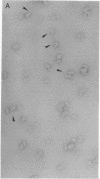
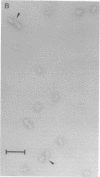
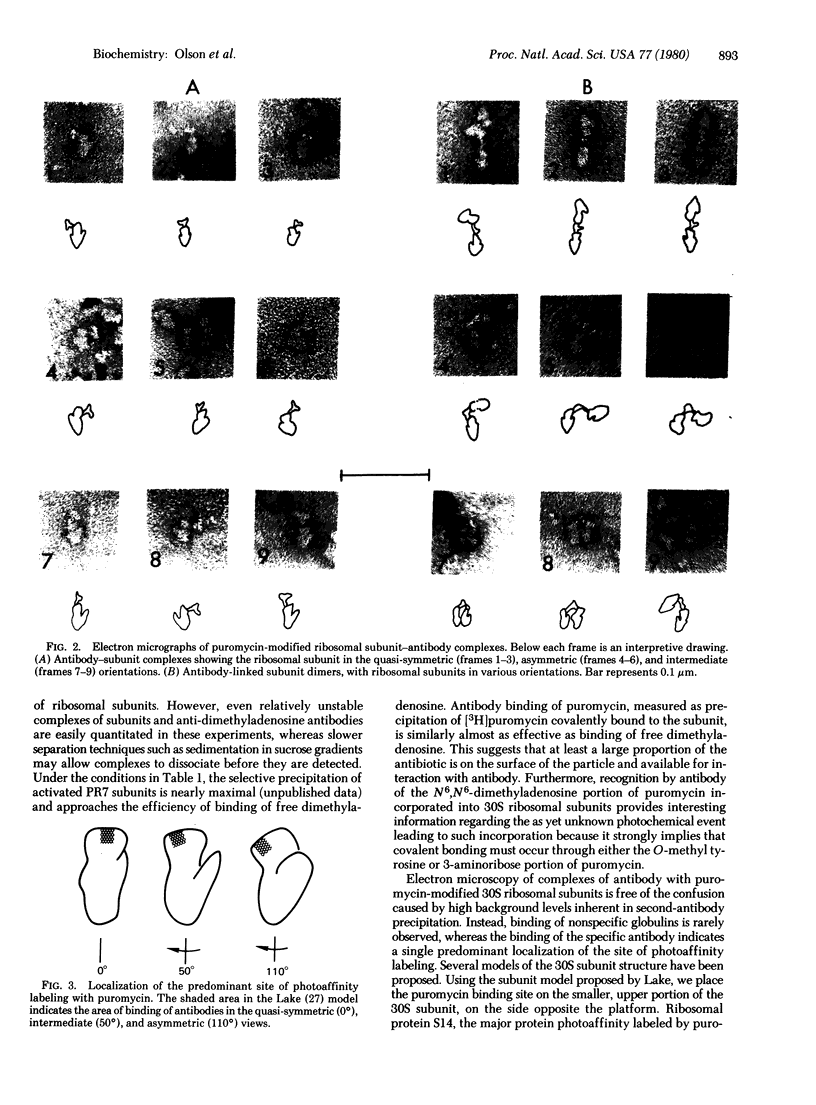
Abstract
Ribosomes from Escherichia coli strain TPR201 (which lack N6,N6-dimethyladenosine) have been photoaffinity labeled with [3H]puromycin in the presence of chloramphenicol. Puromycin-modified 30S ribosomal subunits appear to be identical to untreated subunits in electron micrographs and are efficiently precipitated by antibodies to the puromycin analog N6,N6-dimethyladenosine. Electron micrographs of subunit-antibody complexes show ribosomal subunits to which an individual antibody molecule is bound and pairs of 30S subunits which appear to be crosslinked by a single IgG molecule. A predominant site of puromycin photoaffinity labeling has been identified from the apparent point of contact of antibody and ribosomal subunit. The puromycin site is localized to the small upper portion of the particle on the side opposite to the subunit platform. This location is close to that reported for ribosomal protein S14, the major puromycin-labeled protein in the small ribosomal subunit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley H., Knowles J. R. Photoaffinity labeling. Methods Enzymol. 1977;46:69–114. doi: 10.1016/s0076-6879(77)46012-9. [DOI] [PubMed] [Google Scholar]
- Chowdhry V., Westheimer F. H. Photoaffinity labeling of biological systems. Annu Rev Biochem. 1979;48:293–325. doi: 10.1146/annurev.bi.48.070179.001453. [DOI] [PubMed] [Google Scholar]
- Cooperman B. S., Grant P. G., Goldman R. A., Luddy M. A., Minnella A., Nicholson A. W., Strycharz W. A. Photoaffinity labeling of ribosomes. Methods Enzymol. 1979;59:796–815. doi: 10.1016/0076-6879(79)59126-5. [DOI] [PubMed] [Google Scholar]
- Cooperman B. S., Jaynes E. N., Brunswick D. J., Luddy M. A. Photoincorporation of puromycin and N-(ethyl-2-diazomalonyl)puromycin into Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2974–2978. doi: 10.1073/pnas.72.8.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., BEISER S. M. ANTIBODIES SPECIFIC FOR RIBONUCLEOSIDES AND RIBONUCLEOTIDES AND THEIR REACTION WITH DNA. Proc Natl Acad Sci U S A. 1964 Jul;52:68–74. doi: 10.1073/pnas.52.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G. P., Cooperman B. S., Strycharz W. A. On the mechanism of chloramphenicol-induced changes in the photoinduced affinity labeling of Escherichia coli ribosomes by puromycin. Evidence for puromycin and chloramphenicol sites on the 30S subunit. Biochemistry. 1979 May 29;18(11):2154–2160. doi: 10.1021/bi00578a004. [DOI] [PubMed] [Google Scholar]
- Grant P. G., Strycharz W. A., Jaynes E. N., Jr, Cooperman B. S. Antibiotic effects on the photoinduced affinity labeling of Escherichia coli ribosomes by puromycin. Biochemistry. 1979 May 29;18(11):2149–2154. doi: 10.1021/bi00578a003. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Jaynes E. N., Jr, Grant P. G., Giangrande G., Wieder R., Cooperman B. S. Photoinduced affinity labeling of the Escherichia coli ribosome puromycin site. Biochemistry. 1978 Feb 21;17(4):561–569. doi: 10.1021/bi00597a001. [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Cantor C. R. Affinity labeling of multicomponent systems. Methods Enzymol. 1977;46:180–194. doi: 10.1016/s0076-6879(77)46019-1. [DOI] [PubMed] [Google Scholar]
- Keren-Zur M., Boublik M., Ofengand J. Localization of the decoding region on the 30S Escherichia coli ribosomal subunit by affinity immunoelectron microscopy. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1054–1058. doi: 10.1073/pnas.76.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A. Aminoacyl-tRNA binding at the recognition site is the first step of the elongation cycle of protein synthesis. Proc Natl Acad Sci U S A. 1977 May;74(5):1903–1907. doi: 10.1073/pnas.74.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A., Kahan L. Ribosomal proteins S5, S11, S13 and S19 localized by electron microscopy of antibody-labeled subunits. J Mol Biol. 1975 Dec 25;99(4):631–644. doi: 10.1016/s0022-2836(75)80177-x. [DOI] [PubMed] [Google Scholar]
- Lake J. A., Pendergast M., Kahan L., Nomura M. Localization of Escherichia coli ribosomal proteins S4 and S14 by electron microscopy of antibody-labeled subunits. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4688–4692. doi: 10.1073/pnas.71.12.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A. Ribosome structure determined by electron microscopy of Escherichia coli small subunits, large subunits and monomeric ribosomes. J Mol Biol. 1976 Jul 25;105(1):131–139. doi: 10.1016/0022-2836(76)90200-x. [DOI] [PubMed] [Google Scholar]
- Olson H. M., Glitz D. G. Ribosome structure: localization of 3' end of RNA in small subunit by immunoelectronmicroscopy. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3769–3773. doi: 10.1073/pnas.76.8.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politz S. M., Glitz D. G. Ribosome structure: localization of N6,N6-dimethyladenosine by electron microscopy of a ribosome-antibody complex. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1468–1472. doi: 10.1073/pnas.74.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH A., THORNTON E. R., WESTHEIMER F. H. The photolysis of diazoacetylchymotrypsin. J Biol Chem. 1962 Sep;237:3006–3008. [PubMed] [Google Scholar]
- Strycharz W. A., Nomura M., Lake J. A. Ribosomal proteins L7/L12 localized at a single region of the large subunit by immune electron microscopy. J Mol Biol. 1978 Dec 5;126(2):123–140. doi: 10.1016/0022-2836(78)90355-8. [DOI] [PubMed] [Google Scholar]
- Tischendorf G. W., Zeichhardt H., Stöffler G. Architecture of the Escherichia coli ribosome as determined by immune electron microscopy. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4820–4824. doi: 10.1073/pnas.72.12.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischendorf G. W., Zeichhardt H., Stöffler G. Determination of the location of proteins L14, L17, L18, L19, L22, L23 on the surface of the 5oS ribosomal subunit of Escherichia coli by immune electron microscopy. Mol Gen Genet. 1974;134(3):187–208. doi: 10.1007/BF00267715. [DOI] [PubMed] [Google Scholar]
- Tischendorf G. W., Zeichhardt H., Stöffler G. Location of proteins S5, S13 and S14 on the surface of the 3oS ribosomal subunit from Escherichia coli as determined by immune electron microscopy. Mol Gen Genet. 1974;134(3):209–223. doi: 10.1007/BF00267716. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Vasiliev V. D. Morphology of the ribosomal 30S subparticle according to electron microscopic data. Acta Biol Med Ger. 1974;33(5-6):779–793. [PubMed] [Google Scholar]
- Woese C. R., Fox G. E., Zablen L., Uchida T., Bonen L., Pechman K., Lewis B. J., Stahl D. Conservation of primary structure in 16S ribosomal RNA. Nature. 1975 Mar 6;254(5495):83–86. doi: 10.1038/254083a0. [DOI] [PubMed] [Google Scholar]
- Zamir A. Affinity labeling of ribosomal functional sites. Methods Enzymol. 1977;46:621–637. doi: 10.1016/s0076-6879(77)46077-4. [DOI] [PubMed] [Google Scholar]