1.4 ± 0.3 and 1.4 ± 0.2, respectively. The score depended on the dosage of AA or α-tocopherol; however, no dose dependency was observed in mice which had inhaled radon. In summary, treatment with AA, α-tocopherol and radon inhibited lipid peroxidation induced by CCl4 (Table 1).
Table 1.
Inhibitory effects of AA, α-tocopherol and radon inhalation on CCl4 hepatopathy
| Ascorbic Acid [mg/kg] |
α-tocopherol [mg/kg] |
Radon [Bq/m3] |
||||||
|---|---|---|---|---|---|---|---|---|
| 100 | 300 | 500 | 100 | 300 | 500 | 1000 | 2000 | |
| GOT | 1 | 2 | 2 | 0 | 1 | 2 | 1 | 2 |
| GPT | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| T-CHO | 1 | 1 | 1 | 1 | 2 | 1 | 3 | 1 |
| TG (serum) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| TG (liver) | 1 | 1 | 1 | 1 | 3 | 3 | 1 | 2 |
| Lipid peroxide | 1 | 3 | 3 | 2 | 3 | 3 | 2 | 2 |
| Histological score | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Average | 0.9 | 1.3 | 1.4 | 0.7 | 1.4 | 1.6 | 1.4 | 1.4 |
| SEM | 0.1 | 0.4 | 0.3 | 0.3 | 0.5 | 0.4 | 0.3 | 0.2 |
Effects of AA, α-tocopherol and radon on antioxidant-associated substances in liver following CCl4 administration
The effects of CCl4 administration on antioxidant-associated substances in the liver of mice pretreated with AA, α-tocopherol or radon were examined. In mice injected with CCl4 in the absence of AA, α-tocopherol or radon pretreatment, the activities of SOD (P < 0.05), catalase (P < 0.001), GPx (P < 0.01) and t-GSH content (P < 0.05) in the liver were significantly lower than in control animals. However, the activities of SOD and catalase (1000 Bq/m3 or 2000 Bq/m3, SOD; P < 0.05, catalase; P < 0.01 and P < 0.001, respectively) in the liver of mice which had inhaled radon and GPx activity (2,000 Bq/m3, P < 0.01) in the liver of mice which had inhaled radon were significantly higher in CCl4-administered mice. In addition, treatment with AA (100, 300, or 500 mg/kg weight) or α-tocopherol (100, 300, or 500 mg/kg weight) did not result in an increase in SOD, catalase, GPx or t-GSH in the liver (Fig. 6).
Fig. 6.
Effects of AA, α-tocopherol and radon on anti-oxidative-associated parameters in the liver of CCl4-administered mice. Each value is the mean ± SEM. The number of mice per experimental point is 6–8. *P < 0.05, **P < 0.01, ***P < 0.001 vs CCl4 in the absence of AA, α-tocopherol or radon, #P < 0.05 ##P < 0.01, ###P < 0.001 vs Control.
DISCUSSION
We previously reported that 0.5 Gy X-irradiation or radon inhalation activated anti-oxidative function in the liver and inhibited oxidative damage in mice. For example, pretreatment with low-dose X-irradiation inhibits CCl4-induced hepatopathy [24–26] and post-treatment low-dose X-irradiation reduced the oxidative damage associated with CCl4-induced hepatopathy [27]. It appears to be highly likely that low-dose irradiation, including radon inhalation, helps to prevent or reduce ROS-related liver damage [26]. Therefore, we used a model of liver injury in mice to quantitatively compare the anti-oxidative effects of radon inhalation and antioxidant vitamins.
AA and α-tocopherol are well-known antioxidants, easily obtained from foods or supplements. AA is soluble in water, scavenging ROS in the aqueous phase. In contrast, α-tocopherol is a lipophilic antioxidant, so it scavenges ROS in the membrane [28]. Radon has both hydro-soluble and lipophilic radioisotopes; therefore, to elucidate the effects of radon in detail, it is very instructive to compare the antioxidative effects between radon and these two vitamins.
AA reacts readily with superoxide anions and hydrogen peroxide [29], and α-tocopherol has the ability to scavenge peroxyl radicals [3]. Moreover, it is reported that both AA and α-tocopherol are absorbed quickly (within several hours) [30–31]. In one study the AA concentration in the liver of AA-deficient mice peaked 3 hours after AA intake and then decreased [30]. Another report suggested that α-tocopherol in the liver peaked at around 1 hour and then decreased [31]. Although there are differences in the experimental conditions between these studies, they suggest that AA and α-tocopherol are absorbed quickly after administration and increase antioxidant activity in the liver.
SOD activity catalyzes the conversion of superoxide anions into H2O2, and catalase transforms hydrogen peroxide into H2O. GSH reacts directly with ROS, and GPx catalyzes the destruction of H2O2 and hydroxyl radicals [32]. This catalysis generates GSSG and finally GSH; however, GR catalyzes the regeneration of GSH from GSSG. Thus, GR and GPx are both enzymes in the generating GSH pathway, and AA plays the same role as antioxidant enzymes. It has been reported that GSH protects against CCl4-induced microsomal lipid peroxidation [33] and can scavenge trichloromethyl peroxyl radicals but not trichloromethyl radicals [34]. In fact, low-dose irradiation, including radon inhalation, increases t-GSH content in the liver and reduces oxidative damage [24–25]. In addition, AA treatment reduces oxidative damage in the liver [34–35].
The results of this study show that CCl4 administration significantly increases the activities of GOT and GPT and the levels of lipid peroxide and TG in the liver. These findings indicate that CCl4 administration depresses hepatic function and induces oxidation and fatty liver. In addition, CCl4 results in centrilobular necrosis; therefore, we comprehensively examined the inhibitory effects of radon inhalation and treatment with antioxidant vitamins on CCl4-induced hepatopathy using hepatic function-associated and oxidative damage-associated parameters, and observation of histological changes. Treatment with AA or α-tocopherol reduced the activities of GOT and GPT in serum, and the levels of TG and lipid peroxide in the liver of CCl4-administered mice. This inhibitory effect depended on the dosage of AA or α-tocopherol. Radon inhalation at a concentration of 2000 Bq/m3 also decreased the activities of GOT and GPT in serum. These findings suggest that treatment with AA, α-tocopherol or radon can inhibit CCl4-induced hepatopathy. In addition, TG levels in the liver of mice treated with α-tocopherol or radon were significantly decreased in CCl4-administered mice. These results suggest that treatment with α-tocopherol or radon inhibits CCl4-induced fatty liver. Furthermore, AA treatment at a dose of 500 mg/kg weight, or α-tocopherol treatment at a dose of 300 or 500 mg/kg weight, significantly reduced the lipid peroxide level in the liver, suggesting that treatment of antioxidant vitamin such as AA or α-tocopherol inhibits oxidation induced by CCl4.
The inhibition rate of CCl4-induced hepatopathy increased with the dosage of AA (R2 = 0.932, R: correlation coefficient) or α-tocopherol (R2 = 0.871). It seems that the inhibition rate correlates with the vitamin concentration; however, no dose dependency was observed in radon-inhaled groups. According to these results, radon inhalation has an anti-oxidative effect similar to AA treatment at a dose of 500 mg/kg bodyweight, or α-tocopherol at 300 mg/kg bodyweight.
To clarify the mechanisms underlying the inhibitory effect of radon inhalation, we examined antioxidant-associated substances such as SOD, catalase, t-GSH and GPx. Our previous study suggested that radon inhalation at a concentration of 2000 Bq/m3 significantly increased the activities of GPx activity and t-GSH content in mouse liver [19]. In addition, another report suggested that radon inhalation at a concentration of 1000 Bq/m3 significantly increased SOD activity in the liver of BALB/c mice, which are sensitive to radiation [20]. These findings suggest that radon inhalation activates antioxidative functions in mouse liver. In this study, the activity levels of SOD, catalase and GPx in the livers of mice which had inhaled radon were significantly higher than in those of CCl4-treated mice; however, there were no significant differences in the activity levels of SOD, catalase and GPx, and in t-GSH content in livers between the CCl4-administered group and the AA- or α-tocopherol-treated groups. No dose dependency was observed in the increase of antioxidative-associated parameters in the livers of radon-inhaled groups; therefore, no dose dependency may be observed in the scores.
Our radon exposure system is effective in terms of keeping low radon progeny in the mouse cage. The equilibrium equivalent radon concentration, the radon progeny concentration, was quite low compared with the concentration of the coexisting radon, even if the air balance in the mouse cage was considered, because air was supplied through a HEPA filter [36]. According to Sakoda's report [37], the dose absorbed by the lung was 75 to 150 nGy under their experimental conditions. In addition, the dose absorbed by the liver was almost the same as the lung. The absorbed doses were 500 to 1000 times larger than the background level; however, it is difficult to estimate the dose absorbed by the whole body because there are no reports of these models in mice.
Since the 2011 nuclear accident in Fukushima, the effects of low-dose irradiation are at the forefront of the public's attention. There have been many reports of the stimulatory effects of low-dose irradiation; however, there have been no quantitative reports concerning the anti-oxidative effects induced by low-dose irradiation. It is difficult to explain the effects of low-dose irradiation in terms that the public can readily understand; therefore, this report might provide a useful example of the effects of low-dose irradiation in relation to vitamins common in our daily lives.
CONCLUSION
In conclusion, radon inhalation has an anti-oxidative effect against CCl4-induced hepatopathy that is comparable to treatment with AA at a dose of 500 mg/kg weight or α-tocopherol treatment at a dose of 300 mg/kg weight, due to activation of anti-oxidative functions. Radon therapy is performed for various diseases at Misasa Medical Center, Okayama University Hospital. No previous reports on the quantitative effects of radon therapy have been reported, however, quantitative evaluation of the activation of anti-oxidative functions after radon inhalation will provide a basis for future studies aimed at assessing new radon-based therapies. In addition, further study on the therapeutic effects of a combination of radon and AA on liver diseases is warranted.
FUNDING
This work was supported by Okayama University and Japan Atomic Energy Agency.
REFERENCES
- 1.Recknagel RO, Ghoshal AK. Lipoperoxidation as a vector in carbon tetrachloride hepatotoxicity. Lab Invest. 1966;15:132–48. [PubMed] [Google Scholar]
- 2.Durk H, Frank H. Carbon tetrachloride metabolism in vivo and exhalation of volatile alkanes: dependence upon oxygen parital pressure. Toxicology. 1984;30:249–57. doi: 10.1016/0300-483x(84)90096-9. [DOI] [PubMed] [Google Scholar]
- 3.Traber MG, Stevens JF. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–13. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozturk IC, Ozturk F, Gul M, et al. Protective effects of ascorbic acid on hepatotoxicity and oxidative stress caused by carbon tetrachloride in the liver of Wistar rats. Cell Biochem Funct. 2009;27:309–15. doi: 10.1002/cbf.1575. [DOI] [PubMed] [Google Scholar]
- 5.Naziroğlu M, Cay M, Ustündağ B, et al. Protective effects of vitamin E on carbon tetrachloride-induced liver damage in rats. Cell Biochem Funct. 1999;17:253–9. doi: 10.1002/(SICI)1099-0844(199912)17:4<253::AID-CBF837>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka T, Yoshimoto M, Nakagawa S, et al. Basic study on active changes in biological function of mouse liver graft in cold storage after low-dose X-irradiation. J Clin Biochem Nutr. 2009;45:219–26. doi: 10.3164/jcbn.09-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima S, Matsuki O, Kinoshita I, et al. Does small-dose γ-ray radiation induce endogenous antioxidant potential in vivo? Biol Pharm Bull. 1997;20:601–4. doi: 10.1248/bpb.20.601. [DOI] [PubMed] [Google Scholar]
- 8.Yamaoka K, Kojima S, Takahashi M, et al. Change of glutathione peroxidase synthesis along with that of superoxide dismutase synthesis in mice spleen after low-dose X-ray irradiation. Biochem Biophys Acta. 1998;1381:265–70. doi: 10.1016/s0304-4165(98)00021-x. [DOI] [PubMed] [Google Scholar]
- 9.Yamaoka K, Kojima S, Nomura T. Changes of SOD-like substances in mouse organs after low-dose X-ray irradiation. Physiol Chem Phys Med NMR. 1999;31:23–8. [PubMed] [Google Scholar]
- 10.Yamaoka K, Edamatsu R, Mori A. Increased SOD activities and decreased lipid peroxide levels induced by low dose X irradiation in rat organs. Free Radic Biol Med. 1991;11:299–306. doi: 10.1016/0891-5849(91)90127-o. [DOI] [PubMed] [Google Scholar]
- 11.Kataoka T, Sakoda A, Yoshimoto M, et al. Studies on possibility for alleviation of lifestyle diseases by low-dose irradiation or radon inhalation. Radiat Prot Dosim. 2011;146:360–3. doi: 10.1093/rpd/ncr189. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto M, Kataoka T, Toyota T, et al. Inhibitory effects of prior low-dose X-irradiation on cold-induced brain injury in mouse. Inflammation. 2012;35:89–97. doi: 10.1007/s10753-011-9293-9. [DOI] [PubMed] [Google Scholar]
- 13.Kataoka T, Mizuguchi Y, Yoshimoto M, et al. Inhibitory effects of prior low-dose X-irradiation on ischemia-reperfusion injury in mouse paw. J Radiat Res. 2007;48:505–13. doi: 10.1269/jrr.07060. [DOI] [PubMed] [Google Scholar]
- 14.Yamaoka K, Mitsunobu F, Hanamoto K, et al. Study on biologic effects of radon and thermal therapy on osteoarthritis. J Pain. 2004;5:20–5. doi: 10.1016/j.jpain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Mitsunobu F, Yamaoka K, Hanamoto K, et al. Elevation of antioxidant enzymes in the clinical effects of radon and thermal therapy for bronchial asthma. J Radiat Res. 2003;44:95–9. doi: 10.1269/jrr.44.95. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka T, Aoyama Y, Sakoda A, et al. Basic study on biochemical mechanism of thoron and thermal therapy. Physiol Chem Phys Med NMR. 2006;38:85–92. [PubMed] [Google Scholar]
- 17.Becker K. One century of radon therapy. Int J Low Radiat. 2004;1:334–57. [Google Scholar]
- 18.Kataoka T, Teraoka J, Sakoda A, et al. Protective effects of radon inhalation on carrageenan-induced inflammatory paw edema in mice. Inflammation. 2012;35:713–22. doi: 10.1007/s10753-011-9364-y. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka T, Nishiyama Y, Toyota T, et al. Radon inhalation protects mice from carbon-tetrachloride-induced hepatic and renal damage. Inflammation. 2011;34:559–67. doi: 10.1007/s10753-010-9263-7. [DOI] [PubMed] [Google Scholar]
- 20.Kataoka T, Sakoda A, Ishimori Y, et al. Study of the response of superoxide dismutase in mouse organs to radon using a new large-scale facility for exposing small animals to radon. J Radiat Res. 2011;52:775–81. doi: 10.1269/jrr.10072. [DOI] [PubMed] [Google Scholar]
- 21.Baehner RL, Murrmann SK, Davis J, et al. The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest. 1975;56:571–6. doi: 10.1172/JCI108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aebi H, Wyss SR, Scherz B, et al. Properties of erythrocyte catalase from homozygotes and heterozygotes for Swiss-type acatalasemia. Biochem Genet. 1976;14:791–807. doi: 10.1007/BF00485342. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Yamaoka K, Kataoka T, Nomura T, et al. Inhibitory effects of prior low-dose irradiation on carbon tetrachloride-induced hepatopathy in acatalasemic mice. J Radiat Res. 2004;45:89–95. doi: 10.1269/jrr.45.89. [DOI] [PubMed] [Google Scholar]
- 25.Yamaoka K. Activation of antioxidant system by low dose radiation and its applicable possibility for treatment of reactive oxygen species-related diseases. J Clin Biochem Nurt. 2006;39:114–33. [Google Scholar]
- 26.Kataoka T, Yamaoka K. Activation of biodefense system by low-dose irradiation or radon inhalation and its applicable possibility for treatment of diabetes and hepatopathy. ISRN Endocrinology. 2012;2012:1–11. doi: 10.5402/2012/292041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kataoka T, Nomura T, Wang DH, et al. Effects of post low-dose X-ray irradiation on carbon tetrachloride-induced acatalasemic mice liver damage. Physiol Chem Phys Med NMR. 2005;37:109–26. [PubMed] [Google Scholar]
- 28.Niki E. Interaction of ascorbate and α-tocopherol. Ann N Y Acad Sci. 1987;498:186–99. doi: 10.1111/j.1749-6632.1987.tb23761.x. [DOI] [PubMed] [Google Scholar]
- 29.Burton GW, Wayner DDM. The antioxidant role of vitamin C. Adv Free Radic Biol Med. 1986;2:419–44. [Google Scholar]
- 30.Iwama M, Shimokado K, Maruyama N, et al. Time course of vitamin C distribution and absorption after oral administration in SMP30/GNL knockout mice. Nutrition. 2011;27:471–8. doi: 10.1016/j.nut.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Bjørneboe A, Bjørneboe GE, Drevon CA. Serum half-life, distribution, hepatic uptake and biliary excretion of α-tocopherol in rats. Biochim Biophys Acta. 1987;921:175–81. doi: 10.1016/0005-2760(87)90016-6. [DOI] [PubMed] [Google Scholar]
- 32.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 33.Burk RF, Patel K, Lane JM. Reduced glutathione protection against rat liver microsomal injury by carbon tetrachloride dependence on O2. Biochem J. 1983;215:441–5. doi: 10.1042/bj2150441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ergul Y, Erkan T, Uzun H, et al. Effect of vitamin C on oxidative liver injury due to isoniazid in rats. Pediatr Int. 2010;52:69–74. doi: 10.1111/j.1442-200X.2009.02891.x. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee P, Bhattacharyya SS, Bhattacharjee N, et al. Ascorbic acid combats arsenic-induced oxidative stress in mice liver. Ecotoxico Environ Saf. 2009;72:639–649. doi: 10.1016/j.ecoenv.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Ishimori Y, Mitsunobu F, Yamaoka K, et al. Development of a radon test facility for small animals. Jpn J Health Phys. 2010;45:65–71. [Google Scholar]
- 37.Sakoda A, Ishimori Y, Kawabe A, et al. Physiologically-based pharmacokinetic modeling of inhaled radon to calculate absorbed doses in mice, rats and humans. J Nucl Sci Technol. 2010;47:731–8. [Google Scholar]