Abstract
Rationale and Objectives
Patients with atrial fibrillation undergo structural remodeling resulting in increased pulmonary vein sizes. Studies have demonstrated that these changes are reversible following successful ablation therapy. To date, analyses of pulmonary vein structure have focussed on measurements at the pulmonary vein ostia and the full extent of reverse remodeling along the length of the pulmonary veins has not yet been fully characterized.
Materials and Methods
An automated, three-dimensional method is proposed which quantifies pulmonary vein geometry starting at the ostia and extending several centimeters into the veins. A centerline is tracked along the length of the pulmonary vein and orthogonal planes are computed along the curve. The method is validated against manual measurements on each of the four pulmonary veins for 10 subjects. The proposed methodology is used to analyze the pulmonary veins in 21 patients undergoing cardiac ablation therapy with pre- and post-operative CT scans.
Results
Validation results demonstrate that the automated measurements closely follow the manual measurements with an overall mean difference of 11.50 mm2. Significant differences in cross-sectional area at the two time-points were observed at all pulmonary vein ostia and extending for 1.5 cm (excluding the 1.0 cm interval) into the left inferior pulmonary vein, 3.0 cm into the left superior pulmonary vein, and 0.5 cm into the right superior pulmonary vein.
Conclusions
Quantitative analysis along the length of the pulmonary veins can be accomplished using centerline tracking and measurements from orthogonal planes along the curve. The patient study demonstrates that reverse structural remodeling following ablation therapy occurs not only at the ostia, but for several centimeters extending into the pulmonary veins.
Keywords: Cardiac Ablation, Left Atrial Fibrillation, Pulmonary Veins, Left Atrium, Centerline Tracking
1. Rationale and Objectives
Atrial fibrillation is a condition of the heart in which the atria beat rapidly and irregulary, resulting in a lack of synchrony in the beating of the heart. While the exact etiology of the disease is not yet fully understood, it has been shown that the origin of ectopic beats are frequently located in the pulmonary veins [1]. In catheter ablation therapy, a standard treatment strategy, catheters are guided into the left atrium and radio frequency energy is delivered circumferentially to each of the pulmonary veins to create an electrical block to the left atrium. Structural remodeling occurs in conjunction with this disease, with atrial fibrillation patients having larger left atria [2, 3, 4, 5] and pulmonary veins [3, 4, 5, 6] than normal controls. Following ablation therapy, these structural changes are reversible, a process termed reverse remodeling, with left atrial size [7, 8, 9, 10, 11] and pulmonary vein diameters [5, 6, 7, 8, 9] significantly decreasing in patients who have returned to normal sinus rhythm.
Left atrial size has been quantified using either two-dimensional diameter measurements or three-dimensional volume measurements from high-resolution CT and MR datasets. Efforts have also been made to quantify pulmonary vein structure [6, 7, 8, 9, 12], but these techniques primarily include manual line measurements on either volume renderings or within image cross-sections. The number and location of measurements are typically limited to the junction between the pulmonary vein and left atrium and a collection of specified distances within the pulmonary veins. Accurately defining a distance into the pulmonary vein, however, can be difficult since the pulmonary veins traverse a three-dimensional path through the volume. The current lack of three-dimensional quantification tools for pulmonary vein structure limits the ability to assess complex changes that may occur in response to ablation therapy.
In previous work by ourselves and others [13, 14, 15], the pulmonary veins have been modelled using three-dimensional centerline curves. In this paper, we expand upon previous work and propose a technique to quantify the structure of the pulmonary veins using centerline tracking. The approach computes a centerline from the junction between the left atrium and pulmonary vein, called the ostium, to a distal point in the pulmonary vein, as illustrated in Fig. 1. In this figure, the centerline is represented as a dashed line and planes orthogonal to the centerline are shown as solid lines. Each of the planes represents an oblique slice which is extracted from the image volume for making cross-sectional measurements, and distances extending into the pulmonary vein are measured as length along the centerline curve. At each point along the curve, an area measurement is computed allowing for a detailed, quantitative analysis of geometry along the pulmonary vein. The proposed technique is validated against manual measurements for each of the four pulmonary veins in 10 subjects. In addition, the technique is applied to a series of patient scans at both baseline and three months following ablation therapy. The results of the study demonstrate that reverse structural remodeling following ablation therapy occurs not only at the ostia, but for several centimeters extending into the pulmonary veins.
Figure 1.

Back view of left atrium illustrating measurements made along pulmonary vein using successive planes orthogonal to a centerline curve. LSPV=left superior pulmonary vein, RSPV=right superior pulmonary vein, LIPV=left inferior pulmonary vein, RIPV=right inferior pulmonary vein.
2. Materials and Methods
Data Preprocessing
The first step is to segment the left atrium and pulmonary veins from a 3D CT dataset using a previously validated semiautomated approach [16, 17]. The left atrium and pulmonary veins are first segmented as a single region using thresholding and region growing operations. The pulmonary veins are separated from the left atrium using an interactive cut plane as demonstrated in Fig. 2. The cut plane can be visualized in a 3D view and adjusted by the user directly moving the plane with a mouse, or using buttons which rotate the plane about three orthogonal axes. The location of the cut plane is also shown in each of three standard orthogonal views along the bottom panel of the interface. The oblique image slice at the current cut plane is shown in the top right of the interface. Using this combination of interactive visual aids, the user can carefully define an optimal cut plane to separate each pulmonary vein from the left atrium. As a final preprocessing step, any small side branches are manually removed from the pulmonary vein. If a bifurcation of the pulmonary vein occurs, the larger of the two branches is retained leaving a single vein for subsequent analysis.
Figure 2.
3D software for segmentation of left atrium and pulmonary veins. The pulmonary veins are separated from the left atrium using an interactive cut plane which is visualized in the 3D dislay on the top left and the location of the oblique cut plane is shown in three orthogonal planes along the bottom. The oblique image slice is also displayed in the top right panel.
Overall Method
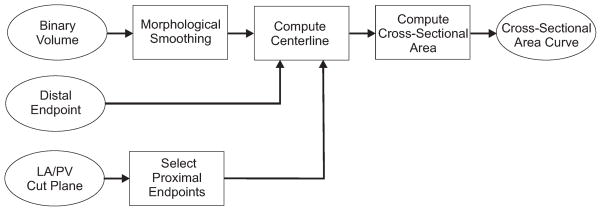
A flowchart for the overall method is shown in Fig. 3. First, a binary volume is created for each pulmonary vein using a thresholding operation on the segmentated volume. The binary volume is smoothed using a series of morphological operations which removes small regions of noisy voxels on the boundaries. Next, the centerline is computed from the smoothed binary volume and two endpoints. The distal endpoint is selected manually by the user as the furthest point along the pulmonary vein included in the segmentation. Proximal endpoints are computed automatically using the boundary between the left atrium and pulmonary vein created by the cut plane during the segmentation process. The cross-sectional area is computed at each point along the curve using an oblique image where the orientation is computed using both the centerline curve and the interactive cut plane defined by the user during the segmentation process. The final output is a 2D curve which gives the cross-sectional area versus distance along the pulmonary vein.
Figure 3.
Flowchart demonstrating the overall method for using a centerline curve to compute the cross-sectional area along a pulmonary vein.
Centerline Calculation
The centerline tracking algorithm follows a previously described method [18] which consists of a series of steps. First, an endpoint preserving skeleton of the object is computed. Next, points on the skeleton are assigned a weight based on the distance to the edge of the original segmented binary object. This distance is inverted so that points on the skeleton closest to the center of the object take on the smallest values and extraneous loops in the skeleton have larger values. A distance-weighted graph is built starting from the proximal endpoint. The final centerline is computed by backtracking along the minimal weight path from the distal endpoint to the proximal endpoint using a simplified version of Djikstra’s shortest path algorithm [19]. The result of the path traversing algorithm is a curve which runs along the center of the pulmonary vein. This path may, however, still contain sharp bends. In order to create a smooth path, the centerline is subsampled and a spline is fit to these points. Results of centerlines tracked for each of the four pulmonary veins from a patient dataset are shown in Fig. 4.
Figure 4.
Centerlines tracked for (a) left inferior, (b) left superior, (c) right inferior, and (d) right superior pulmonary veins.
Cross-Sectional Area Computation
Cross-sectional area along the length of each pulmonary vein is computed by extracting oblique image planes at each point along the centerline curve. The orientation of the oblique plane is computed using both the manually adjusted segmentation cut plane and the plane orthogonal to the centerline curve. As demonstrated in Fig. 5(a), when the pulmonary vein enters the left atrium at a large angle, the plane orthogonal to the centerline curve may cut across both the left atrium and pulmonary vein. When this occurs, the plane orthogonal to the centerline may underestimate the cross-sectional area near the ostium since the area is computed from the binary segmenation of the pulmonary vein. A more accurate estimate of cross-sectional area near the ostium is made using the user-defined cut plane from the segmentation as illustrated in Fig. 5(b). These planes are also demonstrated on a patient dataset in Fig. 6. Thus, the optimal orientation for the plane used to measure cross-sectional area across the pulmonary vein would originate as the user-defined segmentation plane near the ostium and smoothly transition to the orthogonal of the centerline curve further into the vein.
Figure 5.
(a) The centerline curve with orthogonal plane near the ostium. (b) Plane accurately cutting across pulmonary vein near ostium.
Figure 6.
In (a), the orthogonal plane to the centerline curve near the pulmonary vein ostium is shown with (b) its associated image slice. In (c), the manually adjusted cut plane from the segmentation procedure is shown with (d) its associated image slice.
This is accomplished using Eq. 1:
| (1) |
where α1 + α2 = 1 and . c is a constant and n is the point number along the curve. c was empirically set using a single dataset and held constant for all subsequent datasets. v3 is the unit vector used to compute the oblique image plane, v1 is the normal of the segmentation cut plane and v2 is defined in Eq. 2:
| (2) |
where xc is the current point and xn is the next point on the centerline. The effect of Eqs. 1 and 2 is that near the pulmonary vein ostia, v3 is approximately equal to the segmentation plane normal and further into the vein v3 is approximately equal to the vector along the centerline. At each point along the centerline curve, the oblique image plane defined by v3 is extracted from the volume and the cross-sectional area for the specified pulmonary vein is computed as:
| (3) |
where A is cross-sectional area, vi are the voxels within the specificed pulmonary vein Pj, and d is the voxel resolution.
Fig. 7 demonstrates cross-sectional area versus distance along the pulmonary vein. The blue curve is the area measured using the plane orthogonal to v1, the green curve uses the plane orthogonal to v2, and the magenta curve uses the plane orthogonal to v3. When using the vector from the centerline only (green curve), the cross-sectional area first shows an increase followed by a decrease for all four pulmonary veins. This is due to the oblique image intersecting both the pulmonary vein and the left atrium near the ostium, resulting in an underestimation of the cross-sectional area. The orthogonal computed from the user-defined segmentation cut plane (blue curve), can be used to measure the cross-sectional area close to the ostium, but can overestimate the cross-sectional area if the pulmonary vein bends as seen in the left inferior and left superior pulmonary veins. The oblique computed from the combination of the normals (magenta curve) appears to provide a reasonable estimate of cross-sectional area along the entire length of the pulmonary vein. Planes orthogonal to v3 at centerline points along the right superior pulmonary vein, along with the corresponding oblique images extracted from the volume, are shown in Fig. 8.
Figure 7.
Cross-sectional area versus distance for three methods: segmentation cut plane (blue), orthogonal to centerline normal (green), and orthogonal to combined vector (magenta).
Figure 8.
Planes orthogonal to the orientation vector at centerline points along the right superior pulmonary vein (top panel) and corresponding oblique images extracted from volume (bottom panel).
In order to reduce the dependence of the centerline curve on the proximal endpoint, a collection of points are randomly selected and centerline tracking is run for each endpoint. The points are selected by first computing the centroid of the boundary between the left atrium and pulmonary veins and randomly sampling points on the boundary within 3 mm of the centroid. Twenty-five iterations of the centerline tracking are run and the final curve is computed as the median of the collection of cross-sectional area curves sampled at equidistant intervals. The distal endpoint is chosen manually and creates little variation in the cross-sectional measurements since the pulmonary vein is relatively small at this point.
Validation Experiments
In order to validate the proposed technique, a manual method was used to measure the cross-sectional area along the length of the pulmonary vein. First, points were manually selected along the length of the pulmonary vein using a triplanar image view. Points were placed as close as possible to the center of the vein using three orthogonal views simultaneoulsy to guide point placement. Approximately 15 to 30 points were selected for each pulmonary vein depending on its length. At each manually selected point, an initial plane is displayed at an orientation orthogonal to the x-axis as shown in Fig. 9(a). Next, the user adjusts the orientation of the plane to best represent a cross-sectional cut across the pulmonary vein as shown in Fig. 9(b). The user can view the 3D plane on a volume rendering (top left), the plane in the three standard orthogonal views (bottom panel), or the oblique slice cut by the plane (top right). The plane can be adjusted by directly rotating the plane in the 3D view with the mouse, or using three rotation buttons. Once the plane is properly oriented, a cross-sectional measurement is made at that point.
Figure 9.
Interface used to make manual measurements of the cross-sectional area. (a) The initial plane orthogonal to the x axis. (b) The manually adjusted plane.
Patient Study
High-resolution, contrast-enhanced CT data was acquired from sites participating in the CABANA pilot imaging study. Twenty-one patients undergoing cardiac ablation therapy received both a baseline and a three month follow-up scan. All institutional review board approvals were obtained at each participating site prior to data collection and analysis. Each site was provided with a standard imaging protocol requesting the use of prospective gating when possible based on patient condition (sinus rhythm) and hardware constraints. Data was reconstructed with the following parameter ranges: in-plane resolution (0.30 mm to 0.71 mm), slice thickness (0.60 mm to 2.0 mm), R-R interval (approximately 55%–70%). All datasets were subsequently interpolated to isotropic voxels prior to image analysis. For all patient datasets, baseline and follow-up scans were segmented concurrently, however, the analyst was blinded to timepoint. All segmentations were over-read by an experienced radiologist (J.F.B). None of the patients were thought to have a pathological stenosis secondary to the ablation process. The segmented left atrium and pulmonary veins from one patient are shown at both the baseline and three month follow-up scan in Fig. 10. These images provide a qualitative assessment that the pulmonary veins have decreased in size following ablation therapy.
Figure 10.
Back view of segmented left atrium and pulmonary veins from a patient at (a) baseline and (b) three month follow-up. LIPV (green), LSPV (red), RIPV (blue), RSPV (magenta).
In order to assess changes across the entire group of patients, average plots of cross-sectional area versus distance for each pulmonary vein were generated. First, plots of cross-sectional area versus distance for the four pulmonary veins in each subject are computed using the described technique. Next, each pair of baseline and follow-up curves are truncated to the shorter of the two curves so a comparision between the two timepoints can be made at each analyzed point. An average curve is computed at each timepoint by interpolating the cross-sectional area at 0.5 cm intervals and computing the mean area across all patients at each interval. Since the length of each pair of baseline and follow-up pulmonary vein curves will be different, the number of subjects included at each point will vary. At the beginning of the curve, all patients will be included, but the total number of patients will decrease as the distance into the vein increases. A one-sided paired t-test was run at each 0.5 cm distance interval to test for significance in the difference between the baseline and follow-up scan.
3. Results
Validation Results
Validation experiments were conducted for each of the four pulmonary veins in 10 subjects. At each manually selected point, the difference between the manual measurement and the semi-automatic measurement is computed. Results are given in Table 1 as absolute differences. The differences between the manual and automatic methods are small, ranging from 3.71 to 26.67 mm2 with an overall mean difference of 11.50 mm2. Plots of the manual and automatic measurements are shown in Fig. 11 for the subjects with the minimum, median (fifth rank), and maximum error. In these plots, the automatic measurements are shown in red and the manual measurements in blue. The blue circles indicate locations of the manual measurements. As demonstrated in these plots, the automatic measurements closely follow the manual measurements, accurately capturing both the magnitude of the cross-sectional areas as well as the overall geometric shape of the pulmonary vein.
Table 1.
Mean absolute differences in mm2 between manual and automatic measurements for each of the four pulmonary veins across ten subjects.
| LIPV | LSPV | RIPV | RSPV | mean | |
|---|---|---|---|---|---|
|
| |||||
| 1 | 5.75 | 8.35 | 3.71 | 9.59 | 6.85 |
| 2 | 7.84 | 9.80 | 6.93 | 14.40 | 9.74 |
| 3 | 7.15 | 21.33 | 7.70 | 8.89 | 11.27 |
| 4 | 10.86 | 26.67 | 7.17 | 12.87 | 14.39 |
| 5 | 18.75 | 18.35 | 17.19 | 11.68 | 16.49 |
| 6 | 8.42 | 9.83 | 12.38 | 10.88 | 10.38 |
| 7 | 16.28 | 10.62 | 18.82 | 8.24 | 13.49 |
| 8 | 4.11 | 8.35 | 5.94 | 14.87 | 8.32 |
| 9 | 8.82 | 18.41 | 18.06 | 15.30 | 15.15 |
| 10 | 13.95 | 9.17 | 7.25 | 5.17 | 8.89 |
|
| |||||
| mean | 10.19 | 14.09 | 10.52 | 11.19 | 11.50 |
Figure 11.
Plots of manual (blue) and automatic (red) cross-sectional area measurements. Circles indicate locations of manual measurements.
Patient Study
Average plots for each pulmonary vein across all 21 patients are shown in Fig. 12(a). The average baseline plot is shown in blue and the average follow-up plot in green. Data values are computed at 0.5 cm intervals, as indicated by the open circles. Next to each circle, the number of patients included in the mean calculation at that point is indicated. Only data points with at least 5 patients were included, resulting in the left inferior and superior analyzed for 7 cm, the right inferior for 5.5 cm, and the right superior for 8.5 cm. Data was obtained in 15 or more datasets for the first 5 cm in the left inferior and left superior pulmonary vein, for the first 4 cm in the right inferior pulmonary vein and the first 6.5 cm in the right superior pulmonary vein.
Figure 12.
(a) Average cross-sectional area plots at baseline (blue) and follow-up (green) for all 21 patients. Number of patients included in calculation shown next to each data point. (b) Mean(std) of paired differences in cross-sectional area between baseline and follow up scans. Significant differences are indicated by a red star.
Overall, these plots demonstrate that the pulmonary veins decrease in size following ablation therapy. The difference between the baseline and follow-up cross-sectional areas are largest near the pulmonary vein ostia and gradually decreases with distance into the vein. The mean difference for all veins is approximately 20% at the ostium and differences persist for several centimeters into the vein. While the magnitude of the cross-sectional area differs between baseline and follow-up scans, the overall shape of the curves for the two timepoints remain similar. The left inferior vein decreases rapidly in cross-sectional area for the first 4 cm and then reaches a plateau until the end of the measured vein. The left superior vein demonstrates a more linear decrease in area. Both the right inferior and right superior have a rapid decrease in area followed by a plateau with the right inferior plateauing around 2.5 cm and the right superior plateauing around 6 cm.
The mean and standard deviations of the paired differences between baseline and follow-up at each of the 0.5 mm intervals are shown in Fig. 12(b) where statistically significant (p<.05) differences are indicated with a red star. Cross-sectional area at the ostium (distance of 0 cm) decreases significantly following ablation therapy in all pulmonary viens. In addition to ostial changes, cross-sectional area decreases significantly for the first 1.5 cm (excluding the 1.0 cm interval) in the left inferior, 3.0 cm in the left superior, and 0.5 cm in the right superior veins. As indicated by the standard deviation bars, differences in cross-sectional area between timepoints are most variable near the ostium and least variable at more distal regions in the vein. These plots quantitatively demonstrate that the pulmonary veins decrease in size following ablation therapy, and differences can exist for up to several centimeters into the vein.
4. Conclusion
Quantifying pulmonary vein structure is important in the context of treating atrial fibrillation where it has been shown that structural remodeling occurs in response to the disease and reverse remodeling can occur following successful catheter ablation. Previous work in analyzing the pulmonary veins have used lines drawn on either volume renderings or within image slices [6, 7, 8, 9, 12], thereby limiting the ability to fully quantify the 3D structure of the pulmonary veins. The current study is unique in that it utilizes a three-dimensional centerline tool to make automatic measurements along the length of the pulmonary veins. A handful of other techniques have been proposed to evaluate points along the length of the pulmonary veins. In one study aiming to determine normative values for pulmonary vein size [20], oblique images were extracted at specified intervals from a line drawn along the long axis of the vein from a transverse image. This is similar in concept to the centerline approach, however, it can only be used for the portion of the vein that lies in the transverse plane. Another study [5] used curved multi-planar reconstruction images to create an image along the pulmonary vein to measure diameters near ablation lesions. An advantage of a three-dimensional centerline is that it can be used to track and make measurements along the entire vein as it moves in and out of image planes throughout the volume.
Application of the proposed methodology to a patient study demonstrated that reverse structural remodeling occurs in the pulmonary veins following ablation therapy. Previous work has demonstrated decreases in pulmonary vein ostial size following cardiac ablation therapy [5, 6, 9, 7, 8, 20], however, the full extent of reverse remodeling along the length of the pulmonary veins has not yet been fully characterized. In the current work, differences in cross-sectional area at the two timepoints were observed at all pulmonary vein ostia with this trend holding for up to 3.0 cm into the vein. Other studies evaluating cross-sectional area at the ostia have also found decreases in ostial size following ablation therapy [7, 8]. Changes observed in the current study, however, are larger than previously reported values. In the present study, differences in ostial area were found to range from approximately 0.5 to 0.75 cm2, which is larger than the 0.26 to 0.29 cm2 differences reported in [7]. Results from [7] also differed in that significant changes were found only in the superior pulmonary veins and not the inferior veins. The percent decrease in ostial cross-sectional area was approximately 20% in the current study which is also higher than the 10% decrease observed in [8]. Differences in the results of the present study and previous studies could simply be due to different patient populations. Another possibility is the difference in methodolgies for measuring cross-sectional area. In the present study, a plane is adjusted in a three-dimensional visualization with concurrent visualization of the image planes to define the ostium. This method could result in an ostial definition that is closer to the left atrium which potentially undergoes a larger change in size between timepoints.
There are several limitations to the current study. First, the number of patients is relatively small and the current approach will need to be repeated on a larger dataset. Second, the results of this study could be confounded by longitudinal shrinkage along the length of the pulmonary vein, as observed in a prior study [5]. If this shrinkage is substantial, it is possible that different anatomical points along the pulmonary vein may be compared at the two timepoints. Finally, each pulmonary vein was represented using a single curve as opposed to modeling the entire pulmonary tree, including bifurcations. This was done to simplify the analysis of combining results from different patients. In future work, bifurcations could be represented as multiple curves with a more complex algorithm to combine results from individual patients.
The methodology proposed in this work was used to evaluate the extent of reverse structural remodelling along the length of the pulmonary veins following ablation therapy. The quantification of pulmonary vein structure, however, could potentially be utilized in other applications for treating left atrial fibrillation as well. For example, in several recent studies, structural remodeling of the left atrium has been shown to correlate with patient outcomes, with larger left atrial volumes [12, 21, 22, 23, 24] being predictive for recurrence of atrial fibrillation. Larger pulmonary veins, along with the distance into the vein the distension persists, could potentially be a clinical metric complimentary to left atrial size for predicting clinical outcomes. A few studies have investigated this possibility, with one study finding no correlation [12] and another reporting a trend toward reduced efficacy of treatment in patients with large pulmonary vein diameters [25]. More comprehensive techniques for pulmonary vein analysis, such as the currently proposed centerline tracking approach, could provide greater insight into the predictive value of pulmonary vein morphology on treatment outcomes and the utility of using measurements when determining a patient treatment strategy.
Acknowledgments
The authors thank Cindy Ge for assistance with data analysis. This research was supported by NIH grants HL089709 and HL089645 from the National Heart Lung and Blood Institute, and St. Judes’s Foundation.
Appendix
CABANA Pilot Imaging Investigators
Tristram Bahnson, Duke University Medical Center, Durham, NC. Steven Bailin, Mercy Medical Center, Des Moines, IA. Anil Bhandari, Good Samaritan Hospital, Los Angeles, CA. John Day, Intermountain Medical Center, Salt Lake City, UT. John Hummel, Ohio State University, Columbus, OH. Neil Kay, University of Alabama at Birmingham, Birmingham, AL. Kerry Lee, Duke Clinical Research Institute, Durham, NC. Douglas Packer, Mayo Clinic, Rochester, MN. David Wilber, Loyola University Medical Center, Maywood, IL.
Footnotes
Conflict of Interest Statement
Mayo Clinic and R.A. Robb have a financial interest in technology referenced in this article. Dr. D. Packer in the past 12 months has provided consulting services for Biosense Webster, Inc., CardioFocus, Cardiomedics, Cyberheart, Endosense, Johnson & Johnson Healthcare Systems, Medtronic CryoCath, OrthoMcNeill, Siemens, St. Jude Medical, Siemens AG, and Valencia Technologies. Dr. Packer received no personal compensation for these consulting activities. Dr. Packer receives research funding from the Biosense Webster, Boston Scientific, Endosense, EpiEP, EP Advocate, Medtronic CryoCath LP, Minnesota Partnership for Biotechnology and Medical Genomics/ University of Minnesota, NIH, St. Jude Medical, Siemens AcuNav, and Ther-medical (EP Limited). Royalties from Blackwell Publishing and St. Jude Medical. Mayo Clinic and Drs. D. Packer and R. Robb have a financial interest in mapping technology that may have been used at some of the 10 centers participating in this pilot research. In accordance with the Bayh-Dole Act, this technology has been licensed to St. Jude Medical, and Mayo Clinic and Drs. Packer and Robb have received annual royalties greater than $10,000, the federal threshold for significant financial interest. Mayo Clinic and Dr. R. Robb have a financial interest in Analyze-AVW technology that was used to analyze some of the heart images in this research. In accordance with the Bayh-Dole Act, this technology has been licensed to commercial entities, and both Mayo Clinic and Dr. Robb have received royalties greater than $10,000, the federal threshold for significant financial interest. In addition, Mayo Clinic holds an equity position in the company to which the AVW technology has been licensed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Häissaguerre M, Jäis P, Shah D, Takahashi A, Hocini M, Quiniou G, Garrigue S, Mouroux AL, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Kato R, Lickfett L, Meininger G, Dickfeld T, Wu R, Juang G, Angkeow P, LaCorte J, Bluemke D, Berger R, Halperin H, Calkins H. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation. Circulation. 2003;107:2004–2010. doi: 10.1161/01.CIR.0000061951.81767.4E. [DOI] [PubMed] [Google Scholar]
- 3.Knackstedt C, Visser L, Plisiene J, Zarse M, Waldmann M, Mischke K, Koch KC, Hoffmann R, Franke A, Hanrath P, Schauerte P. Dilation of the pulmonary veins in atrial fibrillation: A transesophageal echocardiographic evaluation. PACE. 2003;26:1371–1378. doi: 10.1046/j.1460-9592.2003.t01-1-00196.x. [DOI] [PubMed] [Google Scholar]
- 4.Tsao HM, Yu WC, Cheng HC, Wu MH, Tai CT, Lin WS, Ding YA, Chang MS, Chen SA. Pulmonary vein dilation in patients with atrial fibrillation: Detection by magnetic resonance imaging. J Cardiovasc Electrophysiol. 2001;12:809–813. doi: 10.1046/j.1540-8167.2001.00809.x. [DOI] [PubMed] [Google Scholar]
- 5.Ueda M, Tada H, Kurosaki K, Itoi K, Koyama K, Naito S, Ito S, Komuro I, Oshima S, Taniguchi K. Pulmonary vein morphology before and after segmental isolation in patients with atrial fibrillation. PACE. 2005;28:944–953. doi: 10.1111/j.1540-8159.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- 6.Scharf C, Sneider M, Case I, Chugh A, Lai S, Pelosi F, Jr, Knight B, Kazerooni E, Morady F, Oral H. Anatomy of the pulmonary veins in patients with atrial fibrillation and effects of segmental ostial ablation analyzed by computed tomography. J Cardiovasc Electrophysiol. 2003;14:150–155. doi: 10.1046/j.1540-8167.2003.02444.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsao H, Wu M, Huang B, Lee S, Lee K, Tai C, Lin Y, Hsieh M, Kuo J, Lei M, Chen S. Morphologic remodeling of pulmonary veins and left atrium after catheter ablation of atrial fibrillation: Insight from long-term follow-up of three-dimensional magnetic resonance imaging. J Cardiovasc Electrophysiol. 2005;16:7–12. doi: 10.1046/j.1540-8167.2005.04407.x. [DOI] [PubMed] [Google Scholar]
- 8.Lemola K, Sneider M, Desjardins B, Case I, Chugh A, Hall B, Cheung P, Good E, Han J, Tamirisa K, Bogun F, Pelosi F, Kazerooni E, Morady F, Oral H. Effects of left atrial ablation of atrial fibrillation on size of the left atrium and pulmonary veins. Heart Rhythm. 2004;1:576–581. doi: 10.1016/j.hrthm.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Jayam V, Dong J, Vasamreddy C, Lickfett L, Kato R, Dickfeld T, Eldadah Z, Dalal D, Blumke D, Berger R, Halperin H, Calkins H. Atrial volume reduction following catheter ablation of atrial fibrillation and relation to reduction in pulmonary vein size: an evaluation using magnetic resonance angiography. J Interv Card Electrophysiol. 2005;13:107–14. doi: 10.1007/s10840-005-0215-3. [DOI] [PubMed] [Google Scholar]
- 10.Jeevanantham V, Ntim W, Navaneetham S, Shah S, Johnson A, Hall B, Shah A, Hundley W, Daubert J, Fitzgerald D. Meta-analysis of the effect of radiofrequency catheter ablation on left atrial size, volumes and function in patients with atrial fibrillation. Am J Cardiol. 2010;105:1317–1326. doi: 10.1016/j.amjcard.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 11.Reant P, Lafitte S, Jais P, Serri K, Weerasooriya R, Hocini M, Pillois X, Clementy J, Häissaguerre M, Roudaut R. Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation. 2005;112:2896–2903. doi: 10.1161/CIRCULATIONAHA.104.523928. [DOI] [PubMed] [Google Scholar]
- 12.denUijl D, Tops L, Delgado V, Schuijf J, Kroft L, de Roos A, Boersma E, Trines S, Zeppenfeld K, Schalij M, Bax J. Effect of pulmonary vein anatomy and left atrial dimensions on outcome of circumferential radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol. 2011;107:243–249. doi: 10.1016/j.amjcard.2010.08.069. [DOI] [PubMed] [Google Scholar]
- 13.Karim R, Mohiaddin R, Rueckert D. Left atrium pulmonary veins: Segmentation and quantification for planning atrial fibrillation ablation. Proceedings of SPIE. 7261 [Google Scholar]
- 14.Rettmann M, Holmes D, III, Packer D, Robb R. Identification of left pulmonary vein ostia using centerline tracking. Proceedings of SPIE Medical Imaging. [Google Scholar]
- 15.Rettmann M, Gunawan M, Holmes D, III, Packer D, Robb R. Quantification of pulmonary vein morphology using centerline tracking. Proceedings of the International Symposium on Biomedical Imaging. [Google Scholar]
- 16.Rettmann M, Holmes D, III, Camp J, Packer D, Robb R. Validation of semi-automatic segmentation of the left atrium. Proceedings of SPIE; [Google Scholar]
- 17.Rettmann M, Holmes D, III, Gunawan M, Karwoski R, Breen J, Packer D, Robb R. Validation of geometric measurements of the left atrium and pulmonary veins for analysis of structural remodeling following ablation therapy. Proceedings of SPIE; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadleir R, Whelan P. Fast colon centreline calculation using optimised 3D topological thinning. Computerized Medical Imaging and Graphics. 2005;29:251–258. doi: 10.1016/j.compmedimag.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Dijkstra E. A note on two problems in connexion with graphs. Numer Math. 1959;1:269–271. [Google Scholar]
- 20.Kim Y-H, Marom E, Herndon J, II, McAdams HP. Pulmonary vein diameter, cross-sectional area, and shape: CT analysis. Radiology. 2005;235:43–50. doi: 10.1148/radiol.2351032106. [DOI] [PubMed] [Google Scholar]
- 21.Akutsu Y, Kaneko K, Kodama Y, Suyama J, Li HL, Hamazaki Y, Tanno K, Gokan T, Kobayashi Y. Association between left and right atrial remodeling with atrial fibrillation recurrence after pulmonary vein catheter ablation in patients with paroxysmal atrial fibrillation: A pilot study. Circ Cardiovasc Imaging. 2011;4:524–531. doi: 10.1161/CIRCIMAGING.110.962761. [DOI] [PubMed] [Google Scholar]
- 22.Hof I, Chilukuri K, Arbab-Zadeh A, Scherr D, Dalal D, Nazarian S, Henrikson C, Spragg D, Berger R, Marine J, Calkins H. Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J Cardiovasc Electrophysiol. 2009;20:1005–1010. doi: 10.1111/j.1540-8167.2009.01504.x. [DOI] [PubMed] [Google Scholar]
- 23.Nedios S, Tang M, Roser M, Solowjowa N, Gerds-Li JH, Fleck E, Kriatselis C. Characteristic changes of volume and three-dimensional structure of the left atrium in different forms of atrial fibrillation: predictive value after ablative treatment. J Interv Card Electrophysiol. 2011;32:87–94. doi: 10.1007/s10840-011-9591-z. [DOI] [PubMed] [Google Scholar]
- 24.Parikh S, Jons C, Mcnitt S, Daubert J, Schwarz K, Hall B. Predictive capability of left atrial size measured by CT, TEE, and TTE for recurrence of atrial fibrillation following radiofrequency catheter ablation. PACE. 2010;33:532–540. doi: 10.1111/j.1540-8159.2010.02693.x. [DOI] [PubMed] [Google Scholar]
- 25.Mulder A, Wijffels M, Wever E, Boersma L. Pulmonary vein anatomy and long-term outcome after multi-electrode pulmonary vein isolation with phased radiofrequency energy for paroxysmal atrial fibrillation. Europace. 2011;13:1557–61. doi: 10.1093/europace/eur236. [DOI] [PubMed] [Google Scholar]