Abstract
This revision of the classification of eukaryotes, which updates that of Adl et al. (2005), retains an emphasis on the protists and incorporates changes since 2005 that have resolved nodes and branches in phylogenetic trees. Whereas the previous revision was successful in re-introducing name stability to the classification, this revision provides a classification for lineages that were then still unresolved. The supergroups have withstood phylogenetic hypothesis testing with some modifications, but despite some progress, problematic nodes at the base of the eukaryotic tree still remain to be statistically resolved. Looking forward, subsequent transformations to our understanding of the diversity of life will be from the discovery of novel lineages in previously under-sampled areas and from environmental genomic information.
Keywords: Algae, amoebae, biodiversity, ciliates, flagellates, fungi, parasites, protozoa, systematics, taxonomy
The classification proposed by Adl et al. (2005) on behalf of The Society established name stability as well as a synthesis of the overall structure of the classification of eukaryotes, based on the information available at that time, and after the upheaval introduced by molecular phylogenetic studies over the preceding two decades. Overall, the system proposed was conservative enough to largely avoid erroneous or premature groupings, whilst eliminating wherever possible known polyphyletic groups or groups of convenience, encouraging correction of many of the errors in text books. The current revision reflects the need to have a classification of protistan eukaryotes that incorporates recent advances wrought both by the widespread use of phylogenomic-scale phylogenetic analyses and by massively increased taxon sampling in rRNA-based phylogenies, partly due to a renaissance in novel organism discovery. With the current revision, we have again tried to strike a conservative balance between updating the classification where needed and avoiding formal recognition of uncertain groupings where further investigation would be warranted.
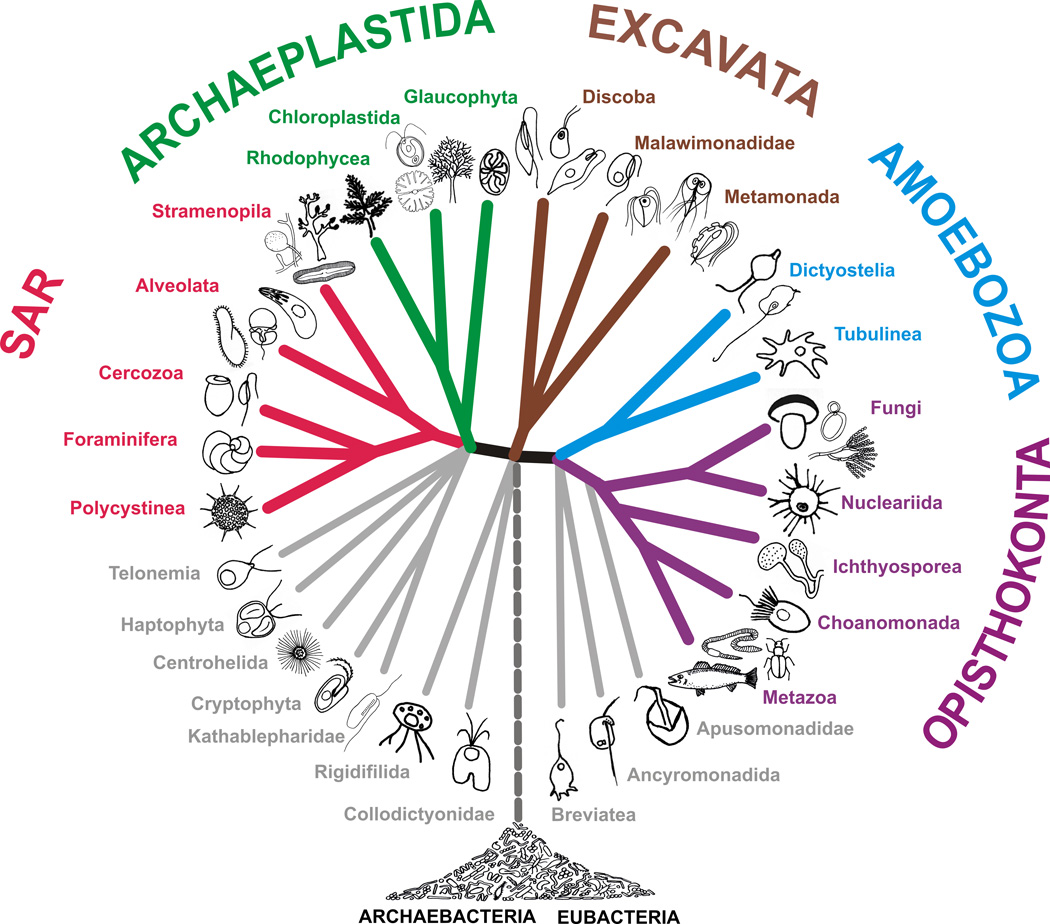
One notable advance since 2005 is the consolidation of a classification founded on robust phylogenetic relatedness. The super-groups formalized by Adl et al. (2005) are mostly retained, though some have been assembled into still higher-order groupings (Table 1, see below). One notable exception is the Chromalveolata, which was retained then as useful though controversial, and with the authors noting concerns with this grouping of Cryptophyceae, Haptophyta, Stramenopiles, and Alveolata. Since then, evidence has mounted that Chromalveolates are probably polyphyletic (Stiller et al. 2009; Baurain et al. 2010; Parfrey et al. 2010). Instead, multigene phylogenetics and phylogenomic studies generally support Stramenopiles and Alveolata as specifically related to Rhizaria (Burki et al. 2009; Burki et al. 2010; Burki et al. 2012; Hampl et al. 2009; Parfrey et al. 2010). The two remaining major lineages that were formerly assigned to the chromalveolates – Haptophyta and Cryptophyceae/cryptomonads – have been more challenging to place phylogenetically (Burki et al. 2010; Burki et al., 2012), and are two examples of several where stable, deep relationships still remain to be established. Analyses with abundant data for each taxon are subject to systematic biases that can lead to high support for incorrect clades (Hedtke et al. 2006, Zwickl and Hillis 2002). Broader taxonomic sampling is likely the most important factor in alleviating these kinds of systematic biases (Heath et al. 2008; Hedtke et al. 2006; Zwickl and Hillis 2002), although adding more taxa may also introduce additional missing sequence data (Wiens 2006). Thus, emerging relationships should be confirmed by multiple studies before proposing revisions to the classification. It is more reasonable first to propose a testable phylogenetic hypothesis.
Table 1.
The classification of eukaryotes at the highest ranks.
| Super-groups | Examples | ||
|---|---|---|---|
| Eukaryota | Amorphea | Amoebozoa | Tubulinea Mycetozoa |
| Opisthokonta | Fungi Choanomonada Metazoa |
||
| Apusomonada | |||
| Breviata | |||
| Excavata | Metamonada Malawimonas Discoba |
||
| Diaphoretickes | Cryptophyceae | ||
| Centrohelida | |||
| Telonemia | |||
| Haptophyta | |||
| Sar | Cercozoa Foraminifera “Radiolaria” |
||
| Alveolata Stramenopiles | |||
| Archaeplastida | Glaucophyta Rhodophyceae Chloroplastida |
||
| Incertae sedis Eukaryota | Incertae sedis, and table 3 |
||
The number of paraphyletic and polyphyletic groups is reduced in this revision. Several new nodes have stabilized with robust support. However, not all robust nodes on a phylogenetic tree need to be named and recognized in a classification. Unlike a phylogenetic tree that tries to reflect correctly the relatedness of lineages, a classification has a utilitarian purpose of categorizing diversity in a practical manner. Thus, we have resisted in this classification creating taxa that were not necessary. The systematic rules used by Adl et al. (2005) were used again unmodified as they helped address problematic issues with codes of nomenclatures (Adl et al., 2007). These issues included: i) placing a focus on clustering genera without creating superfluous ranks; ii) providing stable group names even when a rank changes or a group is moved to another lineage, thus ignoring rank endings; iii) emphasizing name stability in the classification system despite changes; and iv) separating naming clades from assembling nested hierarchies – in contrast to rank-based nomenclature that treats these steps as part of the same process (Adl et al., 2007; Cantino, 2004; Kron, 1997; Pleijel and Rouse 2003). This approach provides a more stable classification that preserves names, while allowing revisions to reflect our changing understanding of evolutionary history. We have also relied on ideas borrowed from phylogenetic nomenclature, distinguishing groups with definitions based on apomorphies, nodes, branches, or combinations of these. These kinds of definitions are more suited to a classification based on phylogenetic trees, and can be written as phylogenetic hypotheses that can be tested.
The most significant changes introduced in this revision are as follows:
First, we recognize the grouping of Amoebozoa with Opisthokonta. Since 2005 this has become a commonly recognized probable clade, and at present it is usually referred to by the informal name “unikont”, sometimes rendered as the more formal-sounding “Unikonta”. However, the underlying hypothesis of a monociliated (with only one ciliated basal body) ancestry for this cluster of organisms (Cavalier-Smith, 2002) is almost certainly incorrect (Kim et al., 2006; Roger and Simpson, 2009). There is no requirement that names of taxa reflect the ancestral state of the clade. However, the name “unikonts” causes confusion because of the apomorphy hypothesized for the ancestral character. To address this we introduce a new formal name for the probable clade. We have formalized this clade as a new taxon, Amorphea, with a node-based phylogenetic definition:
Amorphea: the least inclusive clade containing Homo sapiens Linnaeus 1758, Neurospora crassa Shear & Dodge 1927 (both Opisthokonta), and Dictyostelium discoideum Raper 1935 (Amoebozoa). This is a node-based definition in which all of the specifiers are extant; it is intended to apply to a crown clade; qualifying clause – the name does not apply if any of the following fall within the specified clade – Arabidopsis thaliana (Linnaeus) Heynhold 1842 (Archaeplastida), Tetrahymena thermophila Nanney & McCoy 1976 (Alveolata), Thalassiosira pseudonana Hasle & Hiemdal 1970 (Stramenopiles), Bigelowiella natans Moestrup & Sengco 2001 (Rhizaria), Euglena gracilis Klebs 1883 (Excavata), and Emiliania huxleyi (Lohmann) Hay & Mohler 1967 (Haptophyta).
Note that the term Amorphea (a, Gr. – without; morphe, Gr. – form, shape) relates to the cells of most taxa in this cluster not having fixed form unless restricted by an external layer (e.g. cell wall, lorica, test, extracellular matrix). The clade Amorphea is composed of Opisthokonta, Amoebozoa, Apusomonadida, Breviata, Subulatomonas, and probably Ancyromonadida and Mantamonas, as defined here. The primary reference phylogeny for Amorphea is Minge et al. (2009, Fig. 2). However, this figure is not intended to communicate that the root of the eukaryote tree falls within Amorphea/unikonts. The tree figure is clearly intended to be viewed either as an un-rooted tree or as a rooted-tree that reflects the hypothesis that the root falls between Amorphea (Unikonta in Fig. 2, Minge et al. 2009) and other eukaryotes (Minge et al., 2009; see Richards and Cavalier-Smith, 2005).
Second, we recognize the clustering of the Stramenopiles, Alveolates, and Rhizaria (see above). The term SAR (Burki et al., 2007) is in common usage, and we have formalized the cluster as the taxon ‘Sar’, using a node-based definition:
Sar: the least inclusive clade containing Bigelowiella natans Moestrup & Sengco 2001 (Rhizaria), Tetrahymena thermophila Nanney & McCoy 1976 (Alveolata), and Thalassiosira pseudonana Cleve 1873 (Stramenopiles). This is a node-based definition in which all of the specifiers are extant; it is intended to apply to a crown clade; qualifying clause – the name does not apply if any of the following fall within the specified clade – Homo sapiens Linnaeus 1758 (Opisthokonta), Dictyostelium discoideum Raper 1935 (Amoebozoa), Arabidopsis thaliana (Linnaeus) Heynhold 1842 (Archaeplastida), Euglena gracilis Klebs 1883 (Excavata), and Emiliania huxleyi (Lohmann) Hay & Mohler in Hay et al. 1967 (Haptophyta).
Note that the name is derived from the acronym of the three groups united in this clade – Stramenopiles, Alveolata, and Rhizaria (SAR), as defined here. The primary reference phylogeny is Burki et al. (2008, Fig. 1).
Third, we recognize a larger clade that includes most eukaryotes with the exception of the Amorphea and the Excavata, and a few other lineages currently listed as incertae sedis in the eukaryotes. As for the previous two clusters, there are no known morphological synapomorphies. The cluster is named Diaphoretickes and it is defined as follows:
Diaphoretickes: The most inclusive clade containing Bigelowiella natans Moestrup & Sengco 2001 (Rhizaria), Tetrahymena thermophila Nanney & McCoy 1976 (Alveolata), Thalassiosira pseudonana Cleve 1873 (Stramenopiles), and Arabidopsis thaliana (Linnaeus) Heynhold, 1842 (Archaeplastida), but not Homo sapiens Linnaeus 1758 (Opisthokonta), Dictyostelium discoideum Raper 1935 (Amoebozoa) or Euglena gracilis Klebs 1883 (Excavata). This is a branch-based definition in which all of the specifiers are extant, and it is intended to apply to a crown clade.
Note that the name will not be applicable under phylogenetic hypotheses in which the root of eukaryotes falls 'within' or 'between' Sar or Archaeplastida, since then there would be no clade fitting the definition. The primary reference phylogeny is Burki et al. (2008, Fig. 1). Diaphoretickes (Diaforetikés, Gr.– diverse) refers to the diversity of morphology and basic cellular features among members of this clade since diversifying from a common ancestor. The composition of Diaphoretickes includes at least Archaeplastida, Stramenopiles, Alveolata, and Rhizaria, each as defined here, and probably other clades placed as Eukaryota incertae sedis (see Table 2 below).
Table 2.
Classification of the higher ranks of the protists and multicellular groups. The authority to whom the taxon name is attributed appears immediately after the taxon name. In the square bracket following are names used by some that were not accepted, usually because of historical precedence for a name already in common usage that could be retained with an emended description. Selected references to the literature since 2005 can be found in Appendix 1. Citations in the notes to this table can be found in the LITERATURE CITED. If the taxon name has been emended herein, the authority is indicated and the reference is to this manuscript (e.g. “emend. Adl et al. 2012”). Abbreviations: (M) monotypic group with only one described species; (P) paraphyletic group; and (R) a ribogroup assembled from phylogenetic studies.
| Amoebozoa Lühe 1913, emend. Cavalier-Smith 1998 [Eumycetozoa Zopf 1884, emend Olive 1975] Cells “naked” or testate; tubular mitochondrial cristae, often branched (ramicristate), secondarily lost in some; uninucleate, binucleate or multinucleate; cysts common, morphologically variable; sexual or asexual; many taxa exhibit either sporocarpic (single amoeboid cell differentiates into a usually stalked, subaerial structure that supports one to many propagules termed spores) or sorocarpic (amoebae aggregate into a multicellular mass that develops into a multicellular fruiting body) fruiting; or myxogastroid ciliate stages; when amoeboid locomotion with non-eruptive morphologically variable pseudopodia; ancestraly bikont with many taxa exhibiting reduction of the bikinetid. Note 1, Note 2. |
| Note 1. Kinetids ancestrally bikont (Spiegel et al. 1995), consisting of a long, anteriorly-directed cilium, extending from BB2 (terminology of Andersen et al. 1991, Spiegel 1991, Spiegel et al. 1995, and Wright et al. 1979), a reflexed cilium extending from BB1 and lying in a ventral groove, microtubular rootlets 3 and 4 associated with BB2 – rootlet 3 forming an open cloak of microtubules that is arranged in a left handed spiral in cross section and rootlet 4 as a band of microtubules that arises orthogonally to rootlet 3 and extends along the left side of the groove, microtubular rootlets 1 and 2 associated with BB1 – rootlet 1 as a band of microtubules associated with a nonmicrotubular posterior parakinetosomal structure (Wright et al. 1979) and rootlet 2 as a band of microtubules parallel to BB1, with rootlets1 and 2 extending along the right side of the groove, an MTOC with a cone array extending from a stalk associated with the basal end of BB2 (many taxa have cells with some of these elements missing); several non-microtubular elements may also be present. Though most members of the supergroup have stages of the life cycle that exhibit amoeboid motion and feeding, the morphology of the amoeboid cells is so variable that it is impossible to determine if all amoebae in the group are homologues of each other. It is unlikely that they are not (Spiegel et al. 1995). Many taxa have more than one amoeboid state in the life cycle. It certainly is not presently possible to determine what type of amoebal morphology, if any, is most like that which may have been present in the last common ancestor of the supergroup. |
| Note 2. The term Eumycetozoa was not considered acceptable because it suggests the ancestral condition is the aggregative form and this would be incorrect. The term Amoebozoa is already well-established to identify this group of genera. In addition, there are aggregative forms in lineages outside of the Amoebozoa. |
|
●Tubulinea Smirnov et al. 2005 (R) Tubular, subcylindrical pseudopodia or capable of altering the locomotive form from a flattened, expanded one to a subcylindrical one; with monoaxial flow of the cytoplasm in every pseudopodium or in the entire cell. |
|
●● Euamoebida Lepşi 1960, emend. Smirnov et al. 2011 (R) Naked with subcylindrical pseudopodia in locomotion (or the entire cell is monopodial and subcylindrical); without alteration of the locomotive form to a flattened expanded and branched one; without adhesive uroid; glycocalyx amorphous, filamentous or consisting of prismatic, cup-shaped structures. Amoeba, Cashia, Chaos, Copromyxa, Copromyxella, Deuteramoeba, Glaeseria, Hartmannella, Hydramoeba, Parachaos, Polychaos, Saccamoeba, Trichamoeba. |
|
●●Leptomyxida Pussard and Pons 1976, emend. Page 1987 (R) Naked with locomotive form altering from a flattened expanded or reticulate one, when slowly moving, to a subcylindrical monopodial one when in rapid movement or under specific conditions; adhesive uroid; uninucleate with tendency to have more and with Leptomyxa always multinucleate; glycocalyx amorphous; Rhizamoeba saxonica has collosomes under cell membrane. Flabellula, Gephyramoeba, Leptomyxa, Paraflabellula, Rhizamoeba. |
|
●●Arcellinida Kent 1880 [= Testacealobosia De Saedeleer 1934] (R) Testate, inside an organic or mineral extracellular test of either self-secreted elements (calcareous or siliceous) or recycled mineral particles bound together, with a single main opening. |
|
●●●Arcellina Haeckel 1894 Test rigid or more or less flexible, chitinoid or membranous, sometimes with attached debris; without scales or plates; pseudopodia digitate, finely granular. Amphizonella, Arcella, Spumochlamys. |
|
●●●Difflugina Meisterfeld 2002 Test either completely chitinoid or comprising mineral particles, diatom frustules, or recycled scales or plates (often from Euglyphida), or composed of siliceous or calcite self-secreted plates (idiosomes) held together by an organic cement; granular, digitate pseudopodia. Bullinularia, Centropyxis, Difflugia, Hyalosphenia, Lesquereusia, Nebela, Paraquadrula, Pontigulasia, Plagiopyxis, Quadrulella, Trigonopyxis. |
|
●●●Phryganellina Bovee 1985 Test proteinaceous, with calcified inner layer, or completely chitinoid with recycled mineral particles; pseudopodia conical, pointed, clearly ectoplasmatic, sometimes branched and may anastomose; Cryptodifflugia stands out by having orthomitosis, but it is unclear if this feature is characteristic for the group. Cryptodifflugia, Phryganella, Wailesella. |
|
●●●
Nolandella Page 1980 (R) Clavate, monopodial amoebae with pronounced hyaline cap; glycocalyx basally of discrete units, forming truncated pyramids. |
|
●●● Echinamoebida Cavalier-Smith 2004 (R) Flattened limax locomotion with or without spine-like subpseudopodia; if spiny subpseudopodia absent, then length/breadth ratio >6; glycocalyx amorphous. Echinamoeba, Vermamoeba. |
| ●●● Incertae sedis Arcellinida: Cryptodifflugia, Geamphorella. |
|
●Discosea Cavalier-Smith et al. (2004) (R) Flattened naked amoebae, never producing tubular, subcylindrical pseudopodia and never altering the locomotive form; cytoplasmic flow polyaxial or without a pronounced axis; subpseudopodia short or absent, never both pointed and branched. |
|
●● Flabellinia Smirnov et al. 2005 (R) Flattened generally fan-shaped, discoid or irregularly triangular, never with pointed subpseudopodia or centrosomes. |
|
●●● Pellitida Smirnov et al. 2011 (R) Thick cell coat does not contain scales, is integrated with the cell membrane, and envelops the entire cell or part of the cell, leaving dorsal surface free. Endostelium, Gocevia, Paragocevia, Pellita. Kudryavtsev (2012) show that both Gocevia and Endostelium group within Pellitida in small subunit (SSU) rRNA trees. |
|
●●●
Trichosphaerium Schaudinn 1899 Cell enveloped with flexible membranous shell (smooth form) or rigid envelope bearing spicules (spicule-bearing form); both types of envelopes are separated from the cell membrane; the amoeba protrudes through this envelope with temporary openings, producing finger-shaped dactylopodia. |
|
●●● Dactylopodida Smirnov et al. 2005 (R) Locomotory form as irregular triangle with basement directed forward; wide anterior hyaloplasm; parasomes in Paramoeba and Neoparamoeba; cysts unknown; without fibrous axial cores both in dactylopodia and in the floating pseudopodia; cortex with extracellular scales, pentagonal or hexagonal glycostyles or a complex fibrous “cuticle”. Korotnevella, Neoparamoeba, Paramoeba, Pseudoparamoeba, Squamamoeba, Vexillifera. |
| ●●●●Incertae sedis Dactylopodida: Boveella, Dactylosphaerium, Oscillodignum, Podostoma, Strioluatus, Subulamoeba,Trienamoeba. |
|
●●● Vannellida Smirnov et al. 2005 (R) Locomotion as fan-shaped to spatulate cell; without discrete pseudopodia or subpseudopodia; wide anterior hyaloplasm up to half of the cell; posterior granuloplasm concentrated in a “hump”, often raised over the substratum; cell coat is a layer of hexagonal prismatic structures (Platyamoeba), with short glycostyles on top (Clydonella, Lingulamoeba) or pentagonal glycostyles with or without simple filaments (Vannella); one taxon known to be sporocarpic and protosteloid). Clydonella, Lingulamoeba, Pessonella, Platyamoeba, Protosteliopsis fimicola, Ripella, Vannella. |
| ●●●●Incertae sedis Vanellida: Discamoeba, Unda. |
|
●●Himatismenida Page 1987 Dorsal surface covered with rigid coat without defined aperture; ventral surface naked. Cochliopodium, Ovalopodium, Gocevia, Paragocevia, Parvamoeba. |
|
●●●Stygamoebida Smirnov et al. 2011 Flattened, elongate amoebae resembling tooth-pick or splinters, temporarily acquiring forked or branched form; extended area of anterior hyaloplasm; flattened, ribbon-like mitochondrial cristae. Stygamoeba, Vermistella. |
|
●●Longamoebia Cavalier-Smith and Smirnov in Smirnov et al. 2011 (R) Flattened, elongated cell with pointed subpseudopodia and centrosomes in one lineage. |
|
●●●Dermamoebida Cavalier-Smith 2004 (R) Oblong, lancet-shaped or irregularly triangular in locomotion; with smooth cell surface or with few wide ridges, never wrinkled; short, wide triangular pseudopodia and, in some, subpseudopodia of dactylopodial type; thick cell coat, multilayered or consisting of tightly packed helical structures. Dermamoeba, Mayorella, Paradermamoeba. |
|
●●● Thecamoebida Schaeffer 1926 (R) Oblong, flattened cell with dorsal folds and/or ridges; anterior hyaloplasm forms antero-lateral crescent and never occupies half or more of the body length; never produce discrete pseudopodia or subpseudopodia; cell coat relatively thin, amorphous or with extra structures based on amorphous layer. Sappinia, Stenamoeba, Thecamoeba. |
|
●●●Centramoebida Rogerson & Patterson 2002, emend. Cavalier-Smith 2004 (R) Fattened with prominent subpseudopodia, flexible and tapering to a fine tip and sometimes furcated near their base (acanthopodia); without adhesive uroid; trilaminate cytoplasmic microtubular organizing center; (one species in culture appears as a branched, flattened sheet without subpseudopodia; at least one species sporocarpic and protosteloid. . Acanthamoeba, Balamuthia, Protacanthamoeba. |
| ●●●●Incertae sedis Centramoebida: one undescribed protosteloid member sister to Protacanthamoeba, and perhaps also “Protostelium” arachisporum and “Protostelium” pyriformis. |
| ● Archamoebae Cavalier-Smith 1983 Mitochondrial converted non-aerobic organelles. |
| ●● Entamoebidae Chatton 1925, emend. Cavalier-Smith 1993 Cilium and centrioles absent; with mitosomes instead of classical mitochondria; peroxisomes absent; mitosis closed with endonuclear centrosome and spindle; reduced Golgi dictyosome. Note that this diverse genus could potentially be subdivided into other genera. Entamoeba. |
| ●●Mastigamoebaea Frenzel 1892 [=Mastigamoebidae Goldschmidt 1907; Rhizoflagellida Saville Kent 1880] Amoeboid with several pseudopodia; sometimes body stiff without amoeboid motion, depending on conditions; single cilium directed forward, with stiff vibrating beat; single kinetosome with cone of microtubules extending to nucleus; uninucleate, but some species multinucleate; large nucleoli persist through division with intranuclear spindle; stages without cilium occur; cysts; occurring in microaerophilic to anaerobic habitats rich in dissolved nutrients. Mastigella, Mastigamoeba. |
| ●●●Incertae sedis Mastigamoebidae: Endolimax, Mastigina. |
| ●●Pelomyxa Greef 1874 [Pelobiontida Page 1976] Multiple cilia; anaerobic; polymorphic life cycle with multinucleate stages; with symbionts. Pelomyxa. |
|
● Gracilipodida Lahr et al. 2011 Amoeboid without cilium or centrosomes; flattened, fan-shaped or irregularly branched, with short conical subpseudopodia or fine hyaline hair-like subpseudopodia; cysts with smooth single-layered. Arachnula, Filamoeba, Flamella. |
|
●Multicilia Cienkowsky 1881 Multiciliate, with ciliated single kinetosome; conical microtubular cytoskeleton extending from every kinetosome; interkinetosomal fibres connect each kinetosome to a neighboring one. |
|
● Protosteliida Olive & Stoianovitch 1966, emend. Shadwick & Spiegel in Adl et al. 2012 Protosteloid sporocarpic amoebae typically with uninucleate amoebae containing light orange lipid drops, with acutely pointed subpseudopodia; one taxon amoebociliated with 1--9 reduced unikont kinetids not associated with nucleus; kinetids with only BB2, rootlets 1, 3, and 4, conical array (CA) of microtubules, and posterior parakinetosomal structure (PPKS); taxa without cilium with ring-shaped component in a nucleus-associated MTOC; mitosis with open spindle and either centrioles (one taxon) or ring-shaped MTOC at poles; cysts thin-walled, spherical to subspherical; amoebae that germinate from spores may fruit whether amoebociliated or not; prespore cells lozenge-shaped when viewed from above; sporocarps with long, delicate stalk supporting single spore, morphology varying by taxon. Planoprotostelium, Protostelium. |
|
●Cavosteliida Shadwick & Spiegel in Adl et al. 2012 Protosteloid sporocarpic with various types of amoebae, from uninucleate to plurinucleate amoebae to multinucleate reticulate plasmodia, all characterized by having long, filose, subspeudopodia, anastomosing in some taxa; one taxon amoebociliate with 1-several, reduced unikont kinetids per cell, not associated with the nucleus; kinetids with only BB2, rootlets 1, 3, and 4; taxon with amoebociliate has life cycle consistent with sex where amoebociliate germinates from spore then differentiate into a uninucleate obligate amoeba (Spiegel and Feldman 1985) lacking kinetids that develops into sporocarp; all other taxa without amoebociliate state; only amoebae with no kinetids germinate from spores and then may develop into sporocarp; cysts thin-walled with various morphologies depending on taxon; prespore cells circular or lozenge-shaped when viewed from above; sporocarps with single, nondeciduous spores, morphology variable depending upon taxon, all with spores bearing some kind of sculpturing that varies by taxon. Cavostelium, Schizoplasmodiopsis (P), Tychosporium. |
|
● Protosporangiida Shadwick & Spiegel in Adl et al. 2012 Protosteloid sporocarpic with sexual life cycles where multiple, apparently haploid amoebociliates develop from germling of spore and then develop into apparently diploid obligate amoebae that are able to develop into sporocarps; germlings and amoebociliates covered with distinctive cell coat of fibers that branch at the apex; amoebociliates biciliate with nucleus-associated kinetids with rootlet 4 consisting of only 2 microtubules; prespore cells site of meiotic prophase; meiosis completed in spores. |
|
●●Protosporangiidae Spiegel in Adl et al. 2012 Amoebociliates with bikont kinetids similar to complete kinetids for Amoebozoa except that rootlet 4 consists of two microtubules; mitosis in amoebociliate with open, centric spindle; obligate amoebae rounded with short subpseudopodia, uninucleate to plurinucleate, with nucleus associated MTOC with microtubules radiating from a relatively large, electron dense core; prespore cells circular in outline when viewed from above; sporocarps with stalks characteristic of the taxa, always with two or more spores. Clastostelium, Protosporangium. |
|
●●
Ceratiomyxa Schröter 1889 Amoebociliates with bikont kinetids similar to that of protosporangiids but missing rootlet 2, MTOC and CA; obligate amoebae multinucleate, reticulate plasmodia that may reach several meters in size in some taxa; plasmodia deposit characteristic extracellular “slime” columns typical of each taxon as platform for fruiting then fragment into uninuclear, circular to lozenge-shaped prespore cells; slime columns microscopic to macroscopic depending on taxon; sporocarps long stalked, 4-nucleate at maturity. Ceratiomyxa. |
| ● Fractovitelliida Lahr et al. 2011 Protosteloid sporocarpic amoebae with no reported amoebociliate stage; amoebae flabellate with acanthopodial subpseudopodia, usually uninucleate with diffuse, multipart nucleolus; cysts thin-walled, spherical to irregular; prespore cells initially have “fried egg” appearance with rounded-up center and thin, flat margin; sporocarps with deciduous spores, morphology varies by species. Grellamoeba (not known to fruit), Soliformovum. |
|
● Schizoplasmodiida L. Shadwick & Spiegel in Adl et al. 2012 Protosteloid sporocarpic amoebae, all with multinucleate, reticulate plasmodia that have no directional streaming and a beaded appearance during mitosis; one taxon with amoebociliates that can develop from zoocysts derived from the plasmodium that germinates from the spore or from a fragment of a feeding plasmodium; one nucleus surviving in the zoocyst undergoes two to three rounds of nuclear division, hinting at meiosis, and cell division giving rise to four to eight scaled amoebociliates; kinetids bikont, lacking MTOC and CA; amoebociliate mitosis with open, centric spindle; prespore cells developing from multinucleate fragments of plasmodia; sporocarp stalk length variable according to taxon, but all stalks with cup-like apophysis that fits into annular hilum on spore; spores always multinucleate, shape varying according to taxon. Ceratiomyxella, Nematostelium, Schizoplasmodium. |
| ●● Incertae sedis Schizoplasmodiida: Phalansterium Stein 1878. |
|
●Myxogastria Macbride 1899 [not Myxomycetes Link 1833, emend. Haeckel 1866] Myxogastroid sporocarpic amoebae with trophic stage a free-living, multinucleate, coenocytic, saprobic multinucleate obligate amoeba (plasmodium); under poor conditions plasmodium sometimes becomes a sclerotium; sporocarps (<1 mm–~1 m) developing from multinucleate obligate amoeba, the plasmodium, or fragment of plasmodium; most with stalked sporangia but also sessile sporangia, plasmodiocarps, aethalia or pseudoaethalia; stalks when present acellular; meiosis in uninucleate spores with sculptured spore walls, with spores produced in masses; spores in some suspended by thread-like acellular capillitium; haploid gametic amoebociliates in sexual species germinate from spores to trophic state that may alternate between a ciliated swarm cell and a non-ciliated myxamoeba, or dormant thin-walled microcysts; kinetids closely associated with nucleus, present until mitosis then regenerating after telophase; kinetids as described for Amoebozoa; suspended amoebociliates twisted and obconic with distinct uroid; anteriorly directed cilium and shorter recurved posterior cilium in groove underlain by microtubule arrays 4, 5; mitosis centric and open; plasmodia developing from zygote in sexual species, directly from amoebociliate in apomictic species; plasmodium small and unveined with 8–100 nuclei (protoplasmodium) or large and veined network with 102--4 × 107 nuclei with thick gel-like cortex shuttle in veins (phaneroplasmodium), or thin transparent veins (aphanoplasmodium); mitosis in plasmodium intranuclear with noncentric poles; dormancy as sclerotia of many macrocysts or as sporocarps. Note that recent phylogenetic work shows two major lineages, light spored (LS) and dark spored (DS), which are not yet formally classified (Fiore-Donno et al. 2005, 2010); some classical taxa clearly paraphyletic and in need of major revision. Arcyria (LS), Badhamia (DS), Barbyella (DS), Brefeldia (DS), Calomyxa (LS), Comatricha (DS), Cribraria (LS), Diachea (DS), Diderma (DS), Dydimium (DS), Echinostelium (DS), Fuligo (DS), Hemitrichia (LS), Lamproderma (DS), Leocarpus (DS), Lepidoderma (DS), Licea (LS), Lycogala (LS), Macbrideola(DS), Metatrichia (LS), Oligonema (LS), Perichaena (LS), Physarella (DS), Physarum DS), Stemonitis (DS), Trichia (LS), Tubifera (LS), Willkommlangea (DS). |
|
●Dictyostelia Lister 1909, emend. Olive 1970 Sorocarpic amoebae, known as the cellular slime moulds, with stalked fruiting bodies developing from aggregation of amoebae; sorocarps of stalks with terminal sori of haploid spores; stalks (sorophores), acellular (acytostelioid), cellular, and unbranched to sparsely branched (dictyostelioid) or cellular with whorls of branches (polysphondylioid); stalk cells forming cell walls and dying; spores usually ellipsoid, occasionally reniform or spherical; trophic amoebae, nonciliated, haploid, uninucleate; nuclei with reticulate peripheral nucleoli; microtubular cytoskeleton of amoebae radiating from lamellar discoid organelle near nucleus; amoebae of some species entering dormant stage as thin-walled microcysts; upon starvation, populations of amoebae becoming aggregation-competent, aggregating into multicellular aggregation centres in response to a chemical attractant called an acrasin; acrasins varying according to taxon; aggregated cells differentiating directly into subaerial sorogens that become sorocarps, or migrating along the substrate as slugs, prior to differentiating into sorogens that culminate as sorocarps; stalks produced by both migrating slugs and sorogens in most species, although a few species have stalkless migration; stalk tubes secreted by inner ends of cells at at least the anterior end of the slug/sorogen; in taxa with cellular stalks an anterior population of prestalk cells becoming enclosed in the stalk tube as the slug/sorogen advances, enlarging, secreting walls, vacuolating, and dying as mature stalk cells; remaining posterior prespore cells developing into spores suspended in a slime matrix; sexual, zygotic amoebae forming and acting as aggregation centres for haploid amoebae, which are ingested by the zygotes; entire small aggregate secreting a thick wall and then becoming a dormant macrocyst once all the haploid amoebae are ingested; meiosis occurring when dormancy of macrocyst is broken; haploid amoebae germinating from macrocyst. Note that classical taxa are not monophyletic and efforts at revision are still ongoing; four major clades recognized but not yet named (Schaap et al. 2006, Romeralo et al. 2009). Acytostelium (P), Dictyostelium (P), Polysphondylium (P). |
| ●●Incertae sedis Dictyostelia: Coenonia. |
| ● Incertae sedis Amoebozoa: Gibbodiscus, Hartmannia, Janickia, Malamoeba, Malpigamoeba, Echinosteliopsis oligospora Reinhardt & Olive 1966, Microglomus paxillus Olive & Stoianovitch 1977, Pseudothecamoeba, Stereomyxa, Thecochaos. |
| OPISTHOKONTA Cavalier-Smith 1987, emend. Adl et al. 2005 Single posterior cilium without mastigonemes, present in at least one life cycle stage, or secondarily lost; with pair of kinetosomes or centrioles, sometimes modified; flat cristae in the unicellular stage. |
| ● Holozoa Lang et al. 2002 (R) The most inclusive clade containing Homo sapiens Linnaeus 1758 (Metazoa), but not Neurospora crass Shear & Dodge 1927 (Fungi). This is a branch-based definition in which all the specifiers are extant; it is intended to apply to a crown clade. Note that the apparent composition of Holozoa is Metazoa, Filasterea (Ministeria, Capsaspora), Ichthyosporea, Corallochytrium, and Choanomonada. The primary reference phylogeny is Brown et al. (2009, Fig. 5). Additional phylogenies are Brown et al. (2009, Fig. 3, 4). |
| ●● Filasterea Shalchian-Tabrizi et al. 2008 Trophic cells naked, unicellular; uninucleate; aerobic with flat mitochondrial cristae; long non-tapering tentacles supported by microfilaments, tentacles not organized into a collar as in choanomonads. |
|
●●●
Ministeria Patterson et al. 1993, emend. Tong 1997 [Ministeriida Cavalier-Smith, 1997] Marine isolates known only; <5 µm with equally spaced, unbranched filopodia radiating from spherical bodies; flat mitochondrial cristae; cilium has been suggested but controversial. |
| ●●● Capsaspora Hertel et al. 2002 [Capsasporidae Cavalier-Smith 2008] (M) Amoeboid 3.0–7.0 µm in diameter; single nucleus one-third to one-half size of cell, with central nucleolus; without cilium; flat cristae; long, straight, unbranched pseudopodia, called “feeding peduncles”; without mucous sheath; capable of penetrating tegument of trematode larvae; cell wall with chitin, elastin or collagen. Capsaspora owczarzaki. |
| ●● Ichthyosporea Cavalier-Smith 1998 [Mesomycetozoea Mendoza et al. 2002] Single-celled trophic organisms, Icthyophonus, with hyphal, multinucleated filaments; flat mitochondrial cristae but some may have tubular cristae; if present, single cilium; without collar or cortical alveoli; some species form only elongate amoeboid cells; most animal parasites, some free-living and saprotrophic (Sphaeroforma, LKM51 isolate); chitin reported but controversial. |
| ●●● Rhinosporideacae Mendoza et al. 2001 [Dermocystida Cavalier-Smith 1998] (R) If present, posterior cilium; flat mitochondrial cristae; when parasite of animals, spherical phenotypes with several 2–20 µm endospores that are eventually released and become mature cells with endospores to continue the parasitic cycle. Amphibiocystidium ranae, Amphibiothecum penneri, Dermocystidium, Rhinosporidium seeberi, Sphaerothecum destruens. |
| ●●● Ichthyophonae Mendoza et al. 2001 [Ichthyophonida Cavalier-Smith 1998; Amoebidiidae Reeves 2003] (R) Parasites of fish, arthropods, and insects, or free-living and saprotrophic; usually with flat cristae but Ichthyophonus with tubular cristae; some characteristic amoeboid cells, but in others amoeboid cells absent or unreported; uniciliated stage only in Pseudoperkinsus tapetis, but controversial. Abeoforma whisleri, Amoebidium parasiticum, Anurofeca richardsi, Astreptonema, Caullerya mesnili, Creolimax fragrantissima, Eccrinidus flexilis, Enterobryus oxidi, Enteropogon sexuale, Ichthyophonus, Palavascia patagonica, Pseudoperkinsus tapetis, Psorospermium haeckeli, Sphaeroforma arctica. |
| ●● Aphelidea Gromov 2000 Intracellular phagotrophic parasites of algae with complex life cycle; amoeboid cell invades host through apophysa of spore, attached to host cell surface; characteristic central food vacuole with excretory body; cell division leads to ciliate or amoeboid dispersal cells released from host; tubular or lamellar cristae. Amoeboaphelidium, Aphelidium, Pseudoaphelidium. |
| ●● Corallochytrium Raghu-Kumar 1987 (M) Spherical single cells 4.5–20.0 µm in diameter; binary fissions releasing numerous elongated amoeboid cells; marine saprotrophic, usually recovered from coral reefs in the Indian Ocean. Corallochytrium limacisporum. |
| ●● Choanomonada Kent 1880 Phagotrophic with collar of microvilli around a single cilium; radial symmetry; solitary or colonial; flat mitochondrial cristae; central filament in kinetosome transition zone. |
| ●●● Craspedida Cavalier-Smith 1997, emend. Nitsche et al. 2011 Extracellular test that is entirely organic and does not project above the anterior end of the extended feeding cell; vegetative stage usually sedentary and stalked; motile stages for dispersal. Astrosiga, Aulomonas, Choanoeca, Cladospongia, Codonocladium, Codonosigopsis, Codosiga (junior synonym Codonosiga), Desmarella (junior synonyms Codonodesmus and Kentrosiga), Dicraspedella, Diploeca, Diplosiga, Diplosigopsis, Kentia, Lagenoeca, Monosiga, Pachysoeca, Proterospongia, Salpingoeca, Salpingorhiza, Sphaeroeca, Stelexomonas, Stylochromonas. |
| ●●● Acanthoecida Norris 1965, emend. Cavalier-Smith 1997, emend. Nitsche et al. 2011 Cells surrounded by a basket-like lorica of siliceous costae comprising rod-shaped costal strips and a partial or entire organic test on inner surface. Acanthoeca, Acanthocorbis, Amoenoscopa, Apheloecion, Bicosta, Calliacantha, Calotheca, Campanoeca, Campyloacantha, Conion, Cosmoeca, Crinolina, Crucispina, Diaphanoeca, Didymoeca, Helgoeca, Kakoeca, Monocosta, Nannoeca, Parvicorbicula, Platypleura, Pleurasiga, Polyfibula, Polyoeca, Saepicula, Saroeca, Savillea, Spinoeca, Spiraloecion, Stephanacantha, Stephanoeca, Syndetophyllum. |
| ●● Metazoa Haeckel 1874 Multicellular; cells typically held together by intercellular junctions; extracellular matrix with fibrous proteins, typically collagens, between two dissimilar epithelia, except in Trichoplax or where secondarily lost; sexual with production of an egg cell that is fertilized by a smaller, often monociliated sperm cell; phagotrophic and osmotrophic; without cell wall. |
| ●●● Porifera Grant 1836 [Parazoa Sollas 1884] Cells without walls; flat mitochondrial cristae; sexual species, mostly hermaphroditic, releasing monociliated sperm or producing amoeboid egg cells at different times; zygotes forming ciliated dispersal larvae that resemble blastulae; sessile adult; asexual reproduction by gemmules; differentiation of larva to a variety of cell types, including choanocytes, amoeboid cells, and digestive secretory cells; cell types transformable into other types as necessary; cells more or less independent; supporting matrix secreted by amoeboid cells; without mesoderm, nervous tissue, desmosomes, localised gonad, or glandular digestive cells. |
| ●●●● Silicispongia Schmidt 1862 [Silicea Bowerbank 1864, emend. Gray 1867] Matrix of siliceous spicules organized around a well-defined axial filament of protein. |
| ●●●●● Hexactinellida Schmidt 1870 Siliceous spicules triaxonic, hexactinic; cells forming extensive multinucleate syncytium, with some differentiated cells; electrical conductance across body; non-contractile body; larvae (poorly known) with medial region of ciliated cells. Euplectella, Farrea, Hyalonema, Monoraphis, Lophocalyx, Semperella. |
| ●●●●● Demospongiae Sollas 1885, emend. Borchiellini et al. 2004 Spongin and siliceous spicules in matrix, except in Myxospongiae; spicules not triaxonic, with hollow triangular canal and four rays, not perpendicular; larva with outer monociliated cells, except at posterior pole; one family (Cladorhizidae) with external digestion, by amoeboid cell aggregation, of captured crustacean prey. Aplysina, Axinella, Cacospongia, Chondrosia, Cliona, Euspongia, Halisarca, Hippospongia, Oscarella, Plakina, Spongilla, Suberites. Excludes Homoscleromorpha, includes Keratosa Borchiellini et al. 2004, Myxospongiae Borchiellini et al. 2004, and Haplosclerida Borchiellini et al. 2004 |
| ●●●●●● Democlavia Sperling et al. 2009, emend. Morrow et al. 2012 Includes the following clades (C): C1 Suberitidae Schmidt 1870, Halichondriidae Gray 1867 emend. Morrow et al. 2012; C2 Polymastiidae Gray 1867; C3 Hemiasterillidae Lenderfeld 1889 emend. Morrow et al. 2012, Tethyidae Gray 1848, Timeidae Topsent 1928, Trachycladidae Hallmann 1917; C4 Clionaidae d’Orbigny 1851, Spirasterillidae Ridley & Dendy 1886; C5 Poecilosclerida Topsent 1928; C6 Agelasida Hartman 1980 emend. Morrow et al. 2012; C7 Axinellida Lévi 1973 emend. Morrow et al. 2012; C10 Dictyonellidae van Soetz, Diaz &Pomponi 1990 emend. Morrow et al. 2012; C11 Tetractinellida Marshall 1876 emend. Morrow et al. 2012; C12 Desmacellidae Ridley &Dendy 1886 emend. Morrow et al. 2012; C13 Spongillidae Gray 1867; C14 Scopalinidae Morrow et al 2012. |
| ●●●● Homoscleromorpha Lévi 1973, emend. Borchiellini et al. 2004 (R) Siliceous spicules without defined axial filament, in some species; thick basi-epithelial basement membrane; supporting matrix with collagen-IV. Node includes Oscarella lobularis, excludes Beroe ovata, Geodia cydonium, Hydra viridis, Leucosolenia variabilis, Oopsacas minuta. |
| ●●●● Calcispongia Johnston 1842 [Calcarea Bowerbank 1864] Calcium carbonate spicules; larvae with outer monociliated cells, larger at posterior; invagination of anterior cells at attachment of posterior to substrate. |
| ●●●●● Calcinea Hartman 1958, emend. Borchiellini et al. 2004 (R) Unambiguous characters congruent with molecular phylogenies unclear. Clathrinida, Murrayona. |
| ●●●●● Calcaronea Hartman 1958, emend. Borchiellini et al. 2004 (R) Unambiguous characters congruent with molecular phylogenies unclear. Grantiopsis-Paralurilla, Vosmacropsis-Sycettusa, includes Heteropiidae, Staurorrhaphidae, Minchinellidae. |
| ●●● Trichoplax von Schulze 1883 [Placozoa Grell 1971] (M) Two layers of epithelial cells, with a middle layer of syncytial contractile fibrous cells, and undifferentiated cells; with digestive glandular cells; belt desmosomes or zonulae adherentes connecting adjacent cells; without extracellular matrix; collagen fibres absent; without endoderm, ectoderm, mesoderm or nerve cells; ventral cells having ciliated kinetosomes with 2 horizontal fibrillar rootlets and one vertical rootlet; egg cell and non-ciliated sperm in mid-layer; asexual binary division of body possible. Trichoplax adhaerens. |
| ●●● Animalia Linnaeus 1758, emend. Adl et al. 2005 [Eumetazoa Bütschli 1910] Reproduction through an egg cell, usually fertilised by a monociliated sperm cell with acrosome; embryonic development with blastula followed by gastrulation that begins the differentiation into endoderm, ectoderm, mesoderm, and neuroderm; tissues organised into organs that share tasks for the individual, unless secondarily lost; some secondarily reduced to small number of cells (eg. Myxozoa Grassé 1970); coordination of cells and tissues by membrane receptors that respond to ligands through elaborate signal transduction; characteristic cell-cell junctions with belt desmosomes or zonulae adherentes; basal lamina and extracellular matrix with collagen and other fibrous proteins (laminin, nidogen, perlecan); heterotrophic nutrition with secretion of digestive enzymes and osmotrophy through a digestive tract; without cell wall; ectoderm completely surrounding body, and endoderm surrounding a digestive tract; sensory cells in epithelium; nervous tissue in organised network; epithelial actin-myosin based contractile cells between endoderm-ectoderm. Subdivisions not shown. |
| ● Nucletmycea Brown et al. 2009 [Holomycota Liu et al. 2009] (R) The most inclusive clade containing Neurospora crassa Shear & Dodge 1927 (Fungi) and not Homo sapiens Linnaeus 1758 (Metazoa). This is a branch-based definition in which all the specifiers are extant; it is intended to apply to a crown clade. Note that the composition of Nucletmycea is Fungi, Nuclearia, and Fonticula. The primary reference is Brown et al. (2009, Fig. 5). Additional phylogenies are Brown et al. (2009, Fig. 3, 4). |
| ●● Nuclearia Cienkowski 1865 Amoeboid with rounded body, from which elongated filopodia extend; flat discoid mitochondrial cristae. Nuclearia. |
| ●● Fonticula Worley et al. 1979 (M) Trophic cells small, amoeboid with rounded body, from which elongated filopodia extend; flat discoid cristae; sorocarpic ("aggregative fruiting") with stalked fruiting bodies formed by aggregation of amoebae; aggregated cells form a hollow gelatinous extracellular stalk supported by fibrillar matrix material; cells within stalk column encyst into walled spores that are forcibly pushed through the apex of stalk in an erupting fashion. Fonticula alba. |
| ●● Fungi R. T. Moore 1980 Heterotrophic, not phagotrophic; often with walls and multinucleate hyphae; walls, when present, with β-glucan and usually chitin, at least in spore walls; lysine biosynthesis by aminoadipic acid (AAA) pathway; mitochondria and peroxisomes present, or secondarily lost as in Microsporidia; flattened mitochondrial cristae; plastids and tubular mastigonemes absent. |
| ●●●Microsporidia Balbiani 1882 Obligate intracellular parasites, usually of animals; without mitochondria and peroxisomes; spores with inner chitin wall and outer proteinaceous wall; without kinetosomes, centrioles or cilia; centrosomal plaque; extrusive specialized polar tube for host penetration; sexual, asexual or both. Subdivisions uncertain at this time. Amblyospora, Amphiacantha, Buxtehudia, Caudospora, Chytridiopsis, Desportesia, Encephalitozoon, Enterocytozoon, Glugea, Hessea, Metchnikovella, Nosema, Spraguea, Vairimorpha. |
| ●●● Neocallimastigaceae Heath 1983, emend. Barr 1989 [= Neocallimastigomycota M. J. Powell 2007; =Neocallimastigales J. L. Li et al. 1993] Thallus monocentric or polycentric; anaerobic, found in digestive system of larger herbivorous mammals and possibly in other terrestrial and aquatic anaerobic environments; hydrogenosomes of mitochondrial origin; uni- and multiciliated cells with a kinetosome-associated complex that includes a skirt, strut, spur, and circumciliary ring, microtubules stretching from the spur and radiating around the nucleus, forming a posterior fan; unikont kinetid and without props; nuclear envelope is retained during mitosis. Anaeromyces, Caecomyces, Cyllamyces, Neocallimastix, Orpinomyces, Piromyces. |
| ●●● Chytridiomycota M. J. Powell in Hibbett et al. 2007 Thallus monocentric, polycentric, or filamentous; uniciliated cells with a posteriorly-directed cilium with unikont kinetid, nine ciliary props, one side-body complex, and a stacked Golgi apparatus (microbody-lipid globule complex); sexual reproduction with zygotic meiosis where known; golgi apparatus with stacked cisternae; nuclear envelope fenestrated at poles during mitosis; aerobic; found in soil and water as saprobes but also parasitic on animals, plants, algae, and other fungi; reproduction asexual by uniciliated cells and where known sexually by zygotic meiosis. |
| ●●●● Chytridiomycetes de Barry 1863, emend. Cavalier-Smith 1998, emend. Powell in Hibbett et al. 2007 Thallus monocentric or rhizomycelial polycentric; uniciliated cells with posterior cilium with unikont kinetid; sexual reproduction not oogamous. |
| ●●●●● Chytridiales Cohn 1879, emend. Schröter 1892, emend. Barr 1980, emend. Barr 2001, emend. Letcher & Powell 2006, emend. Mozley-Standridge 2009, emend. Vélez et al. 2011 Thallus monocentric or polycentric rhizomycelial; cells typically with an electron-opaque plug at base of cilium; microtubules extending from one side of the kinetosome in a parallel array; ribosomes aggregated near the nucleus; ciliated kinetosome parallel to kinetosome without cilium and connected to it by fibrous material; nucleus not associated with kinetosome; fenestrated cisterna (rumposome) adjacent to lipid globule. Chytridium, Chytriomyces. |
| ●●●●●Cladochytriales Mozley-Standridge 2009 Thallus epibiotic or endobiotic; eucarpic, monocentric or polycentric; sporangium is either operculate or inoperculate; rhizoidial axis is either apophysate or non-apophysate, and rhizoids can be catenulate, isodiametric or tapering; cells with up to 25 linked microtubules in a cord-like microtubular root situated between the kinetosome and the fenestrated cisterna. Allochytridium, Cladochytrium, Cylindrochytridium, Endochytrium, Nowakowskiella, Septochytrium. |
| ●●●●●●Incertae sedis Cladochytriales: Catenochytridium, Nephrochytrium. |
| ●●●●● Rhizophydiales James 2006, emend. Letcher 2006, emend. Letcher 2008 Uniciliated with one or more of the following characters: microtubular root with one or more microtubules that may or may not be present but when present extends in a parallal fashion from one side of the kinetosome to a cisterna on the lipid globule; double-membrane bound group of ribosomes; mitochondria; microbodies, lipid globule, and membrane cisterna (MLC); kinetosomes either lie parallel or slightly angled toward each other and are connected by a fibrillar bridge; a kinetosome-associated structure, spur or shield, may or may not be present and adjacent to the kinetosome; no electron-dense plug in the ciliary base. Alphamyces, Angulomyces, Aquamyces, Batrachochytrium, Boothiomyces, Globomyces, Gorgonomyces, Kappamyces, Pateramyces, Protrudomyces, Terramyces, Rhizophydium, Urceomyces. |
| ●●●●●●Incertae sedis Rhizophydiales: Batrachochytrium, Coralloidiomyces. |
| ●●●●●Polychytriales Longcore 2012 Thallus polycentric or monocentric; monocentric species with multiple rhizoidal axes; uniciliated spherical cell, may or may not possess each of the following: a ciliary plug, a kinetosome spur, a fenestrated cisterna and a microtubular root that, if present, may have up to 3 microtubules; one to many lipid globules; the kinetid without cilium is equal or longer in length than the ciliated kinetosome and is attached to this kinetosome throughout its length. Arkaya, Karlingiomyces, Lacustromyces, Neokarlingia, Polychytrium. |
| ●●●●● Spizellomycetales Barr 1980, emend. Barr 1983 Nucleus either closely associated with the kinetosome or connected by its root; ribosomes dispersed in the cytoplasm; rumposome absent; dormant kinetosome at an angle to the ciliated kinetosome; without electron-opaque material in the kinetosome transition zone. Gaertneriomyces, Geranomyces, Kochiomyces, Powellomyces, Spizellomyces, Triparticalcar. |
| ●●●●● Rhizophlyctidales Letcher 2008 Thallus monocentric, eucarpic; interbiotic sporangium that is either inoperculate or endo-operculate with one to several discharge short tubes; multiple rhizoidial axes; uniciliated cell possesses a ciliated kinteosome that is at an acute angle (<40°) to the non-ciliated kinetosome and attached by a fibrillar bridge along the length of the non-ciliated kinetosome; multiple mitochondria; ribosomes either dispersed or aggregated in the cytoplasm; one to many lipid globules; without microtubules. Arizonaphlyctis lemmonensis, Borealophlyctis paxensis, Rhizophlyctis rosea, Sonoraphlyctis ranzonii. |
| ●●●●● Lobulomycetaceae Simmons 2009, emend. Simmons 2011 [Lobulomycetales Simmons 2009] Uniciliated cell with an opaque ciliary plug, anterior or posterior plug extensions; one or two lipid globules; thallus monocentric, eucarpic with endogenous development; rhizoids are isodiametric ranging from 0.5--1.5 µm wide. Note that members of this group lack the following ultrastructural features found in most of the other Chytridiomycota: microtubular root, Golgi apparatus, striated inclusion, opaque bodies near kinetosome, and “rumposome” or fenestrated cisterna associated with the lipid globule. Alogomyces, Clydaea, Lobulomyces, Maunachytrium. |
| ●●●● Monoblepharidales Schröter 1893, emend. Sparrow 1943 Thallus filamentous, either extensive or a simple unbranched thallus, often with a basal holdfast; uniciliated cell possessing a kinteosome parallel to the non-ciliated kinetosome with a striated disk partially extending around the kinetosome; microtubules radiating anteriorly from the striated disk; ribosomal aggregation; fenestrated cisterna adjacent to the microbody; asexual reproduction occurs via production of uniciliated cells or autospores while sexual reproduction is oogamous via fusion of uniciliated antherozoids produced by antheridia and non-ciliated female gametes produced by oogonia. Gonapodya, Harpochytrium, Hyaloraphidium, Monoblepharella, Monoblepharis, Oedogoniomyces. |
| ●●●●Incertae sedis Chytridiomycota: Caulochytrium, Olpidium. |
| ●●● Blastocladiales Petersen 1909 [= Blastocladiineae Petersen 1909, Blastocladiomycota T. Y. James 2007, Blastocladiomycetes T. Y. James 2007] Thallus monocentric or polycentric; aerobic to facultatively anaerobic, found in aquatic and terrestrial environments, saprobic and/or parasitic; uniciliated motile cells with microtubules radiating anteriorly from the proximal end of the kinetosome and continuing on to wrap around a cone-shaped nucleus that also terminates near the kinetosome and is capped by a mass of membrane-bound ribosomes, no electron-opaque plug in kinetosome transition zone; one side-body complex (= microbody lipid globule complex); reproduces asexually by uniciliated cells while sexual reproduction occurs through fusion of planogametes with a sporic type of meiosis. Allomyces Blastocladia, Blastocladiella, Blastocladiopsis, Catenomyces, Catenophlyctis, Caternaria, Coelomomyces, Coelomomycidium, Paraphysoderma, Physoderma, Sorochytrium, Urophlyctis. |
| ●●●● Incertae sedis Blastocladiales: Polycaryum leave Stempell 1903. |
| ●●●Mucoromycotina Benny 2007 Saprobes, or rarely gall-forming, non-haustorial, facultative mycoparasites, or forming ectomycorrhiza; mycelium branched, coenocytic when young, sometimes producing septa that contain micropores at maturity; asexual reproduction by sporangia, sporangiola, or merosporangia, or rarely by chlamydospores, arthrospores, or blastospores; sexual reproduction by more or less globose zygospores formed on opposed or apposed suspensors. |
| ●●●●Mucorales Fritz 1832, emend. Schröter 1897 Filamentous fungi, generally saprotrophic, with exceptions; septa absent except in older hyphae; with plasmodesmata at septal pores; asexual reproduction with one to many spores in merosporangia, sporangiola, or sporangium; reproduction by zygospore, typically with opposed suspensors. Traditional subdivisions artificial. Chaetocladium, Choanephora, Mortierella, Mucor, Phycomyces, Pilobolus, Syncephalestrum, Thamnidium. |
| ●●●● Endogone Link 1809 [Endogonaceae Paoletti 1889; Endogonales Moreau ex R. K. Benjamin 1979] Filamentous, hyphae coenocytic; saprobic and ectomycorrhizal; zygospores with apposed suspensors produced in a subterranean sporocarp. Endogone. |
| ●●●Mortierellaceae A. Fischer 1892 [Mortierellales Cavalier-Smith 1998; Mortierellomycotina Kerst et al. 2011] Mycelium with anastomosing hyphae, dichotomously branching, bearing stylospores; hyphae sporangiferous, sporangiophores basally inflated and elongating towards the sporangiophore apex, erect, coenocytic initially, but irregularily septated at maturity; asexual reproduction via sporangia and sporangiola; sporangia spherical, multi-spored; columella absent; ramifications gracilous, primarily horizontally expanding, erecting hyphae sometimes terminate with sporangiola; spores globose to ellipsoid or irregular, smooth or ornamented; rhizoids only occasional; giant cells absent; zygospores naked. Dissophora, Gamsiella, Haplosporangium, Mortierella. |
| ●●●Entomophthorales G. Winter 1880 [Entomophthoromycotina Humber 2007] Filamentous, primarily without septa; mostly parasites of insects, mites, and spiders; sexual reproduction by thick-walled zygospore, strictly homothallic, where known; asexual reproduction by conidia formed by blastosporogenesis; conidia forcibly discharged and often form secondary conidia. Conidiobolus, Completoria, Entomophthora, Meristacrum, Neozygites. |
| ●●●Zoopagales Bessey ex R.K. Benjamin 1979 [Zoopagomycotina Benny 2007] Filamentous, hyphae coenocytic or septate; parasites of soil fungi, invertebrates, and amoebae; asexual reproduction by conidia or merosporangia; sexual reproduction by globose zygospores with apposed suspensors. Amoebophilus, Piptocephalis, Rhopalomyces, Sigmoideomyces, Stylopage. |
| ●●●Kickxellomycotina Benny 2007 Fungi saprobes, mycoparasites, or obligate symbionts; thallus arising from a holdfast on other fungi as a haustorial parasite, or branched, septate, subaerial hyphae; mycelium branched or unbranched, regularly septate; septa with median, disciform cavities containing plugs; asexual production by 1- or 2-spored merosporangia, trichospores, or arthrospores; sexual reproduction by zygospores that are globose, biconical, or allantoid and coiled. |
| ●●●●Asellariales Manier ex Manier & Lichtwardt 1978 Kickxellomycotina with filamentous, branched thalli; asexual reproduction by arthrospore-like cells that disarticulate from the corresponding thallus; in the digestive tracts of terrestrial, aquatic, and marine isopods, as well as springtails. Asellaria, Baltomyces, Orchesellaria. |
| ●●●●Dimargaritaceae R.K. Benjamin 1959 [Dimargaritales R. K. Benjamin 1979] Hyphae regularly septate; septa containing a lenticular cavity; asexual reproduction by bisporous merosporangia; sexual reproduction by a zygospore, often ornamented; obligate haustorial parasites of fungi, especially Mucorales. Dimargaris, Dispira, Spinalia, Tieghemiomyces. |
| ●●●●Harpellales Lichtwardt & Manier 1978 Endosymbionts of freshwater arthropods with basal cell attached to the host, from which a filamentous thallus develops; hyphae septate, with or without branching; septa contain a lenticular cavity; asexual reproduction occurs by lateral elongate monosporous trichospores; sexual reproduction by conical or biconical zygospores. Note that this group includes taxa previously referred to as trichomycetes. Harpella, Orphella, Smittium, Zygopolaris. |
| ●●●●Kickxellaceae Linder 1943 [Kickxellales Kreisel ex R. K. Benjamin 1979] Filamentous; hyphae possessing septa with a lenticular cavity; asexual reproduction by unispored sporangiola (merosporangia) produced on a sporocladium; saprobic or mycoparasitic, isolated from soil and dung. Coemansia, Dipsacomyes, Kickxella, Linderina, Martensella, Martensiomyces, Spirodactylon, Spiromyces. |
| ●●●●Glomeromycota C. Walker & A. Schüßler 2001 Filamentous; primarily endomycorrhizal, forming arbuscules in roots, sometimes with vesicles; without cilium; presumed asexual spores outside or within roots of host; some complex spores with multiple wall groupings, others simple (blastic chlamydospores); without centrioles, conidia, and airborne spores. |
| ●●●●●Archaeosporales C. Walker & A. Schüßler 2001 [Archaeosporomycetes Sieverding et al. 2011] Known to form symbiosis with plant roots or thalli, or with cyanobacteria; if symbiosis occurs between plants and fungi, fungal spores may have two morphs, but often only one is known; species form vesicular arbuscular or arbuscular mycorrhiza. Archaeospora, Ambispora, Geosiphon. |
| ●●●●●Glomeromycetes Cavalier-Smith 1998, emend. Oehl et al. 2011 Glomoid chlamydospores formed terminally, subterminally or intercalarily in hyphae, either in or on the surface of soils or sometimes in roots, either singly, in spore clusters or multiple-spored loose to compact sporocarps, on subtending hyphae: complex multi-walled spores on sporogenous structures, or laterally or centrally within a sporiferous saccule or intrahyphally in the stalk of sporiferous saccules, forming arbuscular or vesicular-arbuscular mycorrhiza. |
| ●●●●●●Glomerales J. B. Morton & Benny 1990 Spores by blastic expansion of the hyphal tip or intercalarily formed in hyphae, either in soils or occasionally in roots, or other subterranean structures such as rhizomes, either singly, in spore clusters or multiple-spored; sporocarps loose to compact, with a mono-to-multiple layered spore wall; wall of subtending hyphae continuous with the spore wall and coloured the same as or slightly lighter than it or hyaline to subhyaline; subtending hyphae funnel-shaped, cylindrical or constricted; forming arbuscular mycorrhiza. Claroideoglomus, Funneliformis, Glomus, Rhizophagus, Sclerocystis, Septoglomus. |
| ●●●●●●Diversisporales C. Walker & A. Schüßler 2001 Spore formation by blastic expansion of hypha (chlamydosporic), or sometimes with complex spores with up to three walls or wall groups: multiple layered outer wall, and hyaline middle and inner walls that may be of several components or layers; spores with subtending hyphae, sometimes with a conspicuous colour change distant to the septum most proximal to the spore base; spores with 1–3 wall layers; pore rarely open. Acaulospora, Diversispora, Gigaspora, Pacispora, Racocetra, Scutellospora. |
| ●●●●● Paraglomus J.B. Morton & D. Redecker 2001 [Paraglomeraceae J. B. Morton & D. Redecker 2001; Paraglomerales C. Walker & A. Schüßler 2001; Paraglomeromycetes Oehl et al. 2011] Endomycorrhizal, forming arbuscular mycorrhiza; asexual spores (chlamydospores) usually formed in soil, sometimes within roots or other host tissue, sometimes with vesicles; without cilium; without centrioles, conidia, and aerial spores. Paraglomus. |
| ●●●Dikarya Hibbett et al. 2007 Unicellular or filamentous Fungi, lacking cilia, often with a dikaryotic state. The least-inclusive clade that contains Ascomycota and Basidiomycota. |
| ●●●●Ascomycota Cavalier-Smith 1998 Sexual reproduction within asci (saccate structures); meiosis usually followed by mitosis to produce from one to over 1,000 ascospores, but usually eight; ascospore walls form inside ascus; mating types heterothallic, homothallic (selfing) or both; may reproduce sexually (teleomorph) or asexually (anamorph) only, or both sexually and asexually (holomorph); asci cylindrical, fusiform, clavate or globose, persistent or evanescent, with or without a fruiting structure (ascoma, -ata); asci developing directly from ascogenous hyphae, from a crozier or from a single cell; asexual reproduction by conidiospores (mitospores) formed by fragmentation of vegetative hyphae (thallic), blastically from single cells, hyphae, or conidiophores; vegetative body of single cells or tubular, septate filaments (hyphae); septa with simple pores, except for those associated with ascogenous hyphae and asci; cell walls lamellate with a thin electron-dense outer layer and a relatively thick electrontransparent inner layer, consisting of varying proportions of chitin and glucans; saprobes, endophytes, parasites (especially on plants) or lichen forming. |
| ●●●●●Taphrinomycotina O. E. Eriksson & Winka 1997 Mycelium present or absent; asci produced from binucleate cells; do not form croziers or interascal tissue. |
| ●●●●●● Archaeorhizomyces Rosling & T. James 2011 [Archaeorhizomycetes Rosling & T. James 2011; Archaeorhizomycetales Rosling & T. James 2011] Phylogenetically placed among Taphrinomycotina, differing by mycelial growth on MMN agar together with an association with roots of living plants. Distinctive molecular characters (nuclear large subunit rRNA). Synonymous to “Soil Clone Group 1 (SCG1)”. Archaeorhizomyces. |
| ●●●●●●Neolecta Spegazzini 1881 [Neolectomycetes Eriksson & Winka 1997; Neolectales Landvik et al. 1997] Mycelium present, multinucleate; ascomata apothecial, stalked, fleshy; interascal tissue absent; cylindrical asci formed from binucleate cells undergo karyogamy, meiosis, and one mitotic division to produce eight cylindrical ascospores, thin-walled, walls blueing in iodine; ascus apex truncate, slightly thickened below ascus wall, with wide apical slit, persistent; ascospores ellipsoidal to globose, hyaline, aseptate; anamorph unknown; saprobic; found in wet mixed woodlands. Neolecta. |
| ●●●●●●Pneumocystis P. Delanoë & Delanoë 1912 [Pneumocystidales O. E. Eriksson 1994; Pneumocystidomycetes Eriksson & Winka 1997] Mycelium and ascomata absent; vegetative cells thin-walled, irregularly shaped, uninucleate, dividing by fission; sexual reproduction initiated by fusion of two vegetative cells followed by karyogamy, cyst wall formation, meiosis, and in some, one mitotic division, to produce four to eight nuclei that are delimited by the cyst (ascus) vesicle; ascospore walls are deposited between the delimiting membranes; cyst walls rupture to release ascospores; extracellular parasite of mammalian lungs. Pneumocystis. |
| ●●●●●●Schizosaccharomyces [Schizosaccharomycetales O. E. Eriksson et al. 1993; Schizosaccharomycetes O. E. Eriksson & Winka 1997] Mycelium absent or poorly developed; ascomata absent; vegetative cells cylindrical, proliferating by mitosis followed by cell division to produce two daughter cells; cell wall composition differs from that of species of Saccharomycetes; sexual reproduction initiated by fusion of two vegetative cells to form an ascus; karyogamy and meiosis occur within the ascus to produce four nuclei, which may or may not divide once again mitotically; ascospores aseptate, delimited by enveloping membrane system (EMS), wall formed within bilayers of EMS, wall blueing in iodine, hyaline or pigmented; saprophytes in sugary plant exudates; fermentation positive. Schizosaccharomyces. |
| ●●●●●●Taphrina [Taphrinales Gäumann & C. W. Dodge 1928; Taphrinomycetes O. E. Eriksson & Winka 1997] Vegetative mycelium mostly absent; ascomata absent; interascal tissue absent; dikaryotic mycelium infects host and proliferates through host tissue; dikaryotic cells or mycelium develop directly into asci, often forming a palisade layer on the host; asci globose or ellipsoidal, eightspored; ascospores hyaline, aseptate; biotrophic on angiosperms forming galls or lesions; cells bud from ascospores to form a yeast-like, monokaryotic, saprobic anamorph. Taphrina. |
| ●●●●●Saccharomycetales Kudryavtsev 1960 [Saccharomycetes O.E. Eriksson & Winka, 1997; Saccharomycotina O.E. Eriksson & Winka 1997] Mycelium mostly absent or poorly developed; hyphae, when present, septate, with septa having numerous pores rather than a single septal pore; vegetative cells proliferating by budding or fission; walls usually lacking chitin except around bud scars; ascomata absent; sexual reproduction by fusion of two vegetative haploid cells or fusion of two haploid nuclei in a single cell or within diploid cells, followed by meiosis and, in some cases, one mitotic division to produce either four or eight nuclei; cells undergoing meiosis become asci, ascospores delimited by an enveloping membrane system (EMS); ascospore wall formed within bilayers of EMS; ascospores aseptate, colourless or pigmented, often with wall thickenings of various types; most osmotrophic, some species parasitic on animals. Ascoidea, Candida, Cephaloascus, Dipodascus, Endomyces, Lipomyces, Metschnikowia, Pichia, Saccharomyces, Scheffersomyces, Trichomonascus, Wickerhamomyces, Yarrowia. |
| ●●●●●Pezizomycotina O.E. Eriksson & Winka 1997 Mycelium present; hyphae filamentous, septate; septa with simple pores and Woronin bodies; life cycle haploid with a dikaryotic stage immediately prior to sexual reproduction; ascomata discoid, perithecial, cleistothecial or occasionally lacking; antheridium present or absent; ascogonium, ascogenous hyphae, and crosiers present; the penultimate cell of the crozier, in which meiosis and usually one mitotic division occur, becomes the ascus; asci fissitunicate or not fissitunicate, cylindrical, clavate or saccate; asci frequently with ascospore discharge mechanism; usually eight ascospores surrounded by enveloping membrane system; ascospore morphology and pigmentation varied; asexual state present or absent, produced from vegetative hyphae in a thallic or blastic manner; mitospores (conidiospores) varied in morphology and pigmentation. |
| ●●●●●●Arthoniales Henssen & Jahns ex D. Hawksw. & O. E. Eriksson 1986 [Arthoniomycetes O. E. Eriksson & Winka 1997] Ascomata usually apothecial, occasionally closed with an elongated poroid opening; peridium thin- or thick-walled; interascal tissue of branched paraphysoids in a gel matrix; asci thick-walled, fissitunicate, blueing in iodine, with or without a large apical dome; ascospores aseptate or septate, sometimes becoming brown and ornamented; anamorphs pycnidial; forming crustose lichens with green algae, lichenicolous or saprobic on plants. Arthonia, Chrysothrix, Melaspilea, Opegrapha, Roccella, Roccellographa. |
| ●●●●●●Dothideomycetes O. E. Eriksson & Winka 1997 Ascomata variable (apothecial, perithecial, cleistothecial), formed lysigenously from stromatic tissue (ascolocular); interascal tissue present or absent, of branched paraphysoids or pseudoparaphyses; asci cylindrical to saccate, thick-walled, fissitunicate, rarely with apical structures; ascospores mostly septate or muriform, colorless to dark brown; anamorphs hyphomycetous or coelomycetous; saprobes, plant parasites, coprophilous or lichen forming. Note that this group partially includes loculoascomycetes. |
| ●●●●●●●Dothideomycetidae P.M. Kirk et al. ex Schoch et al. 2007 Ascomata immersed, erumpent or sometimes superficial, minute, small or medium-sized; separate or merged or grouped on basal stroma, uni- to multiloculate apical pore mostly present, and, when present, ostiolar canal at times periphysate; stromatic tissues may contain pseudoparenchymatous cells; pseudoparaphyses lacking; periphysoids may be present; asci globose, subglobose, ovoid to ellipsoid, saccate, oblong, clavate or subcylindrical; ascospores hyaline, subhyaline or dark brown, variable in shape and size, one celled or one to several septate or muriform; anamorphs coelomycetous and/or hyphomycetous. Containing Capnodiales (Capnodium, Cladosporium, Piedraia, Mycosphaerella, Teratosphaeria, Scorias), Dothideales (Dothidea, Dothiora), Myriangiales (Elsinoё, Myriangium). |
| ●●●●●●●Pleosporomycetidae C. L. Schoch et al. 2007 Ascomata perithecioid, hysterothecioid or cleistothecioid, conchate or dolabrate, immersed, erumpent or superficial; globose, sphaeroid, turbinate, ovoid, obpyriform, conoid, doliiform, dimidiate; hamathecium of wide to narrow cellular or trabeculate pseudoparaphyses, deliquescing at maturity in some; asci bitunicate, usually basal, at times extending laterally, cylindric, clavate, oblong or saccate; ascospores variable in pigmentation, shape and septation, usually with bipolar asymmetry, but some symmetrical. Containing Hysteriales (Hysterium, Psiloglonium), Jahnulales (Aliquandostipite, Jahnula), Mytilinidiales (Mytilinidion, Lophium), Pleosporales (Aigialus, Cucurbitaria, Delitschia, Didymella, Massaria, Massarina, Melanomma, Montagnula, Morosphaeria, Phaeotrichum, Sporormia, Leptosphaeria, Phaeosphaeria, Pleospora, Tetraplosphaeria). |
| ●●●●●●● Incertae sedis Dothideomycetes: Containing Acrospermales (Acrospermum, Oomyces), Botryosphaeriales (Botryosphaeria, Guignardia, Saccharata), Patellariales (Baggea, Patellaria), Trypetheliales (Laurera, Trypethelium), Venturiales (Apiosporina, Sympoventuria, Venturia). |
| ●●●●●●Eurotiomycetes O. E. Eriksson & Winka 1997, emend. Geiser et al. 2006 (R) Morphologically heterogeneous, circumscribed using phylogenetic re-delimitation to contain Chaetothyriomycetidae, Eurotiomycetidae, and Mycocaliciomycetidae. Important industrially and medically; saprobic, pathogenic on animals and rarely on plants, some lineages lichenized. |
| ●●●●●●●Chaetothyriomycetidae Doweld 2001 Ascomata perithecial, superficial or immersed within a thallus; asci are usually thick-walled and fissitunicate, rarely evanescent and sometimes accompanied by pseudoparaphyses; species lichenized, parasitic (especially on other fungi) or saprobic. Containing Chaetothyriales (Capronia, Chaetothyrium), Pyrenulales (Granulopyrenis, Pyrenula, Pyrgillus), Verrucariales (Endocarpon, Flakea, Staurothele, Verrucaria). |
| ●●●●●●●Eurotiomycetidae Doweld 2001, emend. Geiser & Lutzoni 2007 Ascomata, when present, usually cleistothecial/gymnothecial, globose, often produced in surrounding stromatic tissue and brightly coloured; hamathecial elements lacking; gametangia usually undifferentiated and consisting of hyphal coils; asci usually evanescent, sometimes bitunicate, scattered throughout the ascoma, rarely from a hymenium; ascospores usually single-celled, lenticular, sometimes spherical or elliptical; anamorphs variable, including phialidic and arthroconidial forms; saprotrophic, parasitic and mycorrhizal. Containing Coryneliales (Caliciopsis, Corynelia, Eremascus), Eurotiales (Aspergillus, Elaphomyces, Eurotium, Monascus, Penicillium), Onygenales (Ajellomyces, Arachnomyces, Arthroderma, Ascosphaera, Eremascu, Gymnoascus, Onygena). |
| ●●●●●●●Mycocaliciales Tibell & Wedin 2000 [Mycocaliciomycetidae Tibell 2007] Ascomata disciform, stalked or sessile; excipulum cupulate, and like the stalk hyphae at least in part sclerotized; spore dispersal active, more rarely passive and ascomata then with amoderately developed mazaedium; asci unitunicate, cylindrical, mostly with a distinctly thickened apex, 8-spored; ascospores pale to blackish brown, ellipsoidal or spherical to cuboid, non-septate or transversely 1–7-septate; spore wall pigmented, smooth or with an ornamentation formed within the plasmalemma; vulpinic acid derivatives occur in a few species; a variety of coelomycetous and hyphomycetous anamorphs occur; parasites or commensals on lichens or saprobes. Chaenothecopsis, Mycocalicium, Sphinctrina. |
| ●●●●●●Geoglossaceae Corda 1838, emend. Schoch et al. 2009 [Geoglossales Zheng Wang et al. 2009; Geoglossomycetes Zheng Wang et al. 2009] Ascomata scattered to gregarious, capitate, stipitate; stipe cylindrical, black, smooth to furfuraceous; ascigerous portion capitate, club-shaped to pileate, indistinguishable from stipe; hymenium surface black, continues with stipe at early development stage; asci clavate, inoperculate, thin-walled, J+, usually 8-spored; ascospores elongate, dark-brown, blackish to hyaline, septate when mature; paraphyses filiform, blackish to hyaline; global distribution, terrestrial, habitat usually boggy and mossy. Geoglossum, Trichoglossum. |
| ●●●●●●Laboulbeniomycetes Engler 1898 Mycelium absent except in Pyxidiophorales; cellular thallus hyaline to dark, with basal haustorium present; ascomata perithecial, surrounded by complex appendages, translucent, ovoid, thin-walled; interascal tissue absent; asci few and basal, not fissitunicate, clavate, thin-walled, evanescent, maturing sequentially, usually with four ascospores; ascospores two-celled, hyaline, elongate, one end modified as attachment to host; anamorphs hyphomycetous, spermatial; ectoparasitic on insects, some may be coprophilous. Containing Laboulbeniales (Ceratomyces, Chitonomyces, Euceratomyces, Herpomyces, Laboulbenia), Pyxidiophorales (Mycorhynchidium, Pyxidiophora). |
| ●●●●●●Lecanoromycetes O. E. Eriksson & Winka 2001 Ascomata apothecial, discoid, perithecial or elongated, sometimes stalked or immersed, occasionally evanescent; interascal tissue of simple or branched paraphyses swollen at the apices, often with a pigmented or iodine-staining epithecium; hymenial gel often present; asci not fissitunicate, but thick-walled, with a thickened, cap-like apex, often with an internal apical ocular chamber; ascus walls and thickened apex often stains blue with iodine; ascospores one to several septate, occasionally, multiseptate, rarely plurilocular, hyaline or pigmented; anamorphs pycnidial where known; mostly lichen forming with protococcoid algae, with thallus foliose, fructicose, crustose or occasionally absent; some lichenicolous, some saprobic. |
| ●●●●●●●Acarosporaceae Zahlbruckner 1906 [Acarosporomycetidae Reeb et al. 2004; Acarosporales Reeb et al. 2007] Thallus crustose, squamulose, rarely foliose-umbilicate; photobiont chlorococcoid; ascomata immersed or sessile, in form of apothecia (cryptolecanorine, lecanorine, or lecideine, more rarely biatorine or pseudolecanorine), rarely in form of perithecia; ascospores generally more than a 100 per ascus, simple and colorless, without halo; paraphyses moderately or slightly branched-anastomosed; asci bitunicate, functionally unitunicate, non-amyloid or slightly amyloid tholus, presence of ocular chamber. Acarospora, Pleopsidium. |
| ●●●●●●●Lecanoromycetidae P. M. Kirk et al. ex Miądl 2007 Thallus varied; ascomata almost always apothecial, flat to strongly cup-shaped, with or without a thalline margin, rarely mazaedial; interascal tissue of paraphyses, usually branched and swollen at the apices often with a pigmented or J+ epithecium, rarely absent; asci typically with a single wall layer visible in light microscope but thick-walled, almost always with a conspicuous thick caplike apical part, often with complex apical strucutres, often J+, rarely thin-walled and evanescent; ascospores varied; mainly lichen forming, almost all with protococcoid green photopbionts; some lichenicolous or saprobes, then especially on wood in xeric situations. Containing Lecanorales (Calicium, Cladonia, Lecanora, Parmelia, Porpidia, Physcia, Rhizocarpon, Usnea), Peltigerales (Coccocarpia, Collema, Lobaria, Nephroma, Pannaria, Peltigera, Placynthium), and Teloschistales (Letrouitia, Megalospora, Teloschistes). |
| ●●●●●●●Incertae sedis Lecanoromycetes: Candelariales (Candelaria, Candelariella), Umbilicariales (Lasallia, Umbilicaria). |
| ●●●●●●●Ostropomycetidae Reeb et al. 2004 Thallus crustose, squamulose or filamentose; photobiont chlorococcoid or trentepohlioid; ascomata immersed, sessile or pedunculate in form of apothecia (cryptolecanorine lecanorine, rarely lecideine-immersed) or in form of perithecia; ascospores eight or fewer per ascus, colorless, simple, transversely septate or muriform; paraphyses sensu lato simple or more or less branched-anastomose; asci unitunicate or bitunicate but functionally unitunicate, lacking tholus and, if tholus present, amyloid or not, with or without ocular chamber; lichenized and non-lichenized species. Containing Agyriales (Agyrium, Anamylopsora), Baeomycetales (Baeomyces), Ostropales (Coenogonium, Graphis, Gyalecta, Gyalidea, Odontotrema, Porina, Sagiolechia, Stictis), and Pertusariales (Aspicilia, Ochrolechia, Pertusaria, Icmadophila). |
| ●●●●●●Leotiomycetes O. E. Eriksson &Winka 1997 Ascomata apothecial, discoid, cleistothecial, elongated or rarely absent; apothecia stalked or sessile, frequently fleshy, sometimes hairy or with appendages, occasionally stromatic or sclerotioid; interascal tissue of simple paraphyses or absent; peridium thin-walled; asci typically inoperculate, cylindrical, thin-walled, not fissitunicate, occasionally with apical pore; apical apparatus variable; ascospores aseptate or transversely septate, hyaline or pigmented and longitudinally slightly asymmetrical; anamorphs occasionally present, hyphomycetous or coelomycetous; saprobes or plant parasites, some lichenized or lichenicolous. Containing Cyttariales (Cyttaria), Erysiphales (Blumeria, Erysiphe, Microsphaera, Oidium, Podosphaera), Helotiales (Botryotinia, Bulgaria, Dermea, Hyaloscypha, Lachnum, Leotia, Sclerotinia, Vibrissea), Rhytismatales (Ascodichaena, Cudonia, Rhytisma), and Thelebolales (Thelebolus, Antarctomyces). |
| ●●●●●●Lichinomycetes Reeb et al. 2004 Ascomata apothecial, discoid, sometimes immersed, occasionally clavate, stalked, setose, and fleshy; peridium often not well-defined; interascal tissue varied; hymenium often stains blue with iodine; asci thin-walled or apically thickened, not fissitunicate, without well-defined apical structures, usually with an iodine-staining outer gelatinized layer; ascospores one-septate or occasionally multiseptate, ellipsoidal to fusiform, hyaline or pigmented; anamorphs pycnidial; lichenized with cyanobacteria forming crustose, fruticose or foliose often gelatinized thalli. Containing Eremithallales (Eremithallus) and Lichinales (Lichina, Peltula). |
| ●●●●●●Orbiliaceae Nannfeldt 1932 [Orbiliales Baral et al. 2003; Orbiliomycetes Eriksson & Baral 2003] Ascomata apothecial, small, waxy, translucent or lightly pigmented; interascal tissue of simple paraphyses, usually with knob-like apices, united by a matrix; asci minute, not fissitunicate, apex truncate, with J apical rings, often forked at the base; ascospores minute, cylindrical, hyaline, often aseptate; anamorphs hyphomycetous where known; saprobic, often on wet wood. Halorbilia, Orbilia. |
| ●●●●●●Pezizales J. Schröter 1894 [Pezizomycetes O. E. Eriksson & Winka 1997] Ascomata apothecial or cleistothecial, usually visible with unaided eye, leathery or fleshy; carotenoids as bright colours to dark, sometimes present; interascal tissue present (paraphyses); asci not fissitunicate, usually elongated, cylindrical but more or less globose in cleistothecial species, thin-walled, lacking obvious apical wall thickening or apical apparatus, with operculum or vertical slit except in cleistothecial species, forcibly discharging ascospores except in cleistothecial species; ascospores usually ellipsoidal or globose, aseptate, hyaline to darkly pigmented, smooth or ornamented; anamorphs hyphomycetous, where known; saprobes on soil, dead wood or dung; some species hypogeous and mycorrhizal. Ascobolus, Ascodesmis, Caloscypha, Carbomyces, Chorioactis, Gyromitra, Helvella, Karstenella, Morchella, Peziza, Pyronema, Rhizina, Sarcoscypha, Sarcosoma, Tuber. |
| ●●●●●●Sordariomycetes O. E. Eriksson & Winka 1997 Defined using molecular phylogenetic methods, by a parsimony comparison of small subunit rRNA sequences, containing Boliniales (Bolinia, Camarops), Calosphaeriales (Calosphaeria, Pleurostoma), Chaetosphaeriales (Chaetosphaeria, Melanochaeta), Coniochaetales (Barrina, Coniochaeta), Diaporthales (Cryphonectria, Diaporthe, Gnomonia, Melanconis, Pseudovalsa, Schizoparme, Sydowiella, Valsa, Vialaea), Magnaporthales (Gaeumannomyces, Magnaporthe, Ophioceras), Ophiostomatales (Kathistes, Ophiostoma), Sordariales (Annulatascus, Cephalotheca, Chaetomium, Lasiosphaeria, Neurospora, Sordaria). |
| ●●●●●●●Incertae sedis Sordariomycetes: Koralionastetales (Koralionastes, Pontogeneia), Lulworthiales (Lindra, Lulworthia, Spathulospora), Meliolales (Armatella, Meliola), and Phyllachorales (Phaeochora, Phyllachora). |
| ●●●●●●●Hypocreomycetidae O. E. Eriksson & Winka 1997 Ascomata are perithecia or cleistothecia; interascal tissue consists of periphyses and/or periphysoids; asci, unitunicate or pseudoprototunicate. Containing Coronophorales (Bertia, Chaetosphaerella, Coronophora, Nitschkia, Scortechinia), Glomerellales (Australiasca, Colletotrichum, Glomerella, Reticulascus), Hypocreales (Bionectria, Cordyceps, Claviceps, Fusarium, Hypocrea, Nectria, Sphaerodes, Stachybotrys), Melanosporales (Melanospora, Sphaerodes), Microascales (Ceratocystis, Gondwanamyces, Halosphaeria Microascus, Pseudallescheria), and Savoryellales (Ascotaiwania, Ascothailandia, Savoryella). |
| ●●●●●●●Sordariomycetidae O.E. Eriksson & Winka 1997 (R) Without obvious synapomorphies. Containing Boliniales (Bolinia, Camarops), Calosphaeriales (Calosphaeria, Pleurostoma), Chaetosphaeriales (Chaetosphaeria, Melanochaeta), Coniochaetales (Barrina, Coniochaeta), Diaporthales (Cryphonectria, Diaporthe, Gnomonia, Melanconis, Pseudovalsa, Schizoparme, Sydowiella, Valsa, Vialaea), Magnaporthales (Gaeumannomyces, Magnaporthe, Ophioceras), Ophiostomatales (Kathistes, Ophiostoma), Sordariales (Annulatascus, Cephalotheca, Chaetomium, Lasiosphaeria, Neurospora, Sordaria). |
| ●●●●●●●Xylariales Nannfeldt 1932 [Xylariomycetidae O. E. Eriksson & Winka 1997] Stromata usually well-developed, mostly consisting only of fungal tissue; ascomata perithecial, rarely cleistothecial, globose, superficial or immersed in the stroma, usually black- and thick-walled, the ositole usually papillate, periphysate; interascal tissue well-developed, of narrow paraphyses; asci cylindrical, persistent, relatively thick-walled but without separable layers, with an often complex J+ apical ring, usually 8-spored, spherical in some cleistocarpus taxa and without apical apparatus; ascospores usually pigmented sometimes transversely septate, with germ pores or slits, sometimes with a mucous sheath or mucous appendages; anamorphs varied, usually hyphomycetous, some pycnidial; saprobes and plant parasites, mainly on bark and wood, some associated with termitaria, cosmopolitan. Amphisphaeria, Clypeosphaeria, Diatrype, Graphostroma, Hyponectria, Xylaria. |
| ●●●●Basidiomycota R. T. Moore 1980 Mycelium present, but some with a yeast state primarily in the Tremellomycetes; basidia produced in a fertile layer with or without fleshy sporocarp; basidia whole or divided longitudinally, typically with four spores per basidium but ranging from one to eight; fusion of compatible mycelia of opposite mating types results in a dikaryotic mycelium in which nuclei of the parent mycelia remain paired but not fused; karyogamy quickly followed by meiosis, one or more mitotic divisions and migration of the nuclei into the developing basidiospores; asexual reproduction may occur through production of conidiospores or via spores produced on basidia from nuclei that have not undergone karyogamy and meiosis (secondary homothallism); cell wall with xylose; septa with swelling near pore; septal pore caps (parenthesomes— multilayered endoplasmic reticulum) usually present, elaborate in Tremellomycetes; clamp connections present in hyphae or at base of basidia in some groups; mycelial or yeast states; karyogamy typically in probasidium or teliospore, followed by meiosis in a separate compartment (metabasidia), but in some it occurs in the same compartment (holobasidia); holobasidia remain whole or fragment at septation after meiosis (phragmobasidia); metabasidia typically transversely septate with basidiospore borne laterally; cell wall with xylose; parenthesome pore caps absent but with microbodies at septal pores; septal pores occluded by a plug; centrosome multilayered; many are plant pathogens (rusts), animal pathogens, non-pathogenic endophytes, and rhizosphere species. |
| ●●●●●Pucciniomycotina R. Bauer et al. 2006 [= Urediniomycetes Swann & Taylor 1995] Mycelial or yeast states; karyogamy typically in probasidium or teliospore, followed by meiosis in a separate compartment (metabasidia), but in some it occurs in the same compartment (holobasidia); holobasidia remain whole or fragment at septation after meiosis (phragmobasidia); metabasidia typically transversely septate with basidiospore borne laterally; cell wall with xylose; parenthesome pore caps absent but with microbodies at septal pores; septal pores occluded by a plug; centrosome multilayered; many are plant pathogens (rusts), animal pathogens, non-pathogenic endophytes, and rhizosphere species. |
| ●●●●●●Agaricostilbomycetes R. Bauer et al. 2006 Dimorphic, nonphytoparasitic members of the Pucciniomycotina having fucose as cell wall carbohydrate component, septal pores without associated microbodies, aseptate basidiospores during germination and no colacosomes, teliospores, curved holobasidia, and radiate conidia; septal pores without microbodies, nucleoplasmic spindle-pole body (SPB) separation, metaphasic SPB intranuclear. Containing Agaricostilbales (Agaricostilbum, Chionosphaera) and Spiculogloeales (Mycogloea, Spiculogloea). |
| ●●●●●● Atractiellales Oberwinkler & Bandoni, 1982 [Atractiellomycetes R. Bauer et al. 2006] Members of the Pucciniomycotina having symplechosomes. Atractiella, Phleogena, Saccoblastia. |
| ●●●●●● Classiculales R. Bauer et al. 2003 [Classiculomycetes R. Bauer et al. 2006] Members of Pucciniomycotina having septal pores associated with microbodies and tremelloid haustorial cells. Classicula, Jaculispora. |
| ●●●●●● Cryptomycocolax Oberwinkler & R. Bauer 1990 [Cryptomycocolacales Oberwinkler & R. Bauer 1990; Cryptomycocolacomycetes R. Bauer et al. 2006] Members of the Pucciniomycotina having colacosomes and septal pores with microbodies. Cryptomycocolax. |
| ●●●●●● Cystobasidiomycetes R. Bauer et al. 2006 Members of the Pucciniomycotina having a cell wall carbohydrate composition without fucose; cytoplasmic SPB separation; metaphasic SPBs intranuclear. Containing Cystobasidiales (Cystobasidium), Erythrobasidiales (Bannoa, Erythrobasidium), and Naohideales (Naohidea). |
| ●●●●●● Microbotryomycetes R. Bauer et al. 2006 Members of the Pucciniomycotina and related taxa having colacosomes and septal pores without microbodies; with colacosomes and taxa derived from colacosome fungi; metaphasic SPBs intranuclear. Containing Heterogastridiales (Heterogastridium), Leucosporidiales (Leucosporidiella, Mastigobasidium), Microbotryales (Microbotryum, Ustilentyloma), and Sporidiobolales (Rhodosporidium, Sporidiobolus). |
| ●●●●●● Mixia Kramer 1959 [Mixiales R. Bauer et al. 2006; Mixiomycetes R. Bauer et al. 2006] With multinucleate hyphae and multiple spores produced simultaneously on sporogenous cells. Mixia. |
| ●●●●●● Pucciniomycetes R. Bauer et al. 2006 Members of the Pucciniomycotina and related taxa having a metaphasic intermeiotic SPB duplication. Containing Helicobasidiales (Helicobasidium), Pachnocybales (Pachnocybe), Platygloeales (Eocronartium, Platygloea), Pucciniales (Coleosporium, Cronartium, Dasyspora, Diorchidium, Melampsora, Mikronegeria, Nyssopsora, Ochropsora, Phakopsora, Phragmidium, Pileolaria, Puccinia, Pucciniastrum, Pucciniosira), and Septobasidiales (Auriculoscypha, Septobasidium). |
| ●●●●● Ustilaginomycotina R. Bauer et al. 2006 Mycelial in the parasitic phase, and many with saprobic yeast or ballisticonidial states; plant parasites causing rusts and smuts; meiospores produced on septate or aseptate basidia; cell wall carbohydrates dominated by glucose; xylose absent; parenthesomes absent at septal pores; swellings absent at septal pores except in Tilletia; centrosomes globose, unlayered. |
| ●●●●●● Exobasidiomycetes Begerow et al. 2007 Members of Ustilaginomycotina having local interaction zones and no intracellular hyphal coils. Containing Ceraceosorales (Ceraceosorus),Doassansiales (Doassansia, Melaniella, Rhamphospora), Entylomatales (Entyloma), Exobasidiales (Kordyana, Laurobasidium, Exobasidium, Graphiola), GeorgeFischereriales (Eballistra, GeorgeFischereria, Gjaerumia, Tilletiaria), and Microstromatales (Tilletiales). |
| ●●●●●● Ustilaginomycetes R. Bauer et al. 1997 Members of Ustilaginomycotina having enlarged interaction zones. Containing Urocystales (Doassansiopsis, Floromyces, Thecaphora, Melanotaenium, Urocystis) and Ustilaginales (Anthracoidea, Ustanciosporium, Sporisorium, Ustilago). |
| ●●●●●● Incertae sedis Ustilaginomycotina: Malasseziales (Malassezia). |
| ●●●●● Agaricomycotina Doweld 2001 Members of the Basidiomycota having a type B secondary structure of the 5S RNA; a cell wall carbohydrate composition with dominance of glucose and presence of xylose. |
| ●●●●●● Agaricomycetes Doweld 2001 Fruiting bodies hymenomycetous or gasteroid; basidia two- to eight-spored; parenthesomes perforate or imperforate. The least-inclusive clade containing Auriculariales, Sebacinales, Cantharellales, Phallomycetidae, and Agaricomycetidae. |
| ●●●●●●●Incertae sedis Agaricomycetes: Auriculariales (Auricularia, Exidia, Hyaloria), Cantharellales (Botryobasidium, Cantharellus, Ceratobasidium, Clavulina, Hydnum, Tulasnella), Corticiales (Corticium, Punctularia), Gloeophyllales (Gloeophyllum), Hymenochaetales (Hymenochaete), Polyporales (Antrodia, Coriolopsis, Donkiopora, Ganoderma, Lentinus, Phanerochaete, Phlebia, Polyporus, Sparassis, Trametes), Russulales (Heterobasidion, Lactarius, Peniophora, Russula), Sebacinales (Piriformospora, Sebacina), Thelephorales (Hydnellum, Sarcodon), and Trechisporales (Trechispora). |
| ●●●●●●● Agaricomycetidae Parmasto 1986, emend. Hibbett et al. 2007 Morphologically heterogenous. Circumscribed using phylogenetic re-delimitation, containing Agaricales (Agaricus, Amanita, Armillaria, Cortinarius, Clavaria, Hygrophorus, Marasmius, Pluteus, Pterula, Stephanospora, Schizophyllum, Tricholoma, Hygrophorus), Amylocorticiales (Amylocorticium), Atheliales (Athelia), Boletales (Boletus, Coniophora, Scleroderma, Suillus, Tapinella), Jaapiales (Jaapia). |
| ●●●●●●● Phallomycetidae K. Hosaka et al. 2007 [= Phallales sensu Kirk et al. 2001] Basidioma at first subglobose, ovoid or pyriform and limited by a peridium covering a more or less developed gelatinous layer, but subsequent development varies. Containing Geastrales (Geastrum), Gomphales (Gautieria, Gomphus), Hysterangiales (Hysterangium), Phallales (Clathrus, Phallus). |
| ●●●●●●● Dacrymycetales Hennings 1898 [Dacrymycetes Doweld 2001] Fruiting bodies gelatinous; basidia furcate, rarely unisporous; parenthesomes imperforate. Cerinomyces, Dacrymyces. |
| ●●●●●●● Tremellomycetes Doweld 2001 Dimorphic fungi; fruiting bodies gelatinous or absent; basidia septate or nonseptate; parenthesomes sacculate or absent. Containing Cystofilobasidiales (Cystofilobasidium, Mrakia), Filobasidiales (Filobasidium), Holtermanniales (Holtermannia), and Tremellales (Sirobasidium, Syzygospora, Tremella). |
| ●●●●● Entorrhizales R. Bauer & Oberwinkler 1997 [Entorrhizomycetes Begerow et al. 2007] Phytoparasitic fungi forming intracellular septate hyphal coils with terminal teliospores. Entorrhiza, Talbotiomyces. |
| ●●●●● Wallemia Johan-Olsen 1887 [Wallemiales Zalar et al. 2005; Wallemiomycetes Zalar et al. 2005] Unbranched conidiophores or sympodially proliferating, continuous with conidiogenous cells, smooth-walled; conidiogenous cells verruculose, basauxially extending, distally disarticulating into arthrospore-like conidia; conidia are verruculose, short cylindrical, becoming spherical; hyphal septa have a single pore, flaring out near the periphery of the pore and are barrel-shaped, dolipore-like; typically xerophilic. |
| ●● Rozella Cornu 1872 [=Rozellida Lara et al. 2010; Cryptomycota M. D. M. Jones & T. A. Richards 2011] Unicellular, zoospores single-celled with a single cilium; cysts without a chitin/cellulose cell wall; forming epibiontic associations. Contains numerous diverse lineages currently poorly defined by morphology. Rozella. |
| Sar (R) The least inclusive clade containing Bigelowiella natans Moestrup & Sengco 2001 (Rhizaria), Tetrahymena thermophila Nanney & McCoy 1976 (Alveolata), and Thalassiosira pseudonana Cleve 1873 (Stramenopiles). This is a node-based definition in which all of the specifiers are extant; it is intended to apply to a crown clade; qualifying clause – the name does not apply if any of the following fall within the specified clade – Homo sapiens Linnaeus 1758 (Opisthokonta), Dictyostelium discoideum Raper 1935 (Amoebozoa), Arabidopsis thaliana (Linnaeus) Heynhold 1842 (Archaeplastida), Euglena gracilis Klebs 1883 (Excavata), Emiliania huxleyi (Lohmann) Hay & Mohler in Hay et al. 1967 (Haptophyta). The name is derived from the acronym of the three groups united in this clade. The apparent composition of Sar is: Alveolata, Rhizaria, and Stramenopiles, as defined here. The primary reference phylogeny is Burki et al. (2008: Fig. 1). |
| ●Stramenopiles Patterson 1989, emend. Adl et al. 2005 Motile cells typically biciliate, typically with heterokont ciliation – anterior cilium with tripartite mastigonemes in two opposite rows and a posterior usually smooth cilium; tubular mitochondrial cristae; typically 4 microtubular kinetosomal roots. |
| ●●Opalinata Wenyon 1926, emend. Cavalier-Smith 1997 [Slopalinida Patterson 1985] Pluriciliated with double-stranded transitional helix at the transitional region between kinetosome and cilium; evenly spaced cortical ridges underlain by microtubules, ranging from singlets to ribbons; cyst-forming. |
| ●●●Proteromonadea Grassé 1952 (P?) One or two anterior pairs of anisokont cilia; uninucleate; endobionts in intestinal tract of amphibians, reptiles, and mammals. Karotomorpha, Proteromonas. |
| ●●●Opalinea Wenyon 1926 Multiciliated cells with cilia originating from an anterior morphogenetic center, the falx, and forming oblique longitudinal rows or files; microtubular ribbons supporting longitudinal pellicular ridges between ciliary rows; two to many monomorphic nuclei; endobionts in amphibians and some fish; life cycle complex, with sexual processes induced by hormones of host and linked to the host’s life cycle. Cepedea, Opalina, Protoopalina, Protozelleriella, Zelleriella. |
| ●●Blastocystis Alexeev 1911 Rounded non-ciliated cells, anaerobic commensals/parasites of intestinal tracts; recovered as sister group to Opalinata in molecular phylogenies. Blastocystis. |
| ●●Bicosoecida Grassé 1926, emend. Karpov 1998 Biciliate with or without tripartite mastigonemes, typically lacking transitional helix; without plastids; phagotrophic with cytostome, supported by broad microtubular rootlet No. 2 of posterior cilium; predominantly sedentary, often attach to substrate with posterior cilium; with or without lorica; solitary and colonial. Bicosoeca, Caecitellus, Cafeteria, Pseudobodo, Pseudodendromonas. |
| ●●Placidida Moriya et al. 2002 Biciliate cells without plastids; described species have mastigonemes on anterior cilium, attach to substrates by posterior cilium during feeding; double-stranded transitional helix. Placidia, Wobblia. |
| ●●Labyrinthulomycetes Dick 2001 Producing an ectoplasmic network of branched, anastomosing, wall-less filaments via a specialized organelle known as the bothrosome; Golgi-derived scales; biciliate zoospores with lateral insertion in many species. |
| ●●● Labyrinthula Cienkowski 1867 [Labyrinthulaceae Haeckel 1868] Spindle-shaped vegetative cells distributed in an extensive ectoplasmic net; zoospores with eyespots; sexual reproduction. Labyrinthula. |
| ●●●Thraustochytriaceae Sparrow 1943 Cells producing a small ectoplasmic net; presence of interphase centrioles in vegetative cells; no eyespots; no sexual reproduction. Althornia, Aplanochytrium, Elnia, Japonochytrium, Schizochytrium, Thraustochytrium, Ulkenia. |
| ●●●Incertae sedis Labyrinthulomycetes: Amphitraemidae previously in filose testate amoebae Amphitrema and Archerella. |
| ●●Hyphochytriales Sparrow 1960 Single anteriorly directed cilium. |
| ●●●Anisolpidiaceae Karling 1943, emend. Dick 2001 Thallus holocarpic. Anisolpidium, Canteriomyces. |
| ●●● Hyphochytrium Karling 1939 [Hyphochytridiomycetaceae Fischer 1892, emend. Karling 1939] Thallus eucarpic and polycentric. Hyphochytrium. |
| ●●●Rhizidiomycetaceae Karling 1943 Thallus eucarpic and monocentric. Latrostium, Rhizidiomyces, Rhizidiomycopsis. |
| ●●Peronosporomycetes Dick 2001 [Öomycetes Winter 1897, emend. Dick 1976] Thallus mainly aseptate; cell wall of glucan-cellulose, may have minor amount of chitin; haplomitic-B nuclear cycle; lysine synthesized via the DAP pathway; lanosterol directly from swaline oxide; zoospores biciliate and heterokont but rarely uniciliate; cilia anteriorly inserted; anteriorly directed cilium shorter; transitional plate of kinetosome sitting above the plasma membrane with a central bead; kinetid base structure with six parts, including four roots; oogamous reproduction that results in the formation of thick-walled sexual spores known as öospores, due to contact between male and female gametangia. Achyla, Leptomitus, Peronospora, Pythium, Phytophthora, Rhipidium, Saprolegnia. |
| ●●● Incertae sedis Peronosporomycetes: Ciliomyces, Crypticola, Ectrogella, Eurychasma, Haptoglossa, Lagena, Lagenisma, Myzocytiopsis, Olpidiopsis, Pontisma, Pythiella, Rozellopsis, Sirolpidium. |
| ●●Actinophryidae Claus 1874, emend. Hartmann 1926 Axonemal pseudopodia emerging from amorphous centrosome near nuclei; axonemal microtubules in double interlocking coils; single central nucleus or several peripheral nuclei; tubular mitochondrial cristae; two types of extrusomes for prey-capture along axopodia; cysts covered with siliceous elements; autogamy reported within spores. Actinophrys, Actinosphaerium. |
| ●●Bolidomonas Guillou & Chrétiennot-Dinet 1999 [Bolidophyceae in Guillou et al. 1999] Swimming cells with two cilia, one anteriorly directed and one posteriorly directed; no microtubular or fibrillar kinetosomal roots; ciliary transitional helix absent; no paraciliary rod; chloroplast with girdle lamella; outer chloroplast endoplasmic reticulum membrane with direct membrane connection to the outer nuclear envelope membrane; plastid DNA with ring-type genophore; no eyespot; plastid pigments include chlorophylls a and c1–3, fucoxanthin, 19'-butanoyloxyfucoxanthin, diatoxanthin, and diadinoxanthin. Bolidomonas. |
| ●●Chrysophyceae Pascher 1914 Predominately ciliated cells, but also capsoid, coccoid, filamentous, and parenchymatous forms; swimming cells with biciliated – one anteriorly directed and one laterally directed; tripartite mastigonemes with short and long lateral hairs on the shaft; kinetosome usually with 4 microtubular kinetosomal roots and one large striated root or rhizoplast; ciliary transitional helix with 4--6 gyres located above the major transitional plate; no paraciliary rod; cell coverings, when present, include organic scales, silica scales, organic lorica, and cellulose cell wall; chloroplast with girdle lamella; outer chloroplast endoplasmic reticulum membrane with direct membrane connection to the outer nuclear envelope membrane; plastid DNA with ring-type genophore; eyespots present or absent; plastid pigments include chlorophylls a and c1,2, fucoxanthin, violaxanthin, anthaxanthin, and neoxanthin. |
| ●●●Chromulinales Pascher 1910 Swimming cells with only one cilium visible by light microscopy; four microtubular kinetosome roots. Chromulina, Chrysomonas. |
| ●●●Hibberdia Andersen 1989 [Hibberdiales Andersen 1989] Swimming cells with only one cilium visible by light microscopy; three microtubular kinetosomal roots. Hibberdia. |
| ●●●Ochromonadales Pascher 1910 Swimming cells with two cilia visible by light microscopy. Ochromonas. |
| ●●Dictyochophyceae Silva 1980 Single cells, colonial ciliated cells or amoebae; swimming cells usually with one cilium, anteriorly directed and bearing tripartite tubular hairs; kinetosomes adpressed to nucleus; no microtubular or fibrillar kinetosomal roots; ciliary transitional helix, when present, with 0--2 gyres located below the major transitional plate; paraciliary rod present; cells naked, with organic scales or with siliceous skeleton; chloroplasts, when present, with girdle lamella; plastid DNA with scattered granule-type genophore; no eyespot; plastid pigments include chlorophylls a and c1,2, fucoxanthin, diatoxanthin, and diadinoxanthin. |
| ●●●Dictyochales Haeckel 1894 Silica skeleton present on at least one life stage; with chloroplasts. Dictyocha. |
| ●●●Pedinellales Zimmermann et al. 1984 Naked, organically scaled or loricate ciliates; with or without chloroplasts. Actinomonas, Apedinella, Ciliophrys, Mesopedinella, Palatinella, Pedinella, Pseudopedinella, Pteridomonas. |
| ●●●Rhizochromulinales O'Kelly & Wujek 1994 Vegetative cells amoeboid; zoospore ciliated; with chloroplasts. Rhizochromulina. |
| ●●Eustigmatales Hibberd 1981 Coccoid organisms, single cells or colonies; swimming cells biciliate – one anteriorly directed and one posteriorly directed; 4 microtubular kinetosomal roots and one large striated kinetosome root or rhizoplast; ciliary transitional helix with 6 gyres located above the major transitional plate; no paraciliary rod; cell walls present; chloroplast without girdle lamella; outer chloroplast endoplasmic reticulum membrane with direct membrane connection to the outer nuclear envelope membrane; plastid DNA with ring-type genophore; eyespot present but located outside of the chloroplast; plastid pigments include chlorophylls a, violaxanthin, and vaucherioxanthin. Botryochloropsis, Eustigmatos, Monodopsis, Nannochloropsis, Pseudocharaciopsis, Vischeria. |
| ●●Pelagophyceae Andersen & Saunders 1993 Ciliated, capsoid, coccoid, sarcinoid or filamentous; swimming cells with 1 or 2 cilia – anteriorly directed cilium bearing bipartite or tripartite tubular hairs and second cilium, when present, directed posteriorly; kinetosome(s) adpressed to nucleus; no microtubular or fibrillar kinetosomal roots on uniciliate cells; four microtubular roots on biciliated cells; ciliary transitional helix, when present, with 2 gyres located below the major transitional plate; paraciliary rod present or absent; cells naked or with organic thecae or cell walls; chloroplasts with girdle lamella; plastid DNA with scattered granule-type genophore; no eyespot; plastid pigments include chlorophylls a and c1,2, fucoxanthin, 19'-hexanoyloxyfucoxanthin, 19'-butanoyloxyfucoxanthin, diatoxanthin, and diadinoxanthin. |
| ●●●Pelagomonadales Andersen & Saunders 1993 Ciliated or coccoid organisms; when ciliated, a single cilium without a second kinetosome; no kinetosomal roots. Aureococcus, Aureoumbra, Pelagococcus, Pelagomonas. |
| ●●●Sarcinochrysidales Gayral & Billard 1977 Sarcinoid, capsoid, ciliated or filamentous; cells typically with organic cell wall; ciliate cells biciliated with four microtubular kinetosomal roots. Ankylochrisis, Nematochrysopsis, Pulvinaria, Sarcinochrysis. |
| ●●Phaeothamniophyceae Andersen & Bailey in Bailey et al. 1998 Filamentous, capsoid, palmelloid, ciliated, or coccoid; swimming cells biciliated – anteriorly directed cilium bearing tripartite tubular hairs and posteriorly directed cilium without tripartite hairs; 4 microtubular kinetosomal roots but no striated kinetosomal root or rhizoplast; ciliary transitional helix with 4--6 gyres located above the major transitional plate; no paraciliar rod; cells covered with an entire or two-pieced cell wall; chloroplast with girdle lamella; chloroplast endoplasmic reticulum membrane with direct membrane connection to the outer nuclear envelope membrane; plastid DNA with ring-type genophore; eyespots present; plastid pigments include chlorophylls a and c, fucoxanthin, heteroxanthin, diatoxanthin, and diadinoxanthin. |
| ●●●Phaeothamniales Bourrelly 1954, emend. Andersen & Bailey in Bailey et al. 1998 (R) Distinguished from the Pleurochloridales based upon molecular phylogenetic analyses. Phaeothamnion. |
| ●●●Pleurochloridales Ettl 1956 (R) Distinguished from the Phaeothamniales based upon molecular phylogenetic analyses. Pleurochloridella. |
| ●●Pinguiochrysidales Kawachi et al. 2003 Ciliated or coccoid organisms; swimming cells with one or two cilia; tripartite hairs present or absent on immature cilium; 3--4 microtubular kinetosomal roots and one large striated kinetosomal root or rhizoplast; ciliary transitional helix with 2 gyres located below the major transitional plate; no paraciliary rod; cells naked or enclosed in mineralized lorica; chloroplast with girdle lamella; outer chloroplast endoplasmic reticulum membrane with direct membrane connection to the outer nuclear envelope membrane; plastid DNA with scattered granule-type genophore; eyespots absent; plastid pigments include chlorophylls a and c1,2, fucoxanthin, and violaxanthin. Glossomastix, Phaeomonas, Pinguiochrysis, Pinguiococcus, Polypodochrysis. |
| ●●Raphidophyceae Chadefaud 1950, emend. Silva 1980 Naked swimming biciliates with one anteriorly-directed cilium bearing tripartite tubular hairs and one posteriorly-directed cilium lacking tripartite hairs; microtubular kinetosomal roots present but poorly characterized; one large striated kinetosomal root or rhizoplast present; ciliary transitional helix absent; no paraciliary rod; chloroplast with or without girdle lamella; outer chloroplast endoplasmic reticulum membrane with no or very weak direct membrane connection to the outer nuclear envelope membrane; plastid DNA with scattered granule-type genophore; eyespots absent; plastid pigments include chlorophylls a and c1,2; carotenoid composition distinctly different between marine (M) and freshwater (FW) species - fucoxanthin (M), violaxanthin (M), heteroxanthin (FW), vaucherioxanthin (FW). Chatonella, Fibrocapsa, Goniostomum, Haramonas, Heterosigma, Merotricha, Olisthodiscus, Vacuolaria. |
| ●●Schizocladia Kawai et al. 2003 [Schizocladales Kawai et al. 2003] (M) Filamentous or biciliated; swimming biciliates with one anteriorly-directed cilium bearing tripartite tubular hairs and one laterally- or posteriorly-directed cilium lacking tripartite hairs; microtubular and striated roots undescribed; ciliary transitional helix with 6 gyres located above the major transitional plate; no paraciliary rod; cell walls present, lacking alginates; chloroplasts with girdle lamella; outer chloroplast endoplasmic reticulum membrane with direct membrane connection to the outer nuclear envelope membrane; plastid DNA with ring-type genophore; eyespot present; plastid pigments include chlorophylls a and c and fucoxanthin. Schizocladia ischiensis. |
| ●●Synurales Andersen 1987 Predominately ciliated cells but benthic palmelloid colonies known; swimming cells usually with two anteriorly-directed cilia – one bearing tripartite tubular hairs with short and long lateral hairs on their shafts, two microtubular kinetosomal roots and one large striated kinetosomal root or rhizoplast; ciliary transitional helix with 6--9 gyres located above the major transitional plate; no paraciliar rod; cells covered with bilaterally symmetrical silica scales; chloroplast with girdle lamella; chloroplast endoplasmic reticulum membrane with no (or very weak) direct membrane connection to the outer nuclear envelope membrane; plastid DNA with ring-type genophore; eyespots absent; plastid pigments include chlorophylls a and c1, fucoxanthin, violaxanthin, anthaxanthin, and neoxanthin. Chrysodidymus, Mallomonas, Synura, Tesselaria. |
| ●●Xanthophyceae Allorge 1930, emend. Fritsch 1935 [Heterokontae Luther 1899, Heteromonadea Leedale 1983, Xanthophyta Hibberd 1990] Predominately coccoid or filamentous, rarely amoeboid, ciliated or capsoid; swimming cells with two cilia – one anteriorly-directed and bearing tripartite tubular hairs and one posteriorly-directed and lacking tripartite hairs; 4 microtubular kinetosomal roots and one large striated kinetosome root or rhizoplast; ciliary transitional helix with 6 apparently double gyres located above the major transitional plate; no paraciliar rod; cell walls typical, probably of cellulose and either entire or H-shaped bisectional walls; chloroplast with girdle lamella; outer chloroplast endoplasmic reticulum membrane with direct membrane connection to the outer nuclear envelope membrane; plastid DNA with ring-type genophore; eyespots present or absent; plastid pigments include chlorophylls a and c1,2, violaxanthin, heteroxanthin, and vaucherioxanthin. Note 3. |
| Note 3. Traditional subdivisions do not form monophyletic groups. Two convenient subdivisions are presented here for purpose of classification. |
| ●●●Tribonematales Pascher 1939 Filamentous, coccoid, and capsoid forms, sometimes becoming parenchymatous or multinucleate with age; cell walls, when present, either with H-shaped overlapping cell wall pieces or with complete or entire cell walls; elaborate reproductive structures lacking. Botrydium, Bumilleriopsis, Characiopsis, Chloromeson, Heterococcus, Ophiocytium, Sphaerosorus, Tribonema, Xanthonema. |
| ●●●Vaucheriales Bohlin 1901 Siphonous filaments; elaborate sexual reproductive structures as antheridia and öogonia. Vaucheria. |
| ●●Phaeophyceae Hansgirg 1886 (not Kjellman 1891, not Pfitzer 1894) Filamentous, syntagmatic, parenchymatous or ciliated; swimming cells with two cilia usually inserted laterally – one anteriorly-directed and one posteriorly-directed; usually 4 microtubular kinetosomal roots but no striated kinetosomal root or rhizoplast; ciliary transitional helix typically with 6 gyres located above the major transitional plate; no paraciliary rod; little to no substantial tissue differentiation occurring in parenchymatous forms; cell wall present, containing alginate compounds and cellulose; plasmodesmata or pores between cells in parenchymatous forms; chloroplasts with girdle lamella; outer chloroplast endoplasmic reticulum membrane with direct membrane connection to the outer nuclear envelope membrane; plastid DNA with ring-type genophore; eyespots present or absent; plastid pigments include chlorophylls a and c1,2, fucoxanthin, and violaxanthin. Note that several subdivisions are separated on the basis of life history and gene sequence information, but taxonomic classification is still in flux. |
| ●●●Ascoseirales Petrov 1964 Sporophyte parenchymatous, with intercalary growth; several scattered discoid plastids without pyrenoid; heteromorphic life cycle but gametophyte not free-living; isogamous sexual reproduction. Ascoseira. |
| ●●●Desmarestiales Setchell & Gardner 1925 Gametophyte small and filamentous; sporophyte larger and pseudo-parenchymatous; several scattered discoid plastids with no pyrenoid; trichothallic growth; heteromorphic life cycle; oogamous sexual reproduction. Arthrocladia, Desmarestia (P), Himantothallus, Phaeurus. |
| ●●●Dictyotales Bory de Saint-Vincent 1828 Gametophyte and sporophyte parenchymatous, with apical or marginal growth; several scattered discoid plastids without pyrenoid; isomorphic life cycle; oogamous sexual reproduction. Dictyota, Dilophus, Lobophora, Padina, Stypopodium, Taonia, Zonaria. |
| ●●●Discosporangiales Kawai et al. 2007 Simple branched filaments with apical growth; plastids multiple, discoid, without pyrenoids; species lack heterotrichy and phaeophycean hairs. Note that the early divergence of this group from other brown algae is reflected in their continuous division and elongation of vegetative cells. Choristocarpus, Discosporangium. |
| ●●●Ectocarpales Bessey, 1907, emend. Silva & Reviers, 2000 Gametophyte and sporophyte uniseriate filaments, either branched or unbranched, with diffuse growth; one or more ribbon-shaped plastids with pyrenoid; isomorphic life cycle; isogamous, anisogamous or oogamous sexual reproduction. Adenocystis, Acinetospora, Chordaria, Ectocapus, Scytosiphon. |
| ●●●● Incertae sedis Ectocarpales: Asterocladon, Asteronema. |
| ●●●Fucales Bory de Saint-Vincent 1927 Sporophyte parenchymatous, with apical cell growth; several scattered discoid plastids without pyrenoid; diploid life stage only with meiosis producing gametes; (mostly) oogamous sexual reproduction. Ascophyllum, Bifurcaria, Cystoseira, Druvillaea, Fucus, Hormosira, Sargassum, Turbinaria. |
| ●●●Ishige Yendo 1907 [Ishigeacea Okamura 1935, Ishigeales Cho et al. 2004] Isomorphic alternation of generations, with apical cell growth; scattered discoid plastids without pyreniods; terminal unilocular sporangia or uniseriate plurilocular sporangia; cortex pseudoparenchymatous with assimilatory filaments; phaeophycean hairs in cryptostigmata. Ishige. |
| ●●●Laminariales Migula 1908 Gametophyte small and filamentous with apical growth; sporophyte large and parenchymatous, with intercalary growth; several scattered discoid plastids without pyrenoid; heteromorphic life cycle; oogamous sexual reproduction with eggs sometimes ciliated. Akkesiophycus, Alaria, Chorda, Costaria, Laminaria, Lessonia, Pseudochoda. |
| ●●● Nemoderma Schousboe ex Bonnet 1892 [Nemodermatales Parente et al. 2008] (M) Encrusting heterotrichous thalli; numerous discoid plastids per cell without pyrenoids; isomorphic life cycle; anisogomous gametes; plurilocular reproductive structures are lateral, whereas unilocular sporangia are intercalary. Nemoderma tingitanum. |
| ●●●Onslowiales Draisma & Prud’homme van Reine 2008 An irregularly branched oligostichous thallus, both branches and reproductive structures are the result of lateral divisions from thallus cells; prominent apical cell lacking transverse division of subapical cells; multiple discoid plastids without pyrenoid; isomorphic life cycle; sexual?? reproduction can occur by plurilocular or unilocular sporangia, or via vegetative propagules lacking a central apical cell. Onslowia. |
| ●●●Ralfsiales Nakamura ex Lim & Kawai 2007 Crustose in at least one phase of the life history or via a disc-type germination; plastids without pyrenoids, from one to many per cell; plurilocular sporangium are intercalary and have one or more terminal sterile cells. Lithoderma, Neoralfsia, Pseudolithoderma, Ralfsia. |
| ●●●Scytothamnales Peters & Clayton 1998 Gametophyte large and parenchymatous, with intercalary growth; sporophyte small and filamentous, with apical growth; 1 or more stellate or axial plastids with pyrenoid; heteromorphic alternation of generations; anisogamous sexual reproduction. Scytothamnus, Splachnidium, Stereocladon. |
| ●●●Sphacelariales Migula 1908 Gametophyte and sporophyte branched multiseriate filaments, with apical growth; several scattered discoid plastids without pyrenoid; usually an isomorphic alternation of generations; isogamous, anisogamous or oogamous sexual reproduction. Chaetopteris, Halopteris, Onslowia, Stypocaulon, Sphacelaria, Verosphacella. |
| ●●●Sporochnales Sauvageau 1926 Gametophyte and larger sporophyte pseudoparenchymatous, with trichothallic growth; several scattered discoid plastids without pyrenoid; heteromorphic alternation of generations; oogamous sexual reproduction. Bellotia, Carpomitra, Nereia, Sporochonus, Tomaculopsis. |
| ●●●Syringodermatales Henry 1984 Gametophyte 2--4 cells, sporophyte parenchymatous with apical and marginal growth; several scattered discoid plastids without pyrenoid; heteromorphic alternation of generations but gametophyte not free-living; isogamous sexual reproduction. Syringoderma. |
| ●●●Tilopteridales Bessey 1907 Polystichous construction of the thallus, which grows by a trichothallic meristem; several scattered plastids without pyrenoids; isomorphic alternation of generations; oogamous sexual reproduction. Cutleria, Halosipon, Haplospora, Phaeosiphoniella, Phyllaria Tilopteris. |
| ●●Schizocladia Henry et al. in Kawai et al. 2003 Branched filamentous algae with biciliated zoospores – an immature cilium bearing tubular tripartite hairs; ciliary transitional helix with ~5 gyres located above the transitional plate; ciliary apparatus and kinetosomal roots, if present undescribed; chloroplasts parietal with girdle lamella; outer chloroplast endoplasmic reticulum membrane with direct membrane connection to the outer nuclear envelope membrane; plastid DNA with ring-type genophore; eyespots present; plastids with chlorophylls a and c as well as carotenoids (HPLC data absent); cell wall containing alginates but lacking cellulose and plasmodesmata; eyespot present; major storage product undescribed. Schizocladia. |
| ●● Diatomea Dumortier 1821 [= Bacillariophyta Haeckel, 1878] Vegetative cells cylindrical with a circular, elongate or multipolar cross section, lacking any trace of cilia except as the sperm of centric lineages; cell wall complete, composed of tightly integrated silicified elements and comprised of two valves, one at each end of the cell, with several girdle bands as hoops or segments covering the cylindrical ‘girdle’ lying between the valves; chloroplasts usually present, bounded by 4 membranes, and with lamellae of 3 thylakoids and a ring nucleoid (rarely multiple nucleoids); ciliated sperm cells bearing a single anterior cilium with a 9+0 axoneme and mastigonemes; life cycle diplontic and of unique pattern – slow size reduction over several years during the vegetative phase, caused by an unusual internal wall morphogenesis, alternating with rapid size restitution via a growth phase (auxospore) over several days. Note 4. |
| Note 4. Traditionally, the >> 105 species of diatoms are classified into ‘centric’ and ‘pennate’ on the basis of valve pattern (radial organization vs bilateral organization), pattern centre (ring-like annulus vs elongate sternum), and sexual reproduction (oogamous vs morphologically isogamous). Molecular data show the centrics as a whole to be paraphyletic, but relationships between the principal groups, and whether particular groups are monophyletic or paraphyletic, is currently unclear. Several major molecular clades are cryptic, with no or few morphological or life history traits that can be convincingly argued to be synapomorphies. |
| ●●●Coscinodiscophytina Medlin & Kaczmarska 2004 (P) Valve outline circular (rarely elliptical); valve pattern radiating from a central or subcentral circular annulus; rimoportulae usually present; girdle bands hoop-like or segmental; sexual reproduction oogamous, with non-motile eggs and uniciliate sperm; auxospore with scales but no band-like elements; many small chloroplasts. |
| ●●●●Paralids Mann in Adl et al. 2005 (R) Chain-forming, heavily silicified; valves circular, radially symmetrical; rimoportulae or tube processes small, restricted to the mantle; girdle bands hoop-like. Paralia. |
| ●●●●Melosirids Mann in Adl et al. 2005 (R) Usually chain-forming; valves circular, radially symmetrical; rimoportulae small, scattered on the valve face or marginal; girdle bands hoop-like or segmental. Aulacoseira, Melosira, Stephanopyxis. |
| ●●●●Coscinodiscids Mann in Adl et al., 2005 (R) Solitary; valves generally circular, radiating from a central, subcentral or submarginal circular annulus; rimoportulae central, scattered on the valve face or marginal; girdle bands hoop-like. Actinoptychus, Coscinodiscus. |
| ●●●●Arachnoidiscids Mann in Adl et al., 2005 (R) Solitary, heterovalvar; valves circular, radially symmetrical; valve centre with radial slits, apparently modified rimoportulae; girdle bands hoop-like. Arachnoidiscus. |
| ●●●●Rhizosolenids Mann in Adl et al. 2005 (R) Chain-forming, rarely solitary; valves circular, radially symmetrical or with the pattern centre displaced towards one side; rimoportula single, associated closely with the annulus, sometimes developed into a spine; girdle bands segmental. Guinardia, Leptocylindrus, Rhizosolenia. |
| ●●●●Corethrids Mann in Adl et al. 2005 (R) Solitary; valves circular; radially symmetrical; articulating spines secreted from around the valve margin; rimoportulae absent; girdle bands segmental. Corethron. |
| ●●●Bacillariophytina Medlin & Kaczmarska 2004 Valve outline bipolar or multipolar, sometimes circular; valve pattern radiating from a central circular or elongate annulus or from a sternum; areas of special pores or slits often present, involved in mucilage secretion; rimoportulae present or absent; girdle bands usually hoop-like; sexual reproduction oogamous with non-motile eggs and uniciliate sperm or isogamous with amoeboid gametes; auxospore usually with band-like elements (perizonium or properizonium); chloroplasts many, few or one. |
| ●●●●Mediophyceae Jousé & Proshkina-Lavrenko in Medlin & Kaczmarska 2004 (P) Valve outline bipolar or multipolar, sometimes (secondarily?) circular; valve pattern radiating from a central circular or elongate annulus; rimoportulae central or marginal; sexual reproduction oogamous; auxospore with band-like elements (properizonium) or scales; chloroplasts usually many, small. Chaetoceros, Cymatosira, Ditylum, Odontella, Skeletonema, Thalassiosira. |
| ●●●●Bacillariophyceae Haeckel 1878 Valve outline almost always bipolar; valve pattern organized bilaterally about an elongate axial rib (sternum), as in a feather; rimoportulae generally only one or two per valve or none, sometimes accompanied (or replaced?) by special slits (the ‘raphe’) involved in motility; sexual reproduction morphologically isogamous (though sometimes with behavioural differentiation), involving gametangiogamy; auxospores usually with band-like elements in two series (transverse and longitudinal), forming a ‘perizonium’; chloroplasts usually only 1, 2 or a few and large, but sometimes many and small. Asterionella, Eunotia, Navicula, Nitzschia, Rhaphoneis. |
| ●Alveolata Cavalier-Smith 1991 Cortical alveolae, sometimes secondarily lost; with ciliary pit or micropore; mitochondrial cristae tubular or ampulliform. |
| ●●Protalveolata Cavalier-Smith 1991, emend. Adl et al. 2012 (P) With characters of Alveolata and one or more morphological synapomorphy characteristics of one or more of the Dinoflagellata, Ciliophora or Apicomplexa. |
| ●●●Chromerida Moore et al. 2008 (P?) Single secondary plastid bounded by four membranes; plastids pigmented by chlorophyll a, violaxantnin and β-carotene, but chlorophyll c absent; micropore present; single mitochondrion with tubular cristae; cilia assembled within cytoplasm. Chromera, Vitrella. |
| ●●●Colpodellida Cavalier-Smith 1993, emend. Adl et al. 2005 (P?) Apical complex and rostrum; two cilia in known species; tubular mitochondrial cristae; microtubules beneath alveolae; micropore; cysts at least in some species; predatory on other protists. Alphamonas, Colpodella, Voromonas. |
| ●●●Perkinsidae Levine 1978, emend. Adl et al. 2005 Trophozoites parasitic, dividing by successive binary fissions; released trophozoites (termed hypnospores) developing outside host to form zoospores via the formation of zoosporangia or morphologically undifferentiated mononucleate cells via a hypha-like tube; zoospores with two cilia; apical organelles including an incomplete conoid that is open along one side, rhoptries, micronemes, and micropores, and a microtubular cytoskeleton with both an anterior and posterior polar ring. Parvilucifera, Perkinsus. |
| ●●● Oxyrrhis Dujardin 1841 [Oxyrrhinaceae Sournia 1984] (M) Without true cingulum and sulcus; intranuclear mitotic spindle; with amphiesmal vesicles and trichocysts; cilia inserted laterally. Oxyrrhis marina. |
| ●●●Syndiniales Loeblich III 1976 Motile cells (i.e. dinospores or gametes) with a dinokont-like arrangement of cilia; nucleus possesses histones. Amoebophrya, Duboscquella, Merodinium, Syndinium. |
| ●●Dinoflagellata Bütschli 1885, emend. Fensome et al. 1993, emend. Adl et al. 2005 Cells with two cilia in the motile stage – typically, one transverse cilium ribbon-like with multiple waves beating to the cell’s left and longitudinal cilium beating posteriorly with only one or few waves; nucleus typically a dinokaryon with chromosomes remaining condensed during interphase and lacking typical eukaryotic histones and centrioles; closed dinomitosis with extranuclear spindle. |
| ●●●Noctilucales Haeckel 1894 [Noctiluciphyceae Fensome et al. 1993] Principal life cycle stage comprising a large free-living motile cell inflated by vacuoles; dinokaryon during part of life cycle only. Fossils unknown. Abedinium, Cachonodinium, Craspedotella, Cymbodinium, Kofoidinium, Leptodiscus, Noctiluca, Petalodinium, Pomatodinium, Scaphodinium, Spatulodinium. |
| ●●●Dinophyceae Pascher 1914 Cell cortex (amphiesma) containing alveolae (amphiesmal vesicles) that may or may not contain cellulosic thecal plates, the pattern (tabulation) thus formed being a crucial morphological criterion in recognizing affinities among dinophyceans; with a dinokaryon through the entire life cycle. |
| ●●●●Gymnodiniphycidae Fensome et al. 1993 With numerous amphiesmal vesicles, arranged nonserially or in more than six latitudinal series or with the pellicle as the principal amphiesmal element or the amphiesmal structure uncertain but not comprising a theca divisible into six or fewer latitudinal plates. Few known fossil representatives. |
| ●●●●●Gymnodinium F. Stein 1878, emend. G. Hansen & Moestrup in Daugbjerg et al. 2000 sensu stricto With loop-shaped, anticlockwise apical groove. Dissodinium, Gymnodinium, Lepidodinium, Nematodinium, Polykrikos, Proterythropsis, Warnowia. |
| ●●●●●Amphidinium Claparède & Lachmann 1859, emend. Flø Jørgensen et al. 2004 sensu stricto Minute irregular triangular- or crescent-shaped episome deflected to the left; no apical groove. Amphidinium. |
| ●●●●●Gyrodinium Kofoid & Swezy 1921, emend. G. Hansen & Moestrup in Daugbjerg et al. 2000 sensu stricto Elliptical apical groove, bisected by a ridge; with surface striation/ridges. Gyrodinium. |
| ●●●●●Kareniaceae Bergholtz et al. 2005 Furrow-like straight or sigmoid apical groove; haptophyte-derived chloroplasts. Brachidinium, Karenia, Karlodinium, Takayama. |
| ●●●●●Ptychodiscales Fensome et al. 1993 With wall of the motile cell dominated by a thick pellicle; alveolae, where discernible, usually devoid of thecal plates, forming a tabulation that is non-serially arranged or is organized into numerous latitudinal series. Few known fossil representatives. Achradina, Amphitolus, Balechina, Ptychodiscus, Sclerodinium. |
| ●●●●●Borghiellaceae Moestrup, Lindberg, and Daugbjerg, 2009 With or without apical pair of elongate vesicles (PEV) with furrow; eyespot type B with globules inside the chloroplast associated with external brick-like material in vesicles. Baldinia, Borghiella. |
| ●●●●●Tovelliaceae Moestrup et al. 2005 With alveolae containing light thecal plates with apical line of narrow plates (ALP); eyespot type C with extraplastidal, non-membrane bound pigment globules. Bernardinium, Esopotrodinium, Jadwigia, Tovellia. |
| ●●●●●Suessiaceae Fensome et al. 1993, emend. Moestrup et al. 2009 With alveolae containing light thecal plates and forming a tabulation involving 7--15 latitudinal series, with or without elongate apical vesicle (EAV); eyespot type E with cisternae containing brick-like material. Biecheleria, Polarella, Protodinium, Symbiodinium. |
| ●●●●Peridiniphycidae Fensome et al. 1993 With a tabulation that accords with, or derives from, a pattern in which there are five or six latitudinal plate series; sagittal suture lacking. |
| ●●●●●Gonyaulacales Taylor 1980 Alveolae usually containing thecal plates, forming a tabulation of 5--6 latitudinal series; distinguished by particular tabulation features that can be recognized generally by a strong degree of asymmetry in the anterior (apical) and posterior (antapical) areas. This taxon has fossils extending from the late Triassic period (~210 Ma) to the present day. Alexandrium, Amylax, Ceratium, Coolia, Fragilidium, Gambierdiscus, Goniodoma, Gonyaulax, Lingulodinium, Ostreopsis, Peridinella, Protoceratium, Pyrocystis, Pyrophacus. |
| ●●●●●Peridiniales Haeckel 1894 Alveolae containing thecal plates, forming a tabulation of 6 latitudinal series; distinguished by particular tabulation features that can be recognized generally by a strong degree of symmetry in the anterior (apical) and posterior (antapical) areas. This taxon has fossils extending from the early Jurassic Period (~190 Ma) to the present day. Amphidiniopsis, Archaeperidinium, Blastodinium, Blepharocysta, Diplopelta, Diplopsalis, Diplopsalopsis, Herdmania, Oblea, Peridinium, Peridiniopsis, Preperidinium, Protoperidinium. |
| ●●●●●Thoracosphaeraceae Schiller 1930 Calcareous dinoflagellates and non-calcareous relatives. Amyloodinium, Cryptoperidiniopsis, Ensiculifera, Leonella, Luciella, Paulsenella, Pentapharsodinium, Pfiesteria, Scrippsiella, Stoeckeria, Thoracosphaera, Tyrannodinium. |
| ●●●●●Podolampadaceae Lindemann 1928 No compressed cingulum but homologous plate series. Blepharocysta, Gaarderiella, Heterobractum, Lissodinium, Mysticella, Podolampas. |
| ●●●●Dinophysiales Kofoid 1926 With a cingulum, a sulcus, and a sagittal suture extending the entire length of the cell, one ciliar pore. Apart from one possible fossil representative, only known from present day forms. Amphisolenia, Citharistes, Dinophysis, Histioneis, Ornithocercus, Oxyphysis, Phalacroma, Sinophysis, Triposolenia. |
| ●●●●Prorocentrales Lemmermann 1910 Without cingulum or sulcus; one ciliary pore; cilia apical – one wavy cilium clearly homologous with transverse cilium of other dinoflagellates and one not wavy; thecal plates. Fossils unknown. Mesoporus, Prorocentrum. |
| ●●●Incertae sedis Dinoflagellata: parasitic dinoflagellate with dinokaryon during part of the life cycle only; not highly vacuolated. Fossils unknown. [Blastodiniales Chatton 1906, no longer valid]. Amyloodinium, Apodinium, Cachonella, Crepidoodinium, Haplozoon, Oodinium, Piscinodinium, Protoodinium. |
| ●●Apicomplexa Levine 1980, emend. Adl et al. 2005 At least one stage of the life cycle with flattened sub-pellicular vesicles and an apical complex consisting of one or more polar rings, rhoptries, micronemes, conoid, and sub-pellicular microtubules; sexuality, where known, by syngamy followed by immediate meiosis to produce haploid progeny; asexual reproduction of haploid stages occurring by binary fission, endodyogeny, endopolyogeny, and/or schizogony; locomotion by gliding, body flexion, longitudinal ridges, and/or cilia; parasitic. |
| ●●●Aconoidasida Mehlhorn Peters & Haberkorn 1980 [= Hematozoa Vivier 1982] (P) Apical complex lacking conoid in asexual motile stages; some diploid motile zygotes (ookinetes) with conoid; macrogametes and microgametes forming independently; heteroxenous. |
| ●●●●Haemosporoidia Danilewsky 1885 Zygote motile as öokinete with conoid; ciliated microgametes produced by schizogonous process; öocyst formed in which sporozoites develop. Haemoproteus, Leucocytozoon, Mesnilium, Plasmodium. |
| ●●●●Piroplasmorida Wenyon 1926 Piriform, round, rod-shaped or amoeboid; conoid and cilia absent in all stages; polar ring present; without oocyst; sexuality probably associated with the formation of large axopodium-like “Strahlen”. Babesia, Theileria. |
| ●●●Conoidasida Levine 1988 (P) Complete apical complex, including a conoid in all or most asexual motile stages; cilia, where present, found exclusively in microgametes (male gametes); with the exception of microgametes, motility generally via gliding with possibility of body flexion and undulation of longitudinal pellicular ridges; heteroxenous or homoxenous. This group is not monophyletic with subdivisions artificial and unclear at this time. |
| ●●●●Coccidia Leuckart 1879 (P) Mature gametes develop intracellularly; microgamont typically produces numerous microgametes; syzygy absent; zygote rarely motile; sporocysts usually formed within oocysts. |
| ●●●●●Adeleorina Léger 1911 Microgamonts produce one to four microgametes, which associate with macrogamete in syzygy; endodyogony is absent. Adelina, Adelea, Dactylosoma, Haemolivia, Hepatozoon, Haemoggregarina, Karyolyssus, Klossia, Klossiella. |
| ●●●●●Eimeriorina Léger 1911 Microgametes and macrogametes develop independenty; syzygy is absent; microgamonts produce large number of ciliated microgametes; zygote is non-motile; sporozoites always enclosed in sporocyst within oocyst. Atoxoplasma, Barrouxia, Besnoitia, Caryospora, Caryotropha, Choleoeimeria, Cyclospora, Cystoisospora, Defretinella, Diaspora, Dorisa, Dorisiella, Eimeria, Goussia, Hammondia, Hyaloklossia, Isospora, Lankesterella, Mantonella, Neospora, Nephroisospora, Ovivora, Pfeifferinella, Pseudoklossia, Sarcocystis, Schellackia, Toxoplasma, Tyzzeria, Wenyonella. |
| ●●●●Gregarinasina Dufour 1828 (P) Mature gamonts usually develop extracellularly; syzygy of gamonts generally occurring with production of gametocyst; similar numbers of macrogametes and microgametes maturing from paired gamonts in syzygy within the gametocyst; syngamy of mature gametes leading to gametocyst that contains few to many oocysts, which each contain sporozoites; sporocysts absent; asexual reproduction (merogony) absent in some species. Acuta, Cephalolobus, Gregarina, Levinea, Menospora, Nematocystis, Nematopsis, Steinina, Trichorhynchus. |
| ●●●●●Archigregarinorida Grassé 1953 Trophozoite aseptate; with syzygy; encystment of gamonts; sporocysts contain 4--8 or even more sporozoites. Filipodium, Platyproteum, Rhytidocystis, Selenidium. |
| ●●●●●Eugregarinorida Léger 1900 Trophozoite with epimerite in septate gregarines or mucron in aseptate gregarines; syzygy followed by encystment of gamonts; sporocysts with 8 sporozoites. Amoebogregarina, Ascogregarina, Blabericola, Cephaloidophora, Colepismatophila, Difficilina, Ganymedes, Geneiorhynchus, Gregarina, Heliospora, Hoplorhynchus, Lankesteria, Lecudina, Leidyana, Lithocystis, Neoasterophora, Paraschneideria, Phyllochaetopterus, Prismatospora, Protomagalhaensia, Psychodiella, Pterospora, Pyxinia, Stenophora, Stylocephalus, Syncystis, Thiriotia, Trichotokara, Xiphocephalus. |
| ●●●●●Neogregarinorida Grassé 1953 Trophozoite with epimerite or mucron; multiple rounds of merogony; sporocysts contain 8 sporozoites. Mattesia, Ophryocystis, Schyzocystis, Syncystis. |
| ●●●● Cryptosporidium Oocysts and meronts with attachment “feeder” organelle; microgametes non-ciliated; oocysts without sporocysts, with 4 naked sporozoites; extracytoplasmic localization in host cell. Cryptosporidium. |
| ●●●Incertae sedis Apicomplexa: Agamococcidiorida Levine 1979 Merogony and gametogony both absent; several families described but position within Apicomplexa unclear. Gemmocystis, Rhytidocystis. |
| ●●●Incertae sedis Apicomplexa: Protococcidiorida Kheisin 1956 Merogony absent; extracellular gamogony and sporogony; in some species, gamogony and fertilization in the host, with oocysts released with sporogony in aqueous environment; sporozoites exist inside intestinal epithelium briefly, on their way to coelom or vascular tissues, where development occurs, followed by sporozoite release in the feces. Subdivisions uncertain. Angeiocystis, Coelotropha, Grellia, Eleutheroschizon, Mackinnonia, Myriosporides, Myriospora, Sawayella. |
| ●●●Incertae sedis Apicomplexa: Agreggata – with highly divergent SSU rRNA. |
| ●●●Incertae sedis Apicomplexa: Nephromyces – unclear taxonomic position between coccidians and piroplasmids, of this uniquely mutualistic genus; ciliated stages; most of life cycle extracellular. |
| ●●Ciliophora Doflein 1901 [Ciliata Perty 1852, Infusoria Bütschli 1887] Cells with nuclear dimorphism, including a typically polygenomic macronucleus and at least one diploid micronucleus; somatic kinetids having a postciliary microtubular ribbon arising from triplet 9, a kinetodesmal fibril or striated rootlet homologue arising near triplets 5--8, and a transverse microtubular ribbon arising in the region of triplets 4--6; sexual reproduction, when present, by conjugation typically with mutual exchange of haploid gametic nuclei that fuse to form the synkaryon or zygotic nucleus. |
| ●●●Postciliodesmatophora Gerassimova & Seravin 1976 Somatic dikinetids with postciliodesmata, an arrangement of laterally overlapping postciliary microtubular ribbons associated with somatic dikinetids. |
| ●●●●Karyorelictea Corliss 1974 Two to many macronuclei containing approximately but sometimes slightly more than the diploid amount of DNA; macronuclei not dividing but replaced by division of micronuclei; major postciliary ribbons separated by two groups of microtubules. Kentrophoros, Loxodes, Trachelocerca. |
| ●●●●Heterotrichea Stein 1859 Polygenomic macronucleus dividing by extra-macronuclear microtubules; major postciliary ribbons separated by one microtubule. Blepharisma, Climacostomum, Folliculina, Stentor. |
| ●●●Intramacronucleata Lynn 1996 Polygenomic macronucleus dividing by intra-macronuclear microtubules. |
| ●●●●Cariacothrix Orsi et al. 2011 [Cariacotrichea Orsi et al. 2011] (M) With an archway-shaped kinety surrounding the oral opening and extending to posterior body end; with a unique molecular signature ‘GAAACAGUCGGGGGUAUCAGUA’ (spanning nucleotide positions 283–305 in GenBank accession number GU819615); confirmed only from deep waters of anoxic Cariaco Basin, Venezuela. Cariacothrix caudata. |
| ●●●●Spirotrichea Bütschli 1889 (R) Conspicuous right and left oral and/or pre-oral ciliature; left serial oral polykinetids leading, usually clockwise into the oral cavity, either around a broad anterior end or along anterior and left margins of the body; DNA replication in the macronucleus accomplished by a complicated migrating structure called a replication band in all but Protocruziidia and Phacodiniidia. |
| ●●●●●Protocruzia Faria da Cunha & Pinto 1922 [Protocruziidia de Puytorac et al. 1987] Nuclear apparatus a cluster of similar-sized nuclei with paradiploid macronuclei surrounding one or more micronuclei; each macronucleus possibly organized as a single composite chromosome. Protocruzia. |
| ●●●●●Phacodinium Prowazek 1900 [Phacodiniidia Small & Lynn 1985] Somatic kineties of linear polykinetids; each kinetosome bearing a kinetodesmal fibril, and sometimes accompanied by a partner kinetosome in some regions of the body, thus resembling a cirrus. Phacodinium. |
| ●●●●●Protohypotrichia Shi et al. 1999 With weakly differentiated and non-grouped somatic ciliature – cirri on ventral side generally uniform, no clearly defined marginal cirral rows, ciliature on dorsal side mixed with cirri and dikinetids, no clearly differentiated dorsal kineties. Caryotricha, Kiitricha |
| ●●●●●Licnophoria Corliss 1957 Body hour-glass shaped, both ends discoid; posterior disc adhesive, with peripheral rings of cilia; an anterior disc with serial oral polykinetids around oral region; ectosymbionts, temporarily attached to substrate or host by ciliated, mobile, posterior adhesive disc. Licnophora, Prolicnophora. |
| ●●●●●Euplotia Jankowski 1979 [Hypotrichia Stein 1859 sensu Lynn 2008] Ventral ciliature as cirri and dorsal ciliature as somatic dikinetids with a kinetodesmal fibril; during morphogenetic processes, the ventral somatic infraciliature either turned-over or replaced and dorsal ciliature reorganized. Aspidisca, Discocephalus, Euplotes. |
| ●●●●●Oligotrichia Bütschli 1887 Oral polykinetids forming an open circle, typically with an anterior "collar" and a more ventral "lapel"; somatic kineties reduced in number and variable in pattern, forming bristles, girdles, and spirals. Laboea, Strobilidium, Strombidium. |
| ●●●●●Choreotrichia Small & Lynn 1985 Oral polykinetids forming a closed circle around the anterior end of the body, several often extending into the oral cavity; planktonic tintinnids are all loricate. Strombidinopsis, Strobilidium, Tintinnopsis. |
| ●●●●● Hypotrichia Stein 1859 [for Stichotrichia Small & Lynn 1985 sensu Lynn 2008] Ventral ciliature as cirri and dorsal ciliature as somatic dikinetids without a kinetodesmal fibril; during morphogenetic processes, entire ventral and dorsal somatic infraciliature typically turned-over or replaced. Halteria, Oxytricha, Stylonychia, Urostyla. |
| ●●●●Armophorea Lynn 2004 (R) Typically dependent upon methanogenic endosymbionts, suggesting that hydrogenases within this group may be monophyletic. |
| ●●●●●Armophorida Jankowksi 1964 Body usually twisted to left, often much so; oral region spiralled, with series of 3--5 perioral or perizonal somatic kineties along its edge; typically free-living microaerophils or anaerobes. Caenomorpha, Metopus. |
| ●●●●●Clevelandellida de Puytorac & Grain 1976 Oral polykinetids with a fourth row of kinetosomes directly opposite those of the third, leading to their designation as hetero-membranelles; endosymbionts of animals. Clevelandella, Nyctotherus, Paracichlidotherus. |
| ●●●●Litostomatea Small & Lynn 1981 Somatic monokinetids with two transverse ribbons, a slightly convergent postciliary ribbon, and a laterally directed kinetodesmal fibril that does not overlap those of adjacent kineties; one transverse ribbon tangential to the kinetosome perimeter and extending anteriorly into the somatic ridge to the left of the kinetid and the other transverse ribbon radial to the kinetosome perimeter and extending transversely into the adjacent somatic ridge. |
| ●●●●●Haptoria Corliss 1974 Toxicysts typically between transverse microtubules of oral dikinetids; oral region on body surface bordered by oral dikinetids with some exceptions; typically free-living predators of other protists. Didinium, Lacrymaria, Lagynophrya. |
| ●●●●● Rhynchostomatia Jankowski 1980, emend. Vd'acny et al. 2011 Body partitioned into proboscis and trunk, with or without tail; oral bulge opening ventral, at base of proboscis; right branch of circumoral kinety accompanied by at least one perioral kinety and left branch by many oblique preoral kineties or a single perioral-like kinety. Dileptus, Dimacrocaryon, Trachelius. |
| ●●●●●Trichostomatia Bütschli 1889 Toxicysts absent; oral region or oral cavity densely ciliated, sometimes organized as "polykinetids"; typically endosymbionts in vertebrates. Balantidium, Entodinium, Isotricha, Ophryoscolex. |
| ●●●●CONTHREEP Lynn in Adl et al. 2012 [Ventrata Cavalier-Smith 2004] (R) Group identified by SSU rRNA phylogenies. With a node-based definition: the clade stemming from the most recent common ancestor of the Colpodea (C), Oligohymenophorea (O), Nassophorea (N), Phyllopharyngea (P), Prostomatea (P), and Plagiopylea (P), hence CONTHREEP. «Ventrata» suggests a «ventral» morphological synapomorphy for the group but this does not exist. |
| ●●●●●Phyllopharyngea de Puytorac et al. 1974 The ciliated stage with somatic kineties mostly as monokinetids that each have a lateral kinetodesmal fibril, a reduced or absent transverse microtubular ribbon that is usually accompanied by a left-directed transverse fiber, and a somewhat convergent postciliary ribbon extending posteriorly to accompany ribbons of more anterior monokinetids; ribbon-like subkinetal nematodesmata arising from somatic monokinetids and extending, either anteriorly or posteriorly, beneath kineties as nematodesmata in the Synhymenia or subkinetal ribbons in the other clades; oral region with radially arranged microtubular ribbons, called phyllae. |
| ●●●●●●Synhymenia de Puytorac et al. in Deroux, 1978 Hypostomial frange or synhymenium of dikinetids or small polykinetids (i.e., usually of ~four kinetosomes), extending from right postoral body surface to left dorsal body surface, almost encircling the body in some forms; no atrium; cyrtos, conspicuous. Synhymenia, Zosterodasys. |
| ●●●●●●Cyrtophoria Fauré-Fremiet in Corliss, 1956 Oral ciliature typically composed of one preoral kinety and two circumoral kineties; true cytostome and cytopharynx surrounded by phyllae and rod-shaped nematodesmata; macronucleus heteromerous. Brooklynella, Chilodonella. |
| ●●●●●●Chonotrichia Wallengren 1895 Sedentary and sessile forms with somatic cilia only on walls of perioral funnel or cone-shaped region, which may be flared or compressed; oral cilia absent or only as several inverted kineties next to cytostome; cytopharyngeal apparatus with phyllae, but no nematodesmata; macronucleus, heteromerous; unequal cell division typical, producing “bud” for dispersal; most species are ectosymbionts on crustacean appendages. Chilodochona, Spirochona. |
| ●●●●●●Rhynchodia Chatton & Lwoff 1939 Oral apparatus a suctorial tube supported by radially arranged microtubular ribbons (= phyllae) enclosing toxic (?) extrusomes (acmocysts or haptotrichocysts); predators of other ciliates or endosymbiotic parasites of bivalve molluscs and other marine invertebrates. Ancistrocoma, Ignotocoma, Stegotricha. |
| ●●●●●●Suctoria Claparède & Lachmann 1858 Mature sessile trophonts, usually non-ciliated, with one to many tentacles that ingest prey; extrusomes at tentacle tips as haptocysts; tentacles supported by an outer ring of microtubules and an inner set of microtubular ribbons (=phyllae); unequal cell division typical with ciliated, migratory dispersal "larvae" or swarmers typically bearing neither tentacles nor stalk. Acineta, Discophrya, Ephelota, Tokophrya. |
| ●●●●●Nassophorea Small & Lynn 1981 (P?) Somatic cilia as monokinetids and dikinetids; monokinetid with an anterior, tangential transverse ribbon, a divergent postciliary ribbon, and anteriorly directed kinetodesmal fibril; somatic alveoli well-developed with paired alveolocysts sometimes present; oral nematodesmata are well-developed as the cyrtos in several groups. Microthorax, Nassula, Pseudomicrothorax. Note 5. |
| Note 5. Based on SSU rRNA gene sequences, the Nassophorea with the synhymeniids transferred to the Phyllopharyngea may still not be monophyletic (Gong et al. 2009; Kivimaki et al. 2009). |
| ●●●●●Colpodea Small & Lynn 1981 Ciliated somatic dikinetids with one transverse ribbon and at least one postciliary microtubule associated with the anterior kinetosome and one transverse ribbon, one postciliary ribbon, and one kinetodesmal fibril associated with the posterior kinetosome; posterior transverse ribbons extending posteriorly and overlapping one another, the so-called transversodesmata. |
| ●●●●●●Platyophryida Puytorac et al. 1979 Oral structures on cell surface; paroral as a file of ciliated dikinetids; left oral polykinetids as single rows or brick-shaped organelles; micronucleus in perinuclear space of macronucleus in some species. Platyophrya, Sorogena. |
| ●●●●●●Bursariomorphida Fernández-Galiano 1978 Oral structures in an oral cavity, often very deep or trough-like; paroral as a file of dikinetids or multiple kinetosomal rows on right, sometimes extending to right posterior region of the oral cavity or to almost encircle it; left oral polykinetids, ranging from one to many, which form a conspicuous adoral zone. Bryometopus, Bursaria. |
| ●●●●●●Cyrtolophosidida Foissner 1978 Oral structures in a shallow oral cavity; paroral as a file of dikinetids on right side of oral region; up to 10 brick-shaped, left oral polykinetids; micronucleus enclosed within the perinuclear space of the macronucleus in some species. Aristerostoma, Cyrtolophosis. |
| ●●●●●●Colpodida de Puytorac et al. 1974 Oral structures in an oral cavity; right oral structure as paroral of monokinetids or dikinetids, which may be associated with a few to many somewhat ordered or disordered rows to its right, sometimes forming a polykinetid (e.g. Colpoda), but reduced to a single row of monokinetids in some genera (e.g. Pseudoplatyophrya); left oral structures as one to several polykinetids composed of several to many well-ordered monokinetidal rows; stomatogenesis, merotelokinetal; division, typically palintomic, in reproductive cysts. Colpoda, Pseudoplatyophrya. |
| ●●●●●Prostomatea Schewiakoff 1896 Oral dikinetids, radial to tangential to perimeter of oral area with postciliary microtubular ribbons that extend laterally from each dikinetid, overlapping one another, and, in some species, forming a circular microtubular band that supports the wall of a shallow pre-cytostomal cavity; associated oral ciliature as two or more assemblages of dikinetids, often called a “brush”. Coleps, Cryptocaryon, Holophrya, Prorodon, Urotricha. |
| ●●●●●Plagiopylea Small & Lynn 1985, sensu Lynn 2008 (R) Group identified by SSUrRNA phylogenies. With a node-based definition: The clade stemming from the most recent common ancestor of the Plagiopylida and Odontostomatida. |
| ●●●●●●Plagiopylida Jankowski 1978 Somatic monokinetid with divergent postciliary microtubular ribbon, well-developed anteriorly-directed kinetodesmal fibril, and a transverse ribbon extending laterally or anteriorly; cytoplasm containing conspicuous “sandwich” assemblages of methanogens and ciliate hydrogenosomes. Lechriopyla, Plagiopyla, Sonderia, Trimyema. |
| ●●●●●●Odontostomatida Sawaya 1940 Small body usually laterally compressed, often bearing spines; somatic kineties typically of dikinetids separated into anterior and posterior segments; oral cilia inconspicuous, usually < 10 oral polykinetids. Discomorphella, Epalxella. |
| ●●●●●Oligohymenophorea de Puytorac et al. 1974 Oral apparatus with a distinct right paroral dikinetid and typically three left oral polykinetids, residing in a ventral oral cavity or deeper infundibulum (secondarily lost (?) in Astomatia and some astomatous Hymenostomatia); somatic monokinetids with anteriorly directed overlapping kinetodesmal fibrils, divergent postciliary ribbons, and radial transverse ribbons (except in Peniculia). |
| ●●●●●●Peniculia Fauré-Fremiet in Corliss 1956 Somatic kinetids with tangential transverse ribbons and prominently overlapping kinetodesmal fibrils; cortical alveoli lie between kinetosomal rows of oral polykinetids; extrusome as typical fibrous trichocyst. Frontonia, Paramecium, Stokesia. |
| ●●●●●●Scuticociliatia Small 1967 Paroral dikinetid with a, b, and c segments; stomatogenesis by proliferation of kinetosomes from the c segment or a “scutico”-vestige posterior to a and b segments, with varying involvement of kinetosomes in the a and b segments. Anophryoides, Cyclidium, Loxocephalus, Philasterides, Pleuronema. |
| ●●●●●●Hymenostomatia Delage & Hérouard 1896 Stomatogenesis by proliferation of kinetosomes typically in the mid-ventral region of the cell body, posterior to and some distance from the parental oral apparatus. Colpidium, Glaucoma, Ichthyophthirius, Tetrahymena. |
| ●●●●●●Apostomatia Chatton & Lwoff 1928 Ciliates with a polymorphic life cycle; usually as epibionts of marine Crustacea; novel cortical structures including a “rosette” organelle and the x, y, and z kineties. Foettingeria, Gymnodinioides, Hyalophysa. |
| ●●●●●●Peritrichia Stein 1859 Body divided into three major areas: (1) oral, with a prominent peristome bordered by a dikinetid file (haplokinety) and an oral polykinetid that both originate in an oral cavity (infundibulum) at the base of which is the cytostome; (2) aboral, including kinetosomes as part of the scopula, which secretes the stalk of sessile species; and (3) telotroch, permanently ciliated on mobile species. Carchesium, Epistylis, Trichodina, Vorticella, Zoothamnium. Note 6. |
| Note 6. Zhan et al. (2009) suggest removing the Mobilida from this group and elevating it to a higher rank based on SSUrRNA gene sequence data. This has been supported by tubulin sequence data (Gong et al. 2010). We prefer to await corroboration by multiple genes before recognizing this split. |
| ●●●●●●Astomatia Schewiakoff 1896 Without cytostome; symbionts typically found in the digestive tract of annelids, especially oligochaetes; cortical cytoskeleton in the anterior region may be conspicuously developed as an attachment structure(s). Anoplophrya, Haptophrya. |
| ●●Incertae sedis Alveolata: Ellobiopsidae (Ellobiopsis, Thalassomyces). |
| ●●Incertae sedis Alveolata: Colponema. |
| ●Rhizaria Cavalier-Smith 2002 With fine pseudopodia varying as simple, branching, or anastomosing patterns, often supported by microtubules in those groups examined by electron microscopy. |
| ●● Cercozoa Cavalier-Smith 1998, emend. Adl et al. 2005 (R) Diverse clade lacking distinctive morphological or behavioural characters; biciliated and/or amoeboid, usually with filopodia; most with tubular mitochondrial cristae; cysts common; kinetosomes connecting to nucleus with cytoskeleton; usually with microbodies and extrusomes. |
| ●●● Cercomonadidae Kent 1880, emend. Mylnikov & Karpov 2004 [= Cercomonadida Poche 1913, emend. Vickerman 1983, emend. Mylnikov 1986, emend. Karpov et al. 2006; Cercobodonidae Hollande 1942] Amoebociliates without cell wall; two heterodynamic cilia without mastigonemes; pseudopodia used for feeding; some species have complex life cycle including multinuclear and multiciliary plasmodium stage; cysts occur; kinetosomes connected to the nucleus; tubular mitochondria cristae; with microbodies and extrusomes. Note that with more research new sub-divisions within this group are emerging. Brevimastigomonas, Cavernomonas, Cercomonas, Eoercomonas, Filomonas, Metabolomonas, Neocercomonas, Nucleocercomonas, Paracercomonas. |
| ●●● Pansomonadida Vickerman 2005 Heterotrophic with two heterodynamic cilia, both free from body. Agitata, Aurigamonas. |
| ●●● Glissomonadida Howe & Cavalier-Smith 2009 [Heteromitidae Kent 1880, emend. Mylnikov 1990, emend. Mylnikov & Karpov 2004; Bodomorphidae Hollande 1952] Heterotrophic without cell covering, not strongly amoeboid; ancestrally biciliates, gliding on longer posterior cilium, but includes the derived non-gliding genus Proleptomonas; protoplasmic tails to the cell formed, but to a much less extent than in cercomonads. Note 7. Allantion, Allapsa, Bodomorpha, Dujardina, Flectomonas, Mollimonas, Neoheteromita, Proleptomonas, Sandona, Teretomonas. |
| Note 7. Glissomonadida Howe & Cavalier-Smith 2009 replaces Heteromitidae sensu Cavalier-Smith & Chao 2003, as Heteromita Dujardin 1841 originally contained only two probable euglenozoans and an unidentifiable ciliate, making it inapplicable to Cercozoa. |
| ●●● Tremula
Howe et al. 2011 (M) Heterotrophic and phagotrophic biciliates with long anterior and posterior cilia; glide on surfaces by means of both cilia, one pointing forwards and one backwards; without light microscopically visible theca, scales or cytostome. Tremula longifila. |
| ●●● Metromonadea Cavalier-Smith 2007, emend. Cavalier-Smith 2011 Non-pseudopodial marine gliding biciliate; predator on other eukaryotes; non-thecate but with a dense single or double layered surface coat that may extend up cilium; extrusomes highly elongated. Metromonas, Metopion, Micrometopion. |
| ●●● Granofilosea Cavalier-Smith & Bass 2009 With very fine branching or unbranched granuloreticulopodia bearing obvious extrusomes as the granules at frequent rather regular intervals, or with radiating, sometimes branched, axopodia with similar granules; pseudopodia supported by internal microtubules and typically appressed to the substratum during feeding, in a semi-immobile state; in most species pseudopodia do not anastomose; some with biciliated swimming or gliding stage. Limnofila, Massisteria, Mesofila, Minimassisteria, Nanofila. |
| ●●● Clathrulinidae Claus 1874 [Desmothoracida Hertwig & Lesser 1874] Extracellular capsule or lorica attached to substrate, with axopodia emerging from perforations; kinetocyst extrusomes along axopodia; tubular mitochondrial cristae; biciliated and amoeboid stages; can be colonial. Cienkowskia, Clathrulina, Hedriocystis. |
| ●●●● Incertae sedis Clathrulinidae: Servetia. |
| ●●● Thecofilosea Cavalier-Smith 2003, emend. Cavalier-Smith 2011 Ancestrally with robust organic extracellular theca, unlike most other Cercozoa which are usually naked or with scales; ventral filose pseudopodia emerge from ventral groove; two cilia with divergent centrioles, secondarily lost in Rhizaspidae and the euglyphid amoebae, and restricted to zoospores in phaeodarians; ancestrally benthic gliding on posterior cilium only, but some secondarily planktonic swimmers amongst which ebriids have lost pseudopodia; theca with perforations for cilia and for pseudopodia, and three perforations in phaeodaria (thus also called Tripylea Hertwig 1879), which have surrounded it by a pseudopodial net containing a pigmented phaeodium, thus converting it into a ‘central capsule’, but not homologous with that of Polycystinea of Radiolaria; silica scales absent, unlike many Imbricatea (see below), but hollow silica endoskeleton in all ebriids and most phaeodarians. |
| ●●●● Phaeodarea Haeckel 1879 [Tripylea Hertwig 1879] Central capsule with thickened, double-layered, capsular wall containing two kinds of pores or openings; large opening known as an “astropylum” or oral pore with a protruding mass of cytoplasm, and smaller, typically lateral openings, as “parapylae”, with thinner protruding strands of cytoplasm; dense mass of darkly pigmented granular cytoplasm, the “phaeodium,” containing undigested debris, suspended in the extracapsulum; mineral skeletons, when present, composed of scattered spicules or hollow silica bars, joined by organic material; a wide variety of forms, including geodesic frameworks, spherical to polyhedral shells, or more solid, porous clam-shaped, bivalve shells; tubular mitochondrial cristae. |
| ●●●●● Phaeoconchia Haeckel 1879 Central capsule enclosed within bivalve lattice shell composed of dorsal and ventral boat-shaped valves, which are completely separated and rarely connected by a ligament on the aboral pole. Coelodendrum, Coelographis, Conchellium, Conchopsis. |
| ●●●●● Phaeocystina Haeckel 1879 Central capsule suspended in the centre of the extra-capsular cytoplasmic network; skeleton absent or incomplete, composed of numerous solitary, scattered pieces or spicules without organic connections. Aulacantha, Aulographis, Cannoraphis. |
| ●●●●● Phaeogromia Haeckel 1879 Central capsule located eccentrically, aborally, in simple lattice shell typically provided with large shell opening placed on the oral pole of the main axis; capsule opening surrounded by “teeth” or by peculiar elongate extensions known as “feet”, sometimes with elaborate branches. Castanella, Challengeron, Haeckeliana, Medusetta, Tuscarora. |
| ●●●●● Phaeosphaeria Haeckel 1879 Central capsule located in the centre of a simple or double spherical lattice shell, not bivalve, with a simple shell opening, lacking “feet” or “teeth”. Aulosphaera, Cannosphaera, Sagosphaera. |
| ●●●● Cryomonadida Cavalier-Smith 1993 (R) rDNA trees show Rhogostoma (Rhizaspididae) within the cryomonads, so they evolved after the hypothesised ciliated common ancestor of Ebriida and Cryomonadida by loss of cilia and are unrelated to Pseudodifflugia. Includes Rhogostoma (previously misidentified as Lecythium), Cryothecomonas, Protaspis (called Protaspa in Howe et al. 2011). |
|
●●●●● Rhizaspididae Skuja 1948 Thecate amoebae with ventral cleft that emits fine pseudopodia, which anastomose as reticulopodia in one species; theca thin, flexible, laterally compressed, adherent throughout to cell surface, consisting of single smooth dense layer outside and scarcely thicker than the plasma membrane; thus with bilateral symmetry, unlike other filose testate amoebae which are typically radially symmetric. Type genus Rhizaspis Skuja, 1948; other genera Rhogostoma Belar, 1921; Capsellina Penard, 1909. |
|
●●●●● Protaspidae Cavalier-Smith 1993 (R) Heterodynamic bicilated cells with cilia sub-apical separated by a protrusion; ciliary pit with funnel; dorsoventrally flattened and oval shaped with parallel lateral sides; ventral longitudinal furrow in anterior half of cell; nucleus posterior with permanently condensed chromosomes; thickened cell wall with seven layers with pores fro extrusome discharge; pseudopodia emerge from slits. Protaspis Skuja, 1939, Cryothecomonas Thomsen et al.1991 (P). |
|
●●●● Ventricleftida Cavalier-Smith 2011 Strongly flattened oval cell with rigid theca without scales; two unequal cilia emerging subapically, often from apical notch – posterior cilium used for gliding on surfaces; ventral cleft from which branched filose pseudopods emerge for feeding separate from and posterior to ciliary groove unlike Thaumatomonadida and Auranticordis; extrusomes. Ventrifissura, Verrucomonas. |
| ●●●● Ebriacea Lemmermann 1901 [Ebriidae Poche 1913] Cells with two subapically inserting cilia; open internal skeleton of silica; phagotrophic without plastids. Ebria, Hermesinum. |
| ●●●●Incertae sedis Thecofilosea: Chlamydophryidae de Saedeleer 1934, emend. Meisterfeld 2000 With a more or less flexible test that may be distorted by the contraction of the cell; some with attached foreign particles, scales, spines or spicules; division often longitudinal or by budding. Capsellina, Lecythium. |
| ●●●●● Incertae sedis Chlamydophryidae: Chlamydophrys, Clypeolina, Diaphoropodon, Leptochlamydophrys, Penardeugenia. |
| ●●●●Incertae sedis Thecofilosea: Botuliforma, Mataza, Pseudodifflugia. |
| ●●● Imbricatea Cavalier-Smith 2011 [Cavalier-Smith 2003] Secreted surface silica scales or secondarily lost, except in basal lineages where ancestrally absent; tubular mitochondrial cristae; ciliary transition region longer than in cercomonads and sainouroids, and unlike them with dense distal plate but without the internal dense aggregates and elaborate extra structures opposite the thecal contact zone in cryomonads; groove and cilia secondarily lost by euglyphids; centrioles multiplied and reoriented to make four posteriorly directed gliding cilia in Auranticordis, which also lost pseudopodia; centrioles independently made parallel in the thaumatomonad/ spongomonad subclade. Note 8. |
| Note 8. Ancestral condition probably a gliding heterotrophic cell with two cilia with divergent centrioles, and relatively rigid pellicle that helped define a distinct ventral groove from which filose pseudopods extended, but without the dense extracellular theca of Thecofilosea or internal silica skeleton of ebriids and Phaeodaria. Includes Cercozoa with often imbricate silica scales and their closest non-scaly relatives. |
|
●●●● Spongomonadida Hibberd 1983 [Spongomonadidae Karpov 1990] Biciliate with asymmetrical cell projection at anterior. Rhipidodendron, Spongomonas. |
| ●●●● Nudifila Cavalier-Smith & Howe 2009 (M) Soft-bodied heterotrophic cell with two prominent cilia, forming flattened and/or filose, branching, and reticulose pseudopodia that project from all around cell. Nudifila producta. |
|
●●●● Marimonadida Cavalier-Smith & Bass 2011 Without scales and without theca; marine heterotrophic biciliate swimming cells (Pseudopirsonia: diatom parasites) or interstitial gliding cells with somewhat deformable, semi-rigid pellicle underlain by muciferous bodies and four posterior cilia associated with ventral cleft (Auranticordis), or amoebociliates with two gliding posterior cilia and a non-ciliate feeding stage with broad lobose fan like pseudopods (Rhabdamoeba). Differ from Euglyphida by the absence of silica scales and presence of cilia, and from thaumatomonads, the only other gliding imbricates, by absence of scales. Auranticordis, Pseudopirsonia, Rhabdamoeba. |
| ●●●● Silicofilosea Adl et al. 2005, emend. Adl et al. 2012 Secreted surface silica scales or secondarily lost; tubular mitochondrial cristae. |
| ●●●●● Thaumatomonadida Shirkina 1987 [Thaumatomastigidae Patterson & Zölfell 1991] Heterotrophic usually gliding cells that may swim also; with flattened cell body and with two heterodynamic cilia inserting subapically and/or ventrally; some unikont; with extrusomes; filopodia produced subapically or from ventral groove; cysts; multinucleate and multiciliate stages known. |
|
●●●●●● Thaumatomonadidae Hollande 1952 Biciliated cells with ventral pseudopodia, long ventral posterior pointing cilium used for gliding on surfaces unlike Peregriniidae (see below), and a much shorter anterior cilium, which is naked (Thaumatomonas, Allas) or with small scales (Reckertia, Thaumatomastix); siliceous scales formed in vesicles attached to mitochondria cover the rigid cell surface except for a ventral zone that emits pseudopodia for feeding; unlike Peregriniidae, do not transform completely into an amoeba; all have oval or triangular two-tiered body scales, with an upper plate bearing species-specific perforations supported at the oval ends or triangle corners by discrete struts, unlike Gyromitus; upper plate lacks central cleft with inrolled sides, unlike Peregrinia; Thaumatomastix only additionally has long spine scales with near-circular or rounded triangular bases. Allas, Hyaloselene, Reckertia, Thaumatomonas, Thaumatomastix. |
| ●●●●●● Peregriniidae Cavalier-Smith 2011 With only oval two-tiered body scales and no ciliary scales or spine scales; scales either symmetric ovals with heavily out-turned upper and lower rims as in Gyromitus or asymmetric ovals with concave to flat lower surface and convex upper surface with rims more strongly laterally inrolled than at the ends as in Peregrinia; in contrast to Thaumatomonas, ciliary pit apical not subapical and ventral, or cells so amoeboid as to lack defined shape; cilia not clearly differentiated into anterior and posterior; unlike Thaumatomonadidae no evidence for ciliary gliding; locomotion by swimming or slow amoeboid creeping. Gyromitus, Peregrinia |
| ●●●●●Euglyphida Copeland 1956, emend. Cavalier-Smith 1997 Test of organic material; most taxa with secreted silica scales held together by an organic cement; tubular mitochondrial cristae. |
| ●●●●●●Euglyphidae Wallich 1864, emend Lara et al. 2007 Thin elliptical scales; presence of specialised scales around the pseudostome with typical indentation. Euglypha, Scutiglypha. |
| ●●●●●●Assulinidae Lara et al. 2007 Acrostome test composed of elliptic plates disposed in a regular, alternate pattern; test strongly compressed; no specialised type of scales around pseudostome. Assulina, Placocista, Valkanovia. |
| ●●●●●●Trinematidae Hoogenraad & De Groot 1940, emend Adl et al. 2012 Test with bilateral symmetry; scales oval or round, sometimes of both types; specialised tooth-shaped scales around the aperture; aperture invaginated in some taxa. Corythion, Playfairina, Puythoracia, Trinema. |
| ●●●●●●Cyphoderiidae de Saedeleer 1934 Scales circular to oval; test aperture angled, some with collar. Campascus, Corythionella, Cyphoderia, Messemvriella, Pseudocorythion, Schaudinnula. |
| ●●●●●●Paulinellidae de Saedeller 1934, emend. Adl et al. 2012 Pyriform, uncompressed shape; scales, when present, long, with length perpendicular to aperture. At least one genus (Ovulinata) with totally organic test without silica scales. Ovulinata, Paulinella. |
| ●●●●●●Incertae sedis Euglyphida: Ampullataria, Deharvengia, Euglyphidion, Heteroglypha, Matsakision, Ovulinata, Pareuglypha, Pileolus, Sphenoderia, Tracheleuglypha, Trachelocorythion. |
| ●●●● Incertae sedis Imbricatea: Clautriavia, Discomonas. |
| ●●● Chlorarachniophyta Hibberd & Norris 1984 Amoeboid with plastids of secondary origin; plastid containing chlorophylls a and b, associated with a nucleomorph and surrounded by four membranes in total; usually reticulate pseudopodia with extrusomes; cell bodies often anastomosing; with a biciliated dispersal stage. Bigelowiella, Chlorarachnion, Cryptochlora. |
|
●●● Vampyrellida West 1901, emend. Hess et al. 2012 Exclusively heterotrophic, naked, phagotrophic amoeboid organisms; life cycle includes amoeboid, free-moving trophozoites alternating with an obligatory digestive cyst, in which cell division usually take place; several taxa can fuse to form plasmodia and reach considerable sizes, sexual processes unknown; cytoplasm often differentiated into a finely granular, sometimes highly vacuolated part and structure-less hyaloplasm, the latter often surrounding the main cell body, but at least constituting the pseudopodia; free-living in freshwater, soil, or marine environments. Arachnula, Gobiella, Hyalodiscus, Lateromyxa, Leptophrys, Platyreta, Thalassomyxa, Theratromyxa, Vampyrella, possibly Penardia. |
| ●●● Phytomyxea Engler & Prantl 1897 Amoeboid or plasmodial feeding cells producing biciliate or tetraciliate cells; some with specialized solid extrusome—“satchel”—for penetrating host cells; with distinctive cruciform mitotic profile due to elongate persistent nucleolus lying orthogonal to metaphase plate; parasites or parasitoids of plants or stramenopiles. Includes Plasmodiophorida Cook 1928 (e.g. Plasmodiophora, Phagomyxa, Spongomyxa, Sorosphaera, Spongospora) and Phagomyxida Cavalier-Smith 1993 (e.g. Phagomyxa). |
|
●●●
Filoreta Bass & Cavalier-Smith 2009 (M) Non-ciliate, naked, free-living, mainly bacterivorous reticulose amoebae that form extensive multinucleate open-mesh nets. Filoreta turcica. |
|
●●● Gromia Dujardin 1835 Test of organic material, brown and opaque, with single aperture; filopodia branched, with non-granular cytoplasm; filopodia anastomose but not into a reticulum; multinucleate; tubular mitochondrial cristae; ciliated dispersal cells or gametes. Gromia. |
| ●●● Ascetosporea Sprague 1979, emend. Cavalier-Smith 2009 Complex spore structure - one or more cells, with one or more sporoplasms, without polar capsules or filaments; parasites of invertebrates. |
| ●●●● Haplosporida Caullery & Mesnil 1899 Distinctive lidded spores; during spore development, spore wall produced inside of outer membrane of invaginated area; without polar capsules or polar filaments; spore anterior opening covered by hinged operculum; intra-nuclear spindle, a rudiment of which persists in interphase nuclei (“kernstab”); tubular mitochondrial cristae; Plasmodial endoparasites of marine and sometimes freshwater animals. Bonamia, Haplosporidium, Microcytos, Minchinia, Urosporidium. |
| ●●●● Paramyxida Chatton 1911 Spore bicellular, consisting of a parietal cell and one sporoplasm; without orifice. Marteilia, Paramyxa, Paramarteilia. |
| ●●●● Claustrosporidium Larsson 1987 Uninucleate sporoplasm with haplosporosomes; spore wall with no orifice and formed on sporoplasm surface, not intracellular as in Haplosporidia; spores without operculum and lingula. Claustrosporidium. |
| ●●●● Paradiniidae Schiller 1935 Unlike other ascestosporans have a biciliate dispersal phase with two unequal cilia; marine parasites of Crustacea with multinucleate plasmodial trophic phase. Paradinium, "the spot prawn parasite". |
| ●●● Incertae sedis Cercozoa: Psammonobiotidae Golemansky 1974 This clade is considered as most likely belonging to Euglyphida. However, this position remains to be confirmed as they do not secrete scales. Including Alepiella, Chardezia, Edaphonobiotus, Micramphora, Micropsammella, Nadinella, Ogdeniella, Psammonobiotus, and Propsammonobiotus. |
| ●●● Incertae sedis Cercozoa: Volutellidae Sudzuki 1979 |
| ●● Retaria Cavalier-Smith 2002 Mainly marine heterotrophs, with reticulopodia or axopodia, and usually having various types of skeleton. |
| ●●● Foraminifera d’Orbigny 1826 Filopodia with granular cytoplasm, forming branching and anastomosing network (reticulopodia); bidirectional rapid (10 mm/s) transport of intracellular granules and plasma membrane domains; tubular mitochondrial cristae; fuzzy-coated organelle of unknown function in reticulopodia; polymorphic assemblies of tubulin as (i) conventional microtubules singly or in loosely organized bundles, (ii) single helical filaments, and (iii) helical filaments packed into paracrystalline arrays; majority of forms possess a test, which can be organic walled, agglutinated, or calcareous; wall structure in naked and single-chambered forms quite variable for “naked” athalamids, such as Reticulomyxa, thicker veins vested with an amorphous, mucoid material; for thecate (soft-walled) species, such as members of the genus Allogromia, proteinaceous with little or no foreign material; for agglutinated species, foreign materials bound with an amorphous or fibrous organic matrix; for multi-chambered (polythalamous) forms, walls containing agglutinated material or mineralized with calcite, aragonite, or silica; life cycle often comprising an alternation of asexually reproducing agamont and sexually reproducing gamont. Note 9. |
| Note 9. The Foraminifera classification has been established mainly based on phylogenetic studies, and now includes Xenophyophorea Schulze 1904, and some athalamids, such as Reticulomyxa Nauss 1949. It differs from a traditional morphology-based classification by separating foraminiferans into 3 major groups (class-level), depending on the number of chambers and their form. The first group comprises early lineages of single-chambered (monothalamous) foraminifera, including athalamous freshwater species such as Reticulomyxa and various environmental clades of unknown morphology. The group is clearly paraphyletic with two multi-chambered classes branching among the monothalamous lineages. The systematics of this group, which can be split into several monophyletic lineages, is currently being revised. However, for the moment and for reasons of convenience, we propose to leave it as an undivided group designated by the informal name “monothalamids”.The two multi-chambered classes comprise the groups that usually correspond to traditional foraminiferal orders established by Loeblich and Tappan (1988). According to molecular phylogenetic data, most of these orders are monophyletic, except Textulariida that are probably paraphyletic and Globigerinida that are polyphyletic. |
| ●●●● “Monothalamids” Pawlowski et al. 2003 (P) Single chamber (monothalamous) test with an organic or agglutinated wall; the group comprises all genera traditionally included into the Allogromiida and Astrorhizida, as well as the Xenophyophorea; it also includes freshwater and marine “naked” amoeboid species and environmental clades with unknown morphology; the diversity of this mainly unfossilized group is poorly known and has been largely overlooked in micropaleontologically oriented foraminiferal research. Allogromia, Astrammina, Crithionina, Notodendrodes, Psammophaga, Reticulomyxa. |
| ●●●● Tubothalamea Pawlowski et al. 2012 Bi- or multi-chambered test with tubular chambers at least in the juvenile stage; wall agglutinated or calcareous; in ancestral forms the test is composed of a spherical proloculus followed by a planispirally enrolled tubular chamber in Ammodiscus, Spirillina, and Cornuspira; more derived forms have multi-chambered tests; the highly diverse group of extinct large Fusulinida probably also belong to this clade. |
| ●●●●● Miliolida Delage & Hérouard 1896 Test bi- or multi-chambered, wall agglutinated or calcareous of high magnesium calcite with randomly oriented crystals refracting light in all directions and resulting in a porcelaneous appearance of the test; generally imperforate walls; chambers tubular or elongate, often planispirally coiled; some with complex internal structures adapted to host algal endosymbionts. Alveolina, Cornuspira, Miliammina, Pyrgo, Quinqueloculina, Sorites. |
| ●●●●● Spirillinida Hohenegger & Piller 1975 Test composed of proloculus followed by an enrolled tubular chamber, undivided or with few chambers per whorl; wall of low-magnesium calcite, optically a single crystal. Patellina, Spirillina. |
| ●●●●● Ammodiscidae Reuss 1862 Test composed of globular proloculus followed by a coiled undivided tubular chamber with terminal aperture; wall agglutinated. Ammodiscus, Glomospira. |
| ●●●● Globothalamea Pawlowski et al. 2012 Test multi-chambered, typically trochospirally enrolled but may be triserial, biserial or uniserial; chambers globular or crescent-shaped in early stage; wall agglutinated or calcareous. |
| ●●●●● Rotaliida Delage & Hérouard 1896 Wall of low-magnesium calcite, optically radial, bilamellar, perforate; some with internal canal system. Ammonia, Bolivina, Elphidium, Epistominella, Nummulites, Rosalina. |
| ●●●●● Globigerinida Delage & Hérouard 1896 (P) Wall of low-magnesium calcite, bilamellar, perforate; surface may be covered with fine, elongate spines; planktonic mode of life. Globigerina, Globigerinoides, Globorotalia, Orbulina. |
| ●●●●● Robertinida Loeblich & Tappan 1984 Wall of hyaline, perforate, optical radial aragonite; chambers with internal partition. Hoeglundina, Robertina, Robertinoides. |
| ●●●●● Textulariida Delage & Hérouard 1896 (P) Wall agglutinated, with foreign particles attached to organic lining or cemented by low magnesium calcite. Cyclammina, Eggerella, Reophax, Textularia, Trochammina. |
| ●●●●● Carterina Brady 1884 [Carterinida Loeblich & Tappan 1981] Wall composed of rodlike spicules of low-magnesium calcite held in organic lining; chambers numerous, trochospirally coiled. Carterina. |
| ●●●● Incertae sedis Foraminifera: Lagenida Delage & Hérouard 1896 |
| ●●● Acantharia Haeckel 1881, emend. Mikrjukov 2000 Cell surrounded by fibrillar capsule outside of cell membrane; axopodia, spicules, and amoeboid anastomosing dynamic network of irregular pseudopodia extending from the capsule; this outer network (ectoplasm) surrounded by fibrillar periplasmic cortex; inner cell region inside capsule (endoplasm) holding the organelles; axopodia, supported by microtubular arrays, with kinetocyst extrusomes and with a centroplast-type centrosome at base of each spicule; 20 radial spicules of strontium sulphate merged at cell centre; spicule tips attached to contractile myonemes at periplasm; tubular mitochondrial cristae; often with algal symbionts in endoplasm, and captured prey in ectoplasm network; asexual reproduction unknown; sexual reproduction involving consecutive mitotic and meiotic divisions that ultimately release 102--103 biciliated isogametic cells; only marine isolates known. |
| ●●●●Chaunocanthida Schewiakoff 1926 Pigmented endoplasm, clears towards periphery; many small nuclei in endoplasm; clear ectoplasm with periplasmic cortex; sexual reproduction in gamontocyst; small plaques as lithosomes synthesized in Golgi and forming the gamontocyst wall; litholophus stage prior to reproduction; hexagonal microtubular arrays in axopodia; contractile matrix at base of spicules. Amphiacon, Conacon, Gigartacon, Heteracon, Stauracon. |
| ●●●●Holocanthida Schewiakoff 1926 Pigmented endoplasm, clears towards periphery; many small nuclei in endoplasm; sexual reproduction in gamontocyst; with lithosomes forming the gamontocyst wall; dodecagonal microtubular arrays in axopodia. Acanthochiasma, Acanthocolla, Acanthoplegma. |
| ●●●●Symphyacanthida Schewiakoff 1926 Pigmented endoplasm, clears towards periphery; ectoplasm clear; single large central nucleus; outer endoplasm with anastomosing pseudopodia; capsule and periplasmic cortex visible with light microscopy; sexual reproduction in gamontocyst with lithosomes forming the gamontocyst wall. Amphilithium, Astrolonche, Pseudolithium. |
| ●●●●Arthracanthida Schewiakoff 1926 Thick capsule clearly demarcates pigmented endoplasm from ectoplasm; axopodia with hexagonal microtubular arrays; many nuclei in endoplasm; algal symbionts in all known species, except at reproduction; sexual reproduction without gamontocyst. Acanthometra, Daurataspis, Dictyacantha, Diploconus, Phractopelta, Phyllostaurus, Pleuraspis, Stauracantha. |
| ●●●● Taxopodida Fol 1883 Axopodial pseudopods without kinetocysts, used for motility as oars; axopodial microtubules originate from depressions in nuclear envelope; microtubules in axoneme arranged in irregular hexagons; periplasm of siliceous tangential spicules, with external radial spicules. Sticholonche, several environmental clades. |
| ●●●Polycystinea Ehrenberg 1838, emend. Haeckel 1887 Central capsule spherical to ovate with round pores in the capsular wall either distributed uniformly on the surface of a spherical capsular wall or localized at one pole of an ovate capsular wall; skeleton either absent or when present, composed of spicules or forming elaborate geometric-shaped, porous or latticed shells. |
| ●●●●Spumellaria Ehrenberg 1875, Haeckel 1887, emend. Riedel 1967 Central capsule typically spherical with uniformly distributed round pores in the capsular wall; skeleton either absent or when present, composed of spicules or forming latticed shells (spicules single, or multiple and concentrically arranged). Subdivisions not fully resolved. Actinomma, Didymocyrtis, Euchitonia, Hexacontium, Hexalonche, Hexastylus, Octodendron, Plegmosphaera, Saturnalis, Spongaster, Spongosphaera. |
| ●●●●Nassellaria Ehrenberg 1875, emend. Haeckel 1887 Central capsule ovate with pores localized at one pole; skeleton, when present, composed of a simple tripod, a sagittal ring without tripod or porous helmet-shaped “cephalis” enclosing the central capsule. Artostrobus, Eucyrtidium, Lithomelissa, Pterocanium, Pterocorys. |
| ●●●●Collodaria Haeckel 1887 Skeleton either absent or or when present, composed of scattered spicules within the extra-capsulum; solitary or colonial forms. Acrosphaera, Collosphaera, Collozoum, Sphaerozoum, Rhaphidozoum, Siphonsphaera, Thalassicolla. |
| ●● Incertae sedis Rhizaria: Gymnosphaerida Poche 1913, emend. Mikrjukov 2000 Axopodial microtubules in irregular hexagonal prism; kinetocyst and other types of extrusomes along axopodia; tubular mitochondrial cristae; in some genera, cells attached to substrate with cytoplasmic stalk; free-swimming as amoeboid or motile biciliated cells; one or more nuclei, often located in the amoeboid base of stalk when present; complex life cycle unresolved. Actinocoryne, Gymnosphaera, Hedraiophrys. |
| ●●Incertae sedis Rhizaria: Actinolophus, Biomyxa, Cholamonas, Dictiomyxa, Helkesimastix, Katabia, Myxodictyium, Penardia, Pontomyxa, Protomyxa, Protogenes, Pseudospora, Rhizoplasma Sainouron, Wagnerella. |
| ARCHAEPLASTIDA Adl et al. 2005 Photosynthetic plastid with chlorophyll type a from an ancestral primary endosymbiosis with a cyanobacterium; plastid secondarily lost or reduced in some; usually with cellulose cell wall; flat mitochondrial cristae; starch storage product. Note 10. |
| Note 10. ARCHAEPLASTIDA: Chloroplastida: the terms Chlorobiota and Chlorobionta are not acceptable because there are many “green” genera outside of the Archaeplastida. The term Viridiplantae (green plant) is not acceptable because most of these genera are not plants, traditionally or as defined here. |
| ●Glaucophyta Skuja 1954 [Glaucocystophyta Kies & Kremer 1986] Plastid in the form of a cyanelle which is distinct from the chloroplasts of other organisms in that, like cyanobacteria it has a peptidoglycan wall between its two membranes; chlorophyll type a only, with phycobiliproteins and other pigments; ciliate and non-ciliate species or life cycle stages. Cyanophora, Glaucocystis, Gloeochaete. |
| ●Rhodophyceae Thuret 1855, emend. Rabenhorst 1863 [Rhodophyta Wettstein 1901, Rhodoplantae Saunders & Hommersand 2004] emend. Adl et al. 2005 Without ciliated stages, and without centrioles, or basal bodies, or other 9+2 microtubular structures -- presence of polar rings instead; two-membraned simple chloroplasts, unstacked thylakoids with phycobilisomes, and chlorophyll a only, lacking external endoplasmic reticulum; cytoplasmic carbohydrate reserve floridean starch; chromosomal and inter-zonal microtubules not converging towards polar rings, so spindle poles very broad; telophase spindle and nuclear envelope persisting with closed mitosis surrounded by perinuclear endoplasmic reticulum; cell wall of cellulose; cells in filamentous forms linked by pit plugs, formed between cells after incomplete cell division; sexual reproduction typically oogamous; triphasic life history common. |
| ●● Cyanidiales T. Christensen 1962 [Cyanidiophyceae Merola et al. 1981, Cyanidiophyta Moehn ex Doweld 2001] Unicellular red algae, spherical or elliptical in shape; thick cell wall or lack of cell wall; facultative heterotrophs or obligate photoautotrophs; cell division or endospore formation; inhabiting acidic and high temperature environments. Cyanidioschyzon, Cyanidium, Galdieria. |
| ●● Rhodellophyceae Cavalier-Smith 1998 [Rhodellophytina Cavalier-Smith 1998] Unicellular red algae; a single highly lobed plastid with eccentric or centric pyrenoid; Golgi association with nucleus and ER; contains mannitol; reproduction by cell division. Dixoniella, Glaucosphaera, Rhodella. |
| ●● Stylonematales K. Drew 1956 [Stylonematophyceae H.S. Yoon et al. 2006] Unicellular or pseudofilamentous or filamentous red algae; various plastid morphologies with or without pyrenoid; Golgi association with mitochondria and ER; reproduction by cell division or monospores. Bangiopsis, Chroodactylon, Chroothece, Purpureofilum, Rhodosorus, Rhodospora, Rufusia, Stylonema. |
| ●● Porphyridiophyceae H. S. Yoon et al. 2006 Unicellular red algae with a single branched or stellate plastid, with or without pyrenoid; Golgi association with mitochondria and ER; cells with floridoside as a low molecular weight carbohydrate; reproduction by cell division. Erythrolobus, Flintiella, Porphyridium. |
| ●● Compsopogonales Skuja 1939 [Compsopogonophyceae G. W. Saunders & Hommersand 2004] Red algae with monosporangia and spermatangia usually cut out by curved walls from ordinary vegetative cells; Golgi–ER association; encircling thylakoids in the plastid; life history biphasic if known. Boldia, Compsopogon, Erythrotrichia, Rhodochaete. |
| ●● Bangiales Nägeli 1847 [Bangiophyceae A. Wettstein 1901] Pluricellular red algae with Golgi–ER/mitochondrion association; life history biphasic, heteromorphic, gametophyte macroscopic, initially uniseriate, becoming pluriseriate or foliose by diffuse growth; carposporangia and spermatangia produced in packets by successive perpendicular divisions; sporophyte filamentous, with pit plugs with a single cap layer, but lacking membranes; typically forming conchospores in fertile cell rows. Bangia, Bangiomorpha, Boreophyllum, Dione, Minerva, Porphyra, Pyropia, Pseudobangia. |
| ●●Florideophycidae Cronquist 1960 Pluricellular red algae with Golgi–ER/mitochondrion; growth by means of apical cells and lateral initials forming branched filaments in which the cells are linked throughout by pit connections; life history fundamentally triphasic consisting of gametophytic, carposporophytic, and tetrasporophytic phases; reproductive cells (monosporangia, spermatangia, carposporangia, tetrasporangia) generally terminal or lateral on the filaments; carpogonia terminal or lateral, bearing an apical extension, the trichogyne, to which the spermatangia attach; carposporophyte developing directly from the carpogonium or its derivative. |
| ●●● Hildenbrandia Nardo 1834 [Hildenbrandiophycidae G. W. Saunders & Hommersand 2004] Red algae that are crustose and smooth to tubercular or with erect branches; composed of a basal layer of laterally adhering branched filaments and laterally adhering simple or branched erect filaments; pit plugs with a single cap layer and membrane; tetrasporangia zonately or irregularly divided, apomeiotic, borne in ostiolate conceptacles; sexual reproduction unknown. Hildenbrandia. |
| ●●●Nemaliophycidae Christensen 1978 Pit plugs characterized by two cap layers. Acrochaetium, Balbiania, Ballia, Batrachospermum, Colaconema, Nemalion, Palmaria, Rhodychlya, Thorea. |
| ●●●Corallinophycidae L. Le Gall & G. W. Saunders 2007 Carpogonial branches two-celled; tetrasporangia zonate or cruciate in division; pit plug with two cap layers at cytoplasmic faces, outer dome shaped, membrane absent; calcification in the form of calcite. Corallina, Lithophyllum, Mastophora, Melobesia, Metagoniolithon, Rhodogorgon, Sporolithon. |
| ●●●Ahnfeltiophycidae G. W. Saunders & Hommersand 2004 Carpogonia terminal and sessile; carposporophyte developing outward; pit plugs naked, lacking caps and membranes. Ahnfeltia, Pihiella. |
| ●●●Rhodymeniophycidae G. W. Saunders & Hommersand 2004 Red algae with sexual life histories generally triphasic; carposporophyte developing directly from the carpogonium or carpogonial fusion cell, or indirectly from an auxiliary cell that has received the postfertilization diploid nucleus; pit plugs with membranes only (single inner cap in Gelidiales). Acrosymphytum, Bonnemaisonia, Ceramium, Gelidium, Gigartina, Gracilaria, Halymenia, Nemastoma, Plocamium, Rhodymenia, Sebdenia. |
| ●Chloroplastida Adl et al. 2005 [Viridiplantae Cavalier-Smith 1981; Chlorobionta Jeffrey 1982, emend. Bremer 1985, emend. Lewis & McCourt 2004; Chlorobiota Kendrick & Crane 1997] Plastid with chlorophyll a and b; cell wall with cellulose usually present; with centrioles. |
| ●●Chlorophyta Pascher 1914, emend. Lewis & McCourt 2004 Cilia of swimming cells in pairs or multiples of two, with stellate structure linking 9 pairs of microtubules at basal body transition zone; thylakoids single or stacked; plastid with two membranes without periplastid endoplasmic reticulum; starch inside plastid; glycolate dehydrogenase present; cell wall, when present, of cellulose; cell division without phragmoplast. |
| ●●●Ulvophyceae Mattox and Stewart 1984 (P) Swimming cells with one or two pairs of cilia, without mastigonemes; basal bodies with 4 microtubular rootlets in cruciate arrangement, and smaller roots of two sizes, alternating between 2 or more microtubules; cilia with scales and rhizoplasts; cell wall more or less calcified; cell division by furrowing with mitotic spindle closed, centric and persistent; phycoplast absent; thallus can be branched or unbranched, mono- or distromatic sheet (phyllose), or cushiony forms of compacted tubes; thallus often multinucleate and siphonous; free-living diplobiontic life cycle, iso- or heteromorphic. Acetabularia, Caulerpa, Chladophora, Codium, Pithophora, Pseudonochloris, Rhizoclonium. |
| ●●●Trebouxiophyceae Friedl 1995 [Pleurastrophyceae Mattox et al. 1984, Microthamniales Melkonian 1990] Swimming cells with one or two pairs of cilia, without mastigonemes; basal bodies with 4 microtubular rootlets in cruciate arrangement, including a multi-layered structure, and a smaller root, alternating between 2 or more microtubules; basal bodies with prominent rhizoplast, cruciate, displaced counter-clockwise and counter-clockwise basal body orientation; closed mitosis with metacentric spindle, semi-closed mitosis; cytokinesis with phycoplast; asexual reproduction by autospores or zoospores; sexual reproduction reported but not observed; lichenose and free-living forms; osmotrophy and autotrophy. Botryococcus, Chlorella, Choricystis, Coccomyxa, Microthamnion, Nannochloris, Oocystis, Pabia, Prasiola, Prototheca, Trebouxia. |
| ●●●Chlorophyceae Christensen 1994 Swimming cells with one to hundreds of cilia, without mastigonemes; when 2 or 4 cilia, basal bodies with 4 microtubular rootlets in cruciate arrangement, alternating between 2 and more microtubules; basal bodies displaced clockwise or directly opposed; rhizoplast connects basal bodies and extends to nucleus; in colonial forms, basal bodies re-oriented to face outside of colony; closed mitosis; cytokinesis has phycoplast with microtubules, sometimes with furrowing, with formation of plasmodesmata cell-cell connections; haplobiontic life cycle; sexual reproduction by isogamy, anisogamy or oogamy; asexual reproduction by aplanospores, akinetes, or autosporic; osmotrophy and autotrophy. Bracteacoccus, Chlamydomonas (P), Desmodesmus, Floydiella, Hydrodictyon, Oedegonium, Pediastrum, Scenedesmus, Volvox. |
| ●●●● Incertae sedis Chlorophyceae: Carteria, Cylindrocapsa, Hafniomonas, Mychanastes, Treubaria, Trochiscia. |
| ●●●Pedinophyceae Moestrup 1991, emend. Fawley et al. in Adl et al. 2012 Unicellular, with single cilium; closed mitosis with persistent spindle; phycoplast absent; counter-clockwise basal body orientation; cilia covered with rigid or thin, hair-like appendages; single parietal chloroplast. Marsupiomonas, Pedinomonas. |
| ●●●Nephroselmidophyceae Cavalier-Smith 1993, emend. Yamaguchi 2011 Cells laterally compressed; two cilia inserted laterally; square- or diamond-shaped scales cover cell body and cilia, except in at least one species – Nephroselmis pyriformis; single, cup-shaped chloroplast with pyrenoid and eyespot; contracticle vacuole near ciliary bases in freshwater species; sexual reproduction by hologamy; mostly marine, some freshwater. Nephroselmis. |
| ●●●Mamiellophyceae Marin & Melkonian 2010 Cells typically solitary, with single chloroplast, sometimes two, surrounded by two membranes; chlorophylls a & b; prasinoxanthin commonly present; 2 cilia, single or no cilium present; cilia equal or unequal in length; eyespot posterior if present; cells and/or cilia with 1–2 layers of flattened, rounded or elliptical scales, or scales absent; scales ornamented with spider web-like or uniformly reticulate pattern; mostly marine, some freshwater. Bathycoccus, Dolichomastix, Mamiella, Monomastix. |
| ●●● Prasinophytae Cavalier-Smith 1998, emend. Lewis & McCourt 2004 [Micromonadophyceae Mattox & Stewart 1984] (P) Cells 1–8 cilia, occurring in multiples of two, inserted in a ciliary pit; basal body rootlet structure diverse; rhizoplast extends beyond nucleus; cilia forward and pulling, or undulating and pushing; cilia with lateral mastigonemes; cells with 1–7 distinct types of organic extracellular scales, sometimes elaborate, covering cell wall and cilia; some with extrusomes; cysts in some; mitosis variable, most with persistent telophase spindle; nutrition by autotrophy and osmotrophy. Crustomastix, Halosphaera, Pyramimonas, Pycnococcus, Pseudoscourfieldia, Prasinococcus. |
| ●●●Chlorodendrophyceae Fritsch 1917 Cells with a pair of cilia, inserted in a ciliary pit; cilia beat in breast-stroke pattern; basal body rootlets structure in X2X2 configuration; with organic extracellular scales, outer layer of scales fused to form a theca; metacentric spindle collapses at telophase; nutrition by autotrophy and osmotrophy. Scherffelia, Tetraselmis. |
| ●●●Palmophyllales Zechman et al. 2010 Thallus macroscopic, crustose or erect; subspherical cells in gelatinous matrix make up thallus; cell diameter 6--10 µm; each cell with single cup-shaped chloroplast lacking pyrenoids; benthic marine. Palmophyllum, Palmoclathrus, Verdigellas. |
| ●●Charophyta Migula 1897, emend. Karol et al. 2009 [Charophyceae Smith 1938, Mattox & Stewart 1984] Asymmetric motile cells, when present, with pair of cilia without mastigonemes; basal bodies with distinctive multilayered structure of microtubular rootlet and cytoskeletal anchor; thylakoids stacked; plastid with two membranes without periplastid endoplasmic reticulum; starch inside plastid; open mitosis; usually with phycoplast, but some with phragmoplast and cell plate; with primary plasmodesmata between adjacent cells in filamentous forms; filaments branching or non-branching; with non-motile vegetative phase; some with multinucleate cells; with or without sexual reproduction; sexual species with haplobiontic life cycle; with desiccation-resistant cysts (zygospores); glycolate oxydase in peroxisomes; Cu/Zn superoxide dismutase; ciliary peroxisome. |
| ●●● Chlorokybus Geitler 1942 [Chlorokybophyceae Lewis & McCourt 2004] (M) Sarcinoid packets of cells; subaerial; biciliated zoospores; cilia with hairs; multi-layered structure (MLS) at ciliary root. Chlorokybus atmophyticus. |
| ●●● Mesostigma Lauterborn 1894 [Mesostigmatophyceae Marin & Melkonian 1999, emend. Lewis & McCourt 2004; Mesostigmata Turmel et al. 2002] (M) Asymmetrical cell with pair of lateral cilia without mastigonemes, emerging from a pit; basal body transition region with similarity to Streptophytina; multilayered structure anchor associated with basal body; with chlorophylls a and b; plastid with two membranes without periplastid endoplasmic reticulum; starch inside plastid; with glycolate oxidase; flagellar peroxisome present; cell wall of cellulose; organic scales cover cell wall and flagella. Mesostigma viride. |
| ●●● Klebsormidiophyceae van den Hoek et al. 1995 Coccoid or unbranched filaments; one or two chloroplasts with one pyrenoid; most chloroplasts parietal in shape; cleavage furrow during cell division but no cell plate or phragmoplast; sexual reproduction unknown. Entransia, Interfilum, Klebsormidium. |
| ●●●Phragmoplastophyta Lecointre & Guyander 2006 Cell division by way of some form of phragmoplast; some oogamous, others anisogamous with non-motile female gamete and motile male gamete. |
| ●●●● Zygnematophyceae van den Hoek et al. 1995, emend. Hall et al. 2009 Without ciliated stages; sexual reproduction via conjugation; thalli unicellular or filamentous; no centrioles. Spirogyra, Staurastrum. |
| ●●●●Coleochaetophyceae Jeffrey 1982 Thalli discs of cells or branched filaments; sheathed hairs as extensions of the cell wall. Coleochaete, Chaetosphaeridium. |
| ●●●●Streptophyta Jeffrey 1967 Twisted or spiraled ciliated motile cells. |
| ●●●●● Charophyceae Smith 1938, emend. Karol et al. 2009 [Charales Lindley 1836; Charophytae Engler 1887] Thallus attached to substrate with rhizoids; thallus a central axis of multinucleate internodal cells, with whorls of branchlets radiating from mononucleate cells at nodes; calcium carbonate accumulates in cell wall of many species; haplobiontic life cycle; sexual reproduction oogamous with sperm cells; differentiated sperm and egg producing organs; antheridium with several shield cells and a manubrium that gives rise to spermatogenous filaments. Chara, Nitella, Tolypella. |
| ●●●●●Embryophyta Engler 1886, emend. Lewis & McCourt 2004 [Cormohyta Endlicher 1836; Plantae Haeckel 1866] Ciliated basal bodies, when present, with distinctive multilayered structure of microtubules and cytoskeletal anchor; open mitosis with phragmoplast at cytokinesis; plasmodesmata and other characteristic cell-cell junctions; diplobiontic life cycle, with vegetative propagation possible in many; alternation of generations, with fertilization of egg by sperm inside protective test; embryology with tissue differentiation coordinated by hormones; differentiated sperm and egg cells, may be on different sexual individuals, on different organs of the same individual, or in the same organ. Subdivisions not shown. |
| EXCAVATA Cavalier-Smith 2002, emend. Simpson 2003 Typically with suspension-feeding groove of the “excavate” type, secondarily lost in many taxa; feeding groove used for capture and ingestion of small particles from feeding current generated by a posteriorly directed cilium (F1); right margin and floor of groove are supported by parts of the R2 microtubular root, usually also supported by non-microtubular fibres (B fibre, composite fibre), and the left margin by the R1 microtubular root and C fibre. Grouping somewhat controversial, although recent multigene phylogenies have markedly increased support for monophyly. Apomorphy: Suspension-feeding groove, homologous to that in Jakoba libera. |
| ● Metamonada Cavalier-Smith 1987 [Metamonadina Grassé 1952], emend. Cavalier-Smith 2003 Anaerobic/microaerophilic, with modified mitochondria that lack cristae, are non-respiratory, and lack a genome (e.g. hydrogenosomes or mitosomes); mostly ciliated cells, usually with four kinetosomes per kinetid; some free-living, many endobiotic or parasitic. Apomorphy: mitochondrial organelles anaerobic, and non-respiratory. |
| ●● Fornicata Simpson 2003 With single kinetid and nucleus, or one pair each of kinetids and nuclei; 2–4 kinetosomes per kinetid; usually with a feeding groove or cytopharyngeal tube associated with each kinetid. Apomorphy: “B fibre” originates against R2 microtubular root. |
| ●●● Diplomonadida Wenyon 1926, emend. Brugerolle et al. 1975 Usually with ‘diplomonad’ cell organization, namely a pair of kinetids and two nuclei, each kinetid usually with four ciliated kinetosomes but sometimes only 2 or 3 ciliated; some taxa (‘enteromonads’) secondarily have a single kinetid and nucleus; at least one cilium per kinetid directed posteriorly, associated with a cytopharyngeal tube or groove, or lying axially within the cell; various nonmicrotubular fibres supporting the nucleus and cytopharyngeal apparatus; free-living or endobiotic, often parasitic. Apomorphy: diplomonad cell organization |
| ●●●● Hexamitinae Saville Kent 1880, emend. Brugerolle et al. 1975 With functional feeding apparatuses; with an alternate genetic code – TAR codon for glutamine; several have a single kinetid and nucleus. Enteromonas, Hexamita, Spironucleus, Trepomonas,, Trimitus. |
| ●●●● Giardiinae Kulda and Nohýnková 1978 Without distinct feeding apparatuses; one posteriorly directed cilium from each kinetid (F1) runs through the length of the cell axially and is intra-cytoplasmic; all endobiotic. Giardia, Octomitus. |
| ●●● Retortamonadida Grassé 1952 Single ciliary apparatus with four kinetosomes and either two (Retortamonas) or four (Chilomastix) emergent cilia; posterior cilium has 2–3 vanes and is associated with a ventral feeding groove with posterior cytostome; cell surface underlain by a corset of microtubules; all endobiotic, except one free-living species. Apomorphy: “lapel” structure as an electron-dense sheet supporting the anterior origin of the peripheral microtubules. Chilomastix, Retortamonas. Note that molecular phylogenetic studies currently do not support monophyly, perhaps due to misidentification/polyphyly of Retortamonas spp. |
| ●●● ‘Carpediemonas-like organisms’ (Kolísko et al. 2010) (P) Free-living, marine, anaerobic/microaerophilic ciliated cells with a broad ventral suspension-feeding groove; almost always biciliated, but with 2–4 kinetosomes; posterior cilium with 1–3 vanes and beating within the groove; with relatively large cristae-lacking mitochondrion-related organelles; consistently recovered as a paraphyletic assemblage within Fornicata in molecular phylogenies. Carpediemonas, Dysnectes, Ergobibamus, Hicanonectes, Kipferlia. |
| ●● Parabasalia Honigberg 1973 Cells with a parabasal apparatus; two or more striated parabasal fibres connecting the Golgi apparatus to the ciliary apparatus; kinetid generally with four cilia/kinetosomes, but frequently with additional cilia (one to thousands); one kinetosome bears sigmoid fibres that connect to a pelta–axostyle complex; reduction or loss of the ciliary apparatus in some taxa, or multiplication of all, or parts, of the ciliary apparatus in several taxa; closed mitosis with an external spindle, including a conspicuous microtubular bundle; hydrogenosomes in place of mitochondria. Apomorphy: parabasal apparatus. |
| ●●● Trichomonadea Kirby 1947, sensu Margulis 1974, emend. Čepička et al. 2010 (P?) Four--six cilia with one ciliary axoneme supporting a lamelliform undulating membrane: B-type costa sometimes absent; comb-like structure and infrakinetosomal body absent; axostyle usually of “Trichomonas type”. Hexamastix, Pentatrichomonas, Pseudotrichomonas, Tricercomitus, Trichomonas. |
| ●●● Hypotrichomonadea Čepička et al. 2010 Four cilia with one ciliary axoneme supporting a lamelliform undulating membrane; A-type costa; comb-like structure present, but no infrakinetosomal body; biramous parabasal body; axostyle of “Trichomonas type”. Hypotrichomonas, Trichomitus. |
| ●●● Tritrichomonadea Čepička et al. 2010 (P?) Uninucleate or binucleate; 0--5 (4 ancestrally) cilia; ancestrally with comb-like structure, suprakinetosomal and infrakinetosomal body; if present, undulating membrane typically of rail type; A-type costa; axostyle of “Tritrichomonas type” or “Trichomonas type”. Dientamoeba, Histomonas, Monocercomonas, Tritrichomonas. |
| ●●● Cristamonadea Brugerolle & Patterson 2001, sensu Čepička et al 2010 Uninucleate to multinucleate; akaryomastigonts in addition to karyomastigonts in some multinucleate genera; two-to-thousands of cilia per mastigont; kinetosomes, except for ‘privileged kinetosomes’, often discarded during cell division in highly ciliated taxa; some with cresta and paraxonemal rod associated with the recurrent cilium; axostyle ancestrally of Tritrichomonas type, secondarily thin or reduced in some; multiple axostyles in multinuclear forms; parabasal body single or multiple, ellipsoid or rod-shaped, often spiraled or ramified. Coronympha, Deltotrichonympha, Devescovina, Foaina, Joenia, Mixotricha. |
| ●●● Trichonymphea Poche1913, sensu Cavalier-Smith 2003 Bilaterally or tetraradially symmetrical, with anterior rostrum divided into two hemirostra; each hemirostrum bears one or two ciliary areas with hundreds to thousands of cilia; cilia usually retained during cell division; one hemirostrum goes to each daughter cell; numerous parabasal fibers originate from two or four parabasal plates that form a rostral tube in some; numerous thin axostyles do not protrude outside the cell. Barbulanympha, Hoplonympha, Staurojoenia, Trichonympha. |
| ●●● Spirotrichonymphea Grassé 1952, sensu Čepička et al. 2010 Kinetosomes in counterclockwise spiral rows; cilia retained during cell division with the ciliary rows dividing between daughter cells; axostyle single of Tritrichomonas type, or multiple in thin bands, or reduced. Holomastigotes, Holomastigotoides, Microjoenia, Spirotrichonympha. |
| ●● Preaxostyla Simpson 2003 Heterotrophic with four cilia and kinetosomes per kinetid; lacking classical mitochondria. Apomorphy: “I fibre” with “preaxostylar” substructure – the oxymonad preaxostyle is homologous to the R2 root and I fibre of Trimastix. |
| ●●● Oxymonadida Grassé 1952 Single kinetid (occasionally multiple kinetids) consisting of two pairs of ciliated kinetosomes distantly separated by a pre-axostyle (microtubular root R2, with paracrystalline lamina), from which arises a microtubular axostyle; the axostyle is contractile or motile in some taxa; microtubular pelta usually present; many taxa attach to host using an anterior holdfast; closed mitosis with internal spindle. Oxymonads are gut endosymbionts, mostly in lower termites and Cryptocercus. Apomorphy: axostyle (non-homologous with that of Parabasalia). Dinenympha, Monocercomonoides, Oxymonas, Polymastix, Pyrsonympha, Saccinobaculus, Streblomastix. |
| ●●● Trimastix Saville Kent 1880 Free-living cell with four cilia bearing a broad ventral feeding groove, in which beats the posteriorly directed cilium; posterior cilium with two broad vanes; small dense organelles in place of mitochondria. Trimastix. |
| ● Malawimonas O’Kelly & Nerad 1999 Small free-living biciliated cells, superficially similar to Carpediemonas but not closely related and with a typical respiratory mitochondrion with discoidal cristae and genome; two kinetosomes, a single ventral ciliar vane; typically from freshwater or soil. Malawimonas. |
| ● Discoba Simpson in Hampl et al. 2009 (R) A grouping robustly recovered in multi-gene phylogenetic analyses, containing Heterolobosea, Euglenozoa, Jakobida, and recently, Tsukubamonas. Node-based definition: the clade stemming from the most recent common ancestor of Jakoba libera, Andalucia godoyi, Euglena gracilis and Naegleria gruberi. |
| ●● Jakobida Cavalier-Smith 1993, emend. Adl et al. 2005 With two cilia at the head of a broad ventral feeding groove, in which beats the posterior cilium; posterior cilium with a single dorsal vane that is distinctive among excavates but possibly plesiomorphic. |
| ●●● Jakoba Patterson 1990 (M) Free-swimming cells; can attach temporarily to surfaces by the distal portion of the anterior cilium; flat mitochondrial cristae. Jakoba libera. |
| ●●● Histionidae Flavin & Nerad 1993 Feeding cells sessile and loricate; tubular mitochondrial cristae. Apomorphy: lorica. Histiona, Reclinomonas. |
| ●●●● Incertae sedis Histionidae: Stenocodon, Stomatochone. |
| ●●● Andalucia Lara et al. 2006 Free-swimming cells, some attaching temporarily like Jakoba, with tubular mitochondrial cristae or anaerobic/microaerophilic with cristae-lacking mitochondrial organelles. Andalucia. |
| ●●● Incertae sedis Jakobida: Seculamonas nomen nudum. |
| ●● Discicristata Cavalier-Smith 1998 With discoidal mitochondrial cristae, rarely secondarily altered. Note that this contains two groups – Heterolobosea and Euglenozoa – with little special similarity except presence discoidal cristae, which may be apomorphic, but also may be a plesiomorphy since Malawimonas also has discoidal mitocondrial cristae. |
| ●●● Heterolobosea Page & Blanton 1985 Typically amoebae with eruptive pseudopodia, but with alternate ciliated phase, which is often non-feeding; many species lack the ciliated phase, while some others lack the amoeboid phase; ciliated cells usually with two or four cilia; if capable of feeding usually use a groove-like cytostome. Closed mitosis with internal spindle; mitochondrial cristae flattened, often discoidal; discrete dictyosomes not observed. Apomorphy: eruptive pseudopodia, not homologous to eruptive pseudopodia in Amoebozoa. |
| ●●●● Pharyngomonadidae Cavalier-Smith in Cavalier-Smith & Nikolaev 2008 Cells with four cilia in side-by-side obtuse pairs; feeding using a large groove and cytopharynx; with amoeboid phase; lack helix 17-1 region in SSU rRNA that is typical of Tetramitia. Pharyngomonas. |
| ●●●● Tetramitia Cavalier-Smith 1993, emend. Cavalier-Smith in Cavalier-Smith & Nikolaev 2008 [Vahlkampfiidae Jollos 1917] Ciliated forms usually with four cilia or two per kinetid, with parallel kinetosomes, although Stephanopogon has numerous monokinetids. Apomorphy: distinct helix 17-1 region in SSU rRNA sequence. This group includes the typical vahlkampfiid, and gruberellid amoebae, and acrasid slime molds. Acrasis, Heteramoeba, Naegleria, Percolomonas, Pocheina, Psalteriomonas, Stephanopogon, Tetramitus, Vahlkampfia. |
| ●●●Euglenozoa Cavalier-Smith 1981, emend. Simpson 1997 Cells with two cilia, occasionally one, rarely more, inserted into an apical/subapical ciliar pocket; with rare exceptions, emergent cilia with heteromorphic paraxonemal rods; usually with tubular feeding apparatus associated with ciliary apparatus; basic ciliary apparatus pattern consisting of two functional kinetosomes and three asymmetrically arranged microtubular roots; mostly with discoidal mitochondrial cristae. Apomorphy: Heteromorphic paraxonemal rods, tubular/whorled in anterior cilium F2 and a parallel lattice in posterior cilium F1. |
|
●●●●Euglenida Bütschli 1884, emend. Simpson 1997 With a pellicle of proteinaceous strips, fused in some taxa; when unfused, strips capable of active distortion (metaboly); where known, paramylon is the carbohydrate store. Apomorphy: Pellicle of protein strips. |
| ●●●●● Heteronematina Leedale 1967 (P) With ingestion apparatus capable of phagotrophy; lacking plastids; most glide on surfaces on one or both cilia; a paraphyletic assemblage from which Euglenophyceae/Euglenea and Aphagea are descended. Anisonema, Dinema, Entosiphon, Heteronema, Lentomonas, Metanema, Notosolenus, Peranema, Petalomonas, Ploeotia. Note 11. |
| Note 11. Presently there is not a phylogenetic taxonomy for phagotrophic euglenids as a whole. Traditional systems based on, for example, the presence or absence of conspicuous feeding apparatuses, are artificial. Some possibly/probably monophyletic taxa have been proposed that would cover restricted subsets of the phagotrophic euglenids: for example, the Ploeotiida, including Ploeotia, Entosiphon, Lentomonas, and presumably Keelungia; and the Petalomonadida, including Petalomonas, Notosolenus, and Calycimonas, and presumably also taxa such as Sphenomonas. The limited taxon sampling for molecular sequence data is a significant impediment, especially as many traditional genera are probably polyphyletic. |
| ●●●●●Euglenophyceae Schoenichen 1925, emend. Marin & Melkonian 2003 [Euglenea Bütschli 1884, emend. Busse & Preisfeld 2002] Phototrophic, with one to several plastids of secondary origin with three bounding membranes and chlorophylls a and b; some species secondarily non-photosynthetic; photosensory apparatus associated with cilia; most swim. |
| ●●●●●●Rapaza Yamaguchi et al. 2012 (M) Flexible cell with 2 cilia; has own plastid but also phagotrophic and retains plastids from engulfed algal prey, such as Tetraselmis; apparently sister group to other Euglenophyceae. Rapaza viridis. |
| ●●●●●●Eutreptiales Leedale 1967, emend. Marin & Melkonian 2003 2--4 emergent, heterodynamic cilia of equal or unequal length; cells not rigid, usually capable of metaboly; mostly marine or brackish species, rarely freshwater. Eutreptia, Eutreptiella. |
| ●●●●●● Euglenea Bütschli 1884, emend. Busse & Preisfeld 2002 [Euglenales Leedale 1967, emend. Marin & Melkonian 2003] Single emergent cilium and second cilium within the reservoir, or both cilia nonemergent; phototrophic or heterotrophic; mostly freshwater species. |
| ●●●●●●● Phacaceae Kim et al. 2010 Solitary, with one emergent cilium; palmelloid stages, cysts and envelopes unknown; numerous small discoid chloroplasts without pyrenoids. Discoplastis, Lepocinclis, Phacus. |
| ●●●●●●● Euglenaceae Dujardin 1841, emend. Kim et al. 2010. Cells solitary or colonial, with one emergent cilium; one to several large chloroplasts of various shapes with or without pyrenoid. Ascoglena, Colacium, Cryptoglena, Cyclidiopsis, Euglena, Euglenaria, Euglenopsis, Monomorphina, Strombomonas, Trachelomonas. |
| ●●●●●Aphagea Cavalier-Smith 1993, emend. Busse & Preisfeld 2002 Osmotrophic euglenids lacking photosensory apparatus and plastids; one or two emergent cilia; no ingestion apparatus. Astasia sensu stricto, Distigma, Distigmopsis, Menoidium, Rhabdomonas. |
|
●●●●Diplonemea Cavalier-Smith 1993, emend. Simpson 1997 Heterotrophic cells exhibiting pronounced metaboly; in trophic phase cilia are short and lack paraxonemal rods; sometimes with dispersal phase with longer paraxonemal rod-bearing cilia; apical papilla, feeding apparatus with ‘pseudovanes’; giant, flattened mitochondrial cristae; exhibit mitochondrial RNA trans-splicing. Apomorphy: Paraxonemal rods absent in trophic phase, homologous to that in Diplonema ambulator Larsen & Patterson 1990. Diplonema, Rhynchopus. |
|
●●●● Symbiontida Yubuki et al. 2009 Microaerobic or anaerobic cells that possess rod-shaped epibiotic bacteria; lack euglenid-type pellicle strips. Currently treated as a major taxon within Euglenozoa, but are probably derived phagotrophic euglenids. Apomorphy: Rod-shaped epibiotic bacteria above superficial layer of mitochondrion-derived organelles with reduced or absent cristae, homologous to the organization in Calkinisia aureus. Bihospites, Calkinsia, Postgaardi. |
|
●●●●Kinetoplastea Honigberg 1963 Cells with a kinetoplast, which is a large mass(es) of mitochondrial (=kinetoplast; k) DNA; many mitochondrial pre-mRNAs subject to moderate or extensive editing. Apomorphy: kinetoplast. |
| ●●●●●Prokinetoplastina Vickerman in Moreira et al. 2004 (R) Two genera: Ichthyobodo are polykinetoplastic and biciliated cells with the cilia originating in a pocket that continues as a furrow; ectoparasitic, freshwater and marine. Perkinsela-like organisms (PLOs) - are non-ciliated, oval cells with a single large kinetoplast, which live as endosymbionts (‘parasomes’) of certain amoebae (e.g. Neoparamoeba spp.), but are not enclosed in parasitophorous membrane. Ichthyobodo, Perkinsela. |
| ●●●●●Metakinetoplastina Vickerman in Moreira et al. 2004 (R) Group identified by SSU rRNA phylogenies. Node-based definition: clade stemming from the most recent common ancestor of Neobodonida, Parabodonida, Eubodonida, and Trypanosomatida. |
| ●●●●●●Neobodonida Vickerman in Moreira et al. 2004 (R) Eu- or polykinetoplastic kDNA not in a single network, but in multiple loci throughout mitochondrioplasm; biciliate, without conspicuous hairs; posterior cilium attached or free; phagotrophic or osmotrophic; preciliary rostrum containing apical cytosome. Node: Cruzella, Dimastigella, Neobodo, Rhynchobodo, Rhynchomonas. Other included genera: Actuariola. |
| ●●●●●●Parabodonida Vickerman in Moreira et al. 2004 (R) Pankinetoplastic kDNA not in a single network, but evenly distributed in mitochondrioplasm; biciliate, without hairs; posterior cilium attached or free; phagotrophic or osmotrophic; cytostome, when present, anterolateral; free-living or commensal/parasitic. Node: Cryptobia, Parabodo, Procryptobia, Trypanoplasma. |
| ●●●●●●Eubodonida Vickerman in Moreira et al. 2004 (R) Eukinetoplast with kDNA not in a single network, but in parakinetosomal position; biciliate with anterior cilium with non-tubular hairs; phagotrophic; anterolateral cytostome surrounded by lappets; free-living. Bodo. |
| ●●●●●●Trypanosomatida Saville Kent 1880, emend. Vickerman in Moreira et al. 2004 Eukinetoplastic with kDNA network associated with ciliary basal body; uniciliate with cilium lacking hairs and emerging from anterior pocket, or emerging laterally and attached to body; phagotrophic or osmotrophic; cytostome, when present, simple and close to ciliary pocket; exclusively parasitic. Node: Blastocrithidia, Crithidia, Herpetomonas, Leishmania, Leptomonas, Phytomonas, Rhynchoidomonas, Trypanosoma, Wallaceina. Other included genera: Angomonas, Sergeia, Strigomonas. Note 12. |
| Note 12. Trypanosomatida has certain monophyletic lineages within it, such as Leishmaniinae Maslov & Lukeš 2012 (R) identified by SSU rRNA and GAPDH phylogenies, with relatively slow evolution of gene sequences. Includes monoxenous genera Crithidia, Leptomonas, and Wallaceina, and the dixenous genus Leishmania. |
| ●●●●●Incertae sedis Kinetoplastea: Bordnamonas, Cephalothamnium, Hemistasia. |
|
●● Tsukubamonas Yabuki et al. 2011 (M) Rounded biciliate cell, usually swims; consumes prey through ventral groove. Tsukubamonas globosa. |
| Incertae sedis EUKARYOTA |
| ● Ancyromonadida Cavalier-Smith 1998 Benthic gliding cells, dorsoventrally compressed, with leftward-oriented rostrum at anterior; two unequal cilia, each emerging in separate shallow pocket; short apical anterior cilium may be very thin or terminate at cell membrane; long posterior cilium inserts ventrally/left-laterally; rostrum contains extrusomes in rows; cell membrane supported by a thin single-layered theca; discoidal/flat mitochondrial cristae; bacterivorous. Ancyromonas, Planomonas (regarded as synonyms by Heiss et al. 2010). |
| ● Apusomonadidae Karpov & Mylnikov 1989 Gliding cells; dorsal cell membrane is underlain by thin theca comprising two dense layers, and extending as lateral flanges that delimit a broad ventral region from which pseudopodia develop; with two heterodynamic cilia – the anterior cilium enclosed by sleeve-like extension of flanges to form a proboscis and the posterior cilium lying within the ventral region; tubular mitochondrial cristae; bacterivorous. Amastigomonas, Apusomonas, Manchomonas, Multimonas, Podomonas, Thecamonas. Note 13. |
| Note 13. Apusomonads most likely belong within the Amorphea, based on current data. |
|
● Breviatea Cavalier-Smith et al. 2004 Amoeboid cells with single anteriorly-directed apical cilium; filopodia generally radiating from around the cell, forming at anterior end and often trailing at posterior; anaerobic or microaerophilic, with large mitochondrion-like organelle; ingests bacteria; can form cysts. Breviata, Subulatomonas. Note 14. |
| Note 14. Breviatea most likely belong within the Amorphea, based on current data. |
| ● Collodictyonidae Brugerolle et al. 2002 Free-swimming cells with two or four equal apical cilia orthogonal to each other; ciliary transition zone long, with a two-part axosome; phagocytosis of other eukaryotic cells occurring in a conspicuous longitudinal ventral groove that extends to posterior end, giving a double-lobed appearance. Collodictyon, Diphylleia (= Aulacomonas), Sulcomonas. |
| ● Mantamonas Cavalier-Smith & Glücksman in Glücksman et al. 2011 (M) Gliding biciliated cell; cell body flattened with left side of cell with angled shape and right side plastic; long posteriorly-directed cilium directed straight behind gliding cell; anterior cilium very thin. Mantamonas plastica. |
| ● Rigifilida Cavalier-Smith in Yabuki et al. 2012 Cells rounded, somewhat circular in dorsoventral aspect though somewhat plastic; pellicle underlies cell membrane on dorsal and lateral surfaces; central circular depression on venter of cell, with collar-like margin of reflected pellicle; branching fine pseudopodia arising from ventral depression, used to capture bacteria; flat mitochondrial cristae. Micronuclearia, Rigifila. |
| ● Spironemidae Doflein 1916 [Hemimastigophora Foissner et al. 1988] Cilia arranged in two lateral rows that may or may not run the whole length of the cell, with up to about a dozen cilia per row; submembranous thecal plates separate the cilia; thecal plates rotationally symmetrical, supported by microtubules; anterior differentiated into a capitulum, which is the site of phagocytosis; tubular and saccular mitochondrial cristae; with bottle-shaped extrusomes. Hemimastix, Spironema, Stereonema. |
| ●Cryptophyceae Pascher 1913, emend. Schoenichen 1925, emend. Adl et al. 2012 [Cryptophyta Cavalier-Smith 1986] Autotrophic, mixotrophic or heterotrophic with ejectisomes (trichocysts); mitochondrial cristae flat tubules; two cilia emerging subapically or dorsally from right side of an anterior depression (vestibulum); longitudinal grooves (furrows) and/or tubular channels (gullets) or a combination of both, extending posteriorly from the vestibublum on the ventral side; gullet/furrow complexes lined with large ejectisomes; with or without plastid-nucleomorph complex; chloroplasts when present contain chlorophylls a and c2 and phycobiliproteins, located in thylakoid lumen; chloroplast covering comprised of inner and superficial periplast components (IPC, SPC, respectively); includes heterotrophic species formerly known as Chilomonas and some genera diplomorphic genera, such as Cryptomonas and Proteomonas. |
| ●●Cryptomonadales Pascher 1913 Chloroplasts or leucoplasts present. Chroomonas, Cryptomonas, Hemiselmis, Rhodomonas. |
| ●● Goniomonas Stein 1878 [Goniomonadales Novarino & Lucas 1993] Chloroplasts absent. Goniomonas. |
| ●● Kathablepharidae Vørs 1992 Free-swimming cells with two heterodynamic cilia inserting subapically/medially; cell membrane thickened by lamellar sheath; ingest eukaryotic prey through an apical cytostome supported by bands of longitudinal microtubules; extrusomes are large coiled-ribbon arrayed near kinetosomes, somewhat similar to those of Cryptophyceae; tubular mitochondrial cristae; plastids not observed. Kathablepharis, Leucocryptos, Roombia. |
| ● Haptophyta Hibberd 1976, emend. Edvardsen & Eikrem 2000 Autotrophic, mixotrophic or heterotrophic cells; some in colonies or filaments; motile cells often possessing a haptonema, a filiform appendage situated between cilia; characteristic cell covering of unmineralized and/or mineralized scales; motile cells with two cilia generally without appendages, inserted apically or sub-apically in a papilla or groove, or emerging from a papilla; 1--4 chloroplasts with thylakoids in groups of three; chloroplasts with immersed or bulging pyrenoid; nucleus usually posterior; outer membrane of nuclear envelope continuous with outer chloroplast membrane; major pigments chlorophylls a, c1, and c2 with c3 in prymnesiophyceans, β-carotene, diadinoxanthin, and diatoxanthin; chrysolaminarin often the main storage product; eyespots recorded in a few genera (Pavlova, Diacronema); life cycles include either single phases or alternating stages; in those with alternating stages, palmelloid (colonial) or filamentous stages alternate with motile stages; sexual reproduction may be common in prymnesiophyceans; some species ichthyotoxic. |
| ●● Pavlovophyceae Cavalier-Smith 1986, emend. Green & Medlin 2000 Biciliate with unequal cilia inserted subapically or laterally; scales absent; shorter cilium may have a swelling with densely staining projections on the side adjacent to the cell; haptonema short, tapered and non-coiling; single chloroplast, sometimes with an eyespot beneath the short cilium. Diacronema, Exanthemachrysis, Pavlova. |
| ●● Prymnesiophyceae Hibberd 1976 Unicellular or colonial ciliates with mineralized and/or unmineralized scales covering the cells; some species exhibit two stages in the life cycle, with either a colonial or filamentous stage alternating with a ciliate stage; haptonema may be long and coiling to short and non-coiling; cilia of equal or sub-equal lengths inserted apically or subapically. |
| ●●● Prymnesiales Papenfuss 1955 Motile or non-motile cells, sometimes forming colonies; usually with two cilia and a coiling or flexible haptonema; covering of organic scales, sometimes absent; some alternate stages reported. Chrysochromulina, Phaeocystis, Prymnesium. |
| ●●● Phaeocystis Lagerheim 1893 [Phaeocystales Medlin 2000] Motile cells with two cilia and short non-coiling haptonema; one to four chloroplasts per cell; the cell covered with scales of two sizes; life cycle consisting of non-motile and motile stages; non-motile cells colonial and embedded in gelatinous material. Phaeocystis. |
| ●●● Isochrysidales Pascher 1910 Motile or non-motile cells; haptonema rudimentary or absent; motile cells covered with small organic scales; non-motile cells sometimes covered with coccoliths. Emiliania, Gephyrocapsa, Isochrysis. |
| ●●● Coccolithales Schwarz 1932 Cells with calcified organic scales during some stage of the life cycle; single or alternating stages in the life cycle; haptonema short or highly reduced; some species lack chloroplasts. Balaniger, Calciosolenia, Coccolithus, Hymenomonas, Pleurochrysis, Reticulosphaera, Wigwamma. |
| ● Rappemonads Kim et al. 2011 Marine 5--7 µm cells with 2--4 plastids containing chlorphyll a. Poorly characterised, known only from environmental samples, and no species or genera described. |
| ● Picobiliphytes Not et al. 2007 Slightly oblong marine 2×6 µm cells originally reported to have a plastid, but this is debated. Poorly characterized, known only from environmental samples, and no species or genera described. |
| ● Centrohelida Kühn 1926 Axopodia supported by microtubules in hexagonal or triangular arrays; axopodia retractable by microtubule depolymerization; kinetocyst extrusomes along axopodia; centrosome as trilaminar disc with fibrous electron-dense cortex, called centroplast; flat mitochondrial cristae. |
| ●● Acanthocystidae Claus 1874 Periplast of siliceous elements arranged in internal and external layers; internal layer of scales; external layer of scales possessing central sternum and additional structures or radial spicules with developed shaft. Acanthocystis, Choanocystis, Echinocystis, Pseudoraphidiophrys, Pseudoraphidocystis, Pterocystis. |
| ●● Heterophryidae Poche 1913 Periplasmic mucous coat, with or without organic spicules. Chlamydaster, Heterophrys, Oxnerella, Sphaerastrum. |
| ●● Raphidiophryidae Mikrjukov 1996 Periplast of siliceous scales or spicules arranged in one or more layers. Parasphaerastrum, Polyplacocystis, Raphidiocystis, Raphidiophrys. |
| ● Telonema Griessmann 1913 [Telonemia Shalchian-Tabrizi 2006] Biciliated cells with a proboscis-like structure located at the ciliary pole and a complex cytoskeleton composed of layers of microtubules and microfilaments; tripartite tubular hairs on the long cilium; mitochondria with tubular cristae; peripheral vacuoles located just beneath the cell membrane; chloroplasts not observed. Telonema. |
| ● Palpitomonas Yabuki et al. 2010 (M) Bicilated, both cilia 20 µm long, with anterior cilium moving vigorously and posterior cilium trailing; swimming or sessile, as one cilium can attach to substratum, non-gliding; unilateral bipartite mastigonemes on one cilium; MLS-like structure on one kinetosome root; single mitochondrion lobate with flat cristae; Golgi apparatus located under mastigont; phagotrophic; naked flexible 3×8 µm vacuolated cells. Palpitomonas bilix. |
Several morphological and taxonomic terms were identified as problematic and definitions were provided for these terms by Adl et al. (2005), so these are not repeated here. New problems with terminology have emerged that will need care in usage. These include a variety of terms used to describe pseudopodia and amoeboid locomotion, as well as terms used to refer to the basal body and centrioles. We recommend the following terms, for which we have considered historical usage and application to the details of the morphology implied. Lobopodia, one of several subtypes of pseudopodia, are projections more or less broad, with cytoplasmic streaming; they may have a clear hyaline region at the front; additional finer projections may extend from this hyaline region. If lobopodia are very thin and flat, they can be called lamellipodia. For fine filamentous pseudopodia, the term filopodia must be reserved for fine, pointed hyaline projections, containing no granuloplasm or microtubules, sometimes branching but never anastomosing, as found for example in Nuclearia. Foraminifera and a few other lineages have granuloreticulopodia (with granules) supported by microtubules, while Cercozoa and others have either filopodia or networks of reticulopodia (anastomosing), without granules. Axopodia are stiff with supporting microtubules. For basal bodies and centrioles, a variety of terms exist. We recommend the following usages for clarity. Centrioles should be restricted to a pair of organelles that form the core of the centrosome located near the nucleus, that are without cilium, and that function as a microtubule organizing centre (MTOC) during cell division. Some lineages have an amorphous MTOC usually near the nucleus, instead of either centrioles or basal bodies. Basal bodies or kinetosomes occur below the cell membrane and typically one will be ciliated. A kinetosome plus its cytoskeletal root system are collectively called a kinetid or mastigont, which may or may not be ciliated. In cases where basal bodies are co-ordinated and/or abundant, such as in ciliates, additional terms are used, such as kineties, cirri, and membranelles. We propose that a basal body with a cilium is referred to as a C-kinetosome (with cilium) in contrast to an X-kinetosome (without a cilium). Each kinetosome in a cell can be identified by a numbering system appropriate for the clade; where possible, we recommend a numbering system that reflects cilium development (see Moestrup 2000). Adl et al. (2005) noted that the preferred term for a eukaryotic flagellum is cilium, and we have now used cilium throughout this revision. For additional terms the standard guide remains Andersen et al. (1991), with the term flagellum substituted with cilium.
The highest ranks in this classification are presented in a table with examples of sub-divisions, and in a figure, to help navigation through the classification (Table 1, Fig. 1). The classification (Table 2) includes descriptions for each group, providing apomorphies where possible. Groups that are probably paraphyletic are indicated with (P), and groups that are clustered by molecular phylogenetics and without obvious apomorphies are indicated as being a “ribogroup” (R). Groups known from only one described species are indicated as being monotypic (M). The list of genera with uncertain affiliation has been greatly reduced since 2005 (Table 3).
Figure 1.
Table 3.
Groups or genera with uncertain affiliation within protists, based on Adl et al. (2005).
| Actinastrum | Diplocalium | Luffisphaera |
| Actinocoma | Diplomita | Lymphocytozoon |
| Adinomonas | Diplophysalis | Lymphosporidium |
| Aletium | Diploselmis | Macappella |
| Amphimonas | Dobellia | Magosphaera |
| Amylophagus | Dobellina | Malpighiella |
| Aphelidiopsis | Ducelleria | Martineziella |
| Archaeosphaerodiniopsis | Ectobiella | Megamoebomyxa |
| Artodiscus | Elaeorhanis | Meringosphaera |
| Asterocaelum | Embryocola | Microcometes |
| Asthmatos | Endamoeba | Microgromia |
| Aurospora | Endemosarca | Monochrysis |
| Barbetia | Endobiella | Monodus |
| Belaria | Endomonas | Mononema |
| Bertarellia | Endospora | Myrmicisporidium |
| Bertramia | Enteromyxa | Naupliicola |
| Bjornbergiella | Eperythrocytozoon | Nephrodinium |
| Bodopsis | Errera | Neurosporidium |
| Boekelovia | Fromentella | Ovicola |
| Branchipocola | Gymnococcus | Palisporomonas |
| Camptoptyche | Gymnophrydium | Pansporella |
| Chalarodora | Haematotractidium | Paradinemula |
| Chamydophrys | Hartmannina | Paramastix |
| Cibdelia | Heliobodo | Paramonas |
| Cichkovia | Heliomonas | Paraplasma |
| Cinetidomyxa | Hermisenella | Parastasia |
| Cingula | Heterogromia | Parastasiella |
| Cladomonas | Hillea | Peliainia |
| Codonoeca | Hyalodaktylethra | Peltomonas |
| Coelosporidium | Immnoplasma | Petasaria |
| Copromonas | Isoselmis | Phagodinium |
| Cyanomastix | Janickina | Phanerobia |
| Cyclomonas | Joyeuxella | Phloxamoeba |
| Cytamoeba | Kamera | Phyllomitus |
| Dallingeria | Kibisidytes | Phyllomonas |
| Dictyomyxa | Kiitoksia | Physcosporidium |
| Dimastigamoeba | Komokiacea | Piridium |
| Dimorphids | Lagenidiopsids | Pleuophrys |
| Dinemula | Liegeosia | Pleuromastix |
| Dinoasteromonas | Lithocolla | Protenterospora |
| Protomonas | Serpentoplasma | Thalssomyxa |
| Pseudoactiniscus | Sphaerasuctans | Thaulirens |
| Pseudosporopsis | Spiriopsis | Topsentella |
| Quadricilia | Spirogregarian | Toshiba |
| Rhizomonas | Spongastericus | Toxocystis |
| Rhynchodinium | Spongocyclia | Trichonema |
| Rigidomastix | Stephanomonas | Urbanella |
| Schewiakoffia | Strobilomonas | |
| Sergentella | Tetragonidium |
The consolidation of protist classification, together with the rise of the environmental genomics revolution, has produced impressive quantities of data that can be used to explore their diversity more productively and with more targeted questions. Estimates of protist diversity for each lineage suggest that a huge number remain to be discovered (Adl et al., 2007) and this notion is supported by a separate estimation based on a statistical predictive model (Mora et al., 2011). The latter particularly showed that in classification schemes that best reflected phylogenetic relatedness, the model estimate of diversity was more robust and more accurate. Nevertheless, the databases used to estimate numbers of species were conspicuously poor in their coverage of protists, as they did not use a modern classification scheme that reflected relatedness, and were also substantially incomplete. Thus, the projections under-estimated the likely diversity of protists in the environment. It is a very exciting time to be a protistologist as there is a new wave or organism discovery made possible by technological advances. This provides an argument for a stable classification that preserves names while accommodating changes, and progresses towards clearly representing the evolutionary history.
Supplementary Material
ACKNOWLEDGMENTS
S.M.A. is grateful to the International Society of Protistologists for the opportunity to initiate and mediate discussions among many colleagues towards revising this classification. C.L.S. was supported in part by the Intramural Research Program of the NIH, National Library of Medicine.
Footnotes
This revision was led by the Committee on Systematics and Evolution of The International Society of Protistologists (S.M. Adl (Chair), C.E. Lane, J. Lukes, A.G.B. Simpson). They were joined by colleagues to make the primary contributors to the various sections as follows: Alveolata: S.M. Adl, M. Dunthorn, M. Hoppenrath, J. Lukes, D.H. Lynn, S. Rueckert; Amoebozoa: S.M. Adl, E. Lara, E. Mitchell, L. Shadwick, A.V. Smirnov, F.W. Spiegel; Archaeplastida: C.E. Lane, L. LeGall, H. McManus; Excavata: V. Hampl, J. Lukes, A.G.B. Simpson; Opisthokonta: S.M. Adl, M. Brown, S.E. Mozley-Stanridge, C. Shoch; Rhizaria: S.M. Adl, D. Bass, S. Bowser, E. Lara, E. Mitchell, J. Pawlowski; Stramenopiles: S.M. Adl, C.E. Lane, A.G.B. Simpson; Incertae sedis Eukaryota: S.M. Adl, F. Burki, V. Hampl, A. Heiss, L. Wagener Parfrey, A.G.B. Simpson. While these individuals share authorship of this work, this does not mean that all the authors endorse every aspect of the proposed classification.
LITERATURE CITED
- Adl SM, Simpson AGB, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle G, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, Mccourt RM, Mendoza L, Moestrup O, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MF., JR The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005;52:399–451. doi: 10.1111/j.1550-7408.2005.00053.x. [DOI] [PubMed] [Google Scholar]
- Adl SM, Leander BS, Simpson AGB, Archibald JM, Anderson OR, Bass D, Bowser SS, Brugerolle G, Farmer MA, Karpov S, Kolisko M, Lane CE, Lodge DJ, Mann DG, Meisterfeld F, Mendoza L, Moestrup Ø, Mozley-Standridge SE, Smirnov AV, Spiegel F. Diversity, nomenclature, and taxonomy of protists. Syst. Biol. 2007;56:684–689. doi: 10.1080/10635150701494127. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Barr DJS, Lynn DH, Melkonian M, Moestrup Ø, Sleigh MA. Terminology and nomenclature of the cytoskeletal elements associated with the flagellar apparatus in protists. Protoplasma. 1991;164:1–8. [Google Scholar]
- Baurain D, Brinkmann H, Petersen J, Rodriguez-Ezpelata N, Stechmann A, Demoulin V, Roger AJ, Burger G, Lang BF, Philippe H. Phylogenomic evidence for separate acquisition of plastids in cryptophytes, haptophytes, and stramenopiles. Molec. Biol. Evol. 2010;27:1698–1709. doi: 10.1093/molbev/msq059. [DOI] [PubMed] [Google Scholar]
- Brown MW, Spiegel FW, Silberman JD. Phylogeny of the “forgotten cellular slime mold, Fonticula alba, reveals a key evolutionary branch within Opisthokonta. Mol. Biol. Evol. 2009;26:2699–2709. doi: 10.1093/molbev/msp185. [DOI] [PubMed] [Google Scholar]
- Burki F, Shalchian-Tabrizi K, Pawlowski J. Phylogenomics reveals a new"megagroup"including most photosynthetic eukaryotes. Biol. Lett. 2008;4:366–369. doi: 10.1098/rsbl.2008.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki F, Shalchian-Tabrizi K, Minge M, Skjaeveland A, Nikolaev SI, Jakobsen KS, Pawlowski J. Phylogenomics reshuffles the eukaryotic supergroups. PLoS ONE. 2007;2:e790. doi: 10.1371/journal.pone.0000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki F, Inagaki Y, Bråte J, Archibald JM, Keeling PJ, Cavalier-Smith T, Sakaguchi M, Hashimoto T, Horak A, Kumar S, Klaveness D, Jakobsen KS, Pawlowski J, Shalchian-Tabrizi K. Large-scale phylogenomic analyses reveal that two enigmatic protist lineages, Telonemia and Centroheliozoa, are related to photosynthetic chromalveolates. Genome Biol. Evol. 2009;9:231–238. doi: 10.1093/gbe/evp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki F, Kudryavtsev A, Matz M, Aglyamova G, Bulman S, Fiers M, Keeling PJ, Pawlowski J. Evolution of Rhizaria: new insights from phylogenomic analysis of uncultivated protists. BMC Evol. Biol. 2010;10:377. doi: 10.1186/1471-2148-10-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki F, Okamoto N, Pombert JF, Keeling PJ. The evolutionary history of haptophytes and cryptophytes: phylogenomic evidence for separate origins. Proc. Biol. Sci. 2012;279:2246–2254. doi: 10.1098/rspb.2011.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantino PD. Classifying species versus naming clades. Taxon. 2004;53:795–798. [Google Scholar]
- Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of protozoa. Int. J. Syst. Evol. Biol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- Gong YC, Yu Y-H, Villalobo E, Zhu FY, Miao W. Reevaluation of the phylogenetic relationship between Mobilid and Sessilid peritrichs (Ciliophora, Oligohymenophorea) based on small subunit rRNA genes sequences. J. Euk. Microbiol. 2006;53:397–403. doi: 10.1111/j.1550-7408.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- Hampl V, Hug L, Leigh JW, Dacks JB, Lang BF, Simpson AG, Roger AJ. Phylogenomic analyses support the monophyly of Excavata and resolve relationships among eukaryotic "supergroups". Proc Natl Acad Sci U S A. 2009;106:3859–3864. doi: 10.1073/pnas.0807880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath TA, Hedtke SM, Hillis DM. Taxon sampling and the accuracy of phylogenetic analyses. J. Syst. Evol. 2008;46:239–257. [Google Scholar]
- Hedtke SM, Townsend TM, Hillis DM. Resolution of phylogenetic conflict in large data sets by increased taxon sampling. Syst. Biol. 2006;55:522–529. doi: 10.1080/10635150600697358. [DOI] [PubMed] [Google Scholar]
- Heiss AA, Walker G, Simpson AGB. Clarifying the taxonomic identity of a phylogenetically important group of eukaryotes: Planomonas is a junior synonym of Ancyromonas. J. Eukaryot. Microbiol. 2010;57:285–293. doi: 10.1111/j.1550-7408.2010.00477.x. [DOI] [PubMed] [Google Scholar]
- Howe AT, Bass D, Scoble JM, Lewis R, Vickerman K, Arndt H, Cavalier-Smith T. Novel cultured protists identify deep-branching environmental DNA clades of Cercozoa: new genera Tremula, Micrometopion, Minimassisteria, Nudifila, Peregrina. Protist. 2011;162:332–372. doi: 10.1016/j.protis.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Jeffroy O, Brinkmann H, Delsuc F, Philippe H. Phylogenomics: the beginning of incongruence? Trends Genet. 2006;22:225–231. doi: 10.1016/j.tig.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Kim E, Simpson AGB, Graham L. Evolutionary relationships of Apusomonads inferred from taxon-rich analyses of 6 nuclear encoded genes. Molec. Biol. Evol. 2006;23:2455–2466. doi: 10.1093/molbev/msl120. [DOI] [PubMed] [Google Scholar]
- Kron KA. Exploring alternative systems of classification. Aliso. 1997;15:105–112. [Google Scholar]
- Minge MA, Silberman JD, Orr RJS, Cavalier-Smith T, Shalchia-Tabrizi K, Burki F, Skjaeveland A, Jakobsen K. Evolutionary position of breviate amoebae and the primary eukaryote divergence. Proc. R. Soc. B. 2009;276:597–604. doi: 10.1098/rspb.2008.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moestrup Ø. The flagellate cytoskeleton – Introduction of a general terminology for microtubular flagellar roots in protists. In: Leadbeater BSC, Green JC, editors. Systematics Association Special Volume Series. Vol. 59. Flagellates: Unity, Diversity and Evolution; 2000. pp. 69–94. [Google Scholar]
- Mora C, Tittensor DP, Adl S, Simpson AGB. How many species are there on earth and in the ocean? PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001127. e1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfrey LW, Grant J, Tekle YI, Lasek-Nesselquist E, Morrison HC, Sogin ML, Patterson DJ, Katz L. Broadly sampled multigene analyses yield a well-resolved eukaryotic tree of life. Syst. Biol. 2010;59:518–533. doi: 10.1093/sysbio/syq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleijel F, Rouse GW. Ceci n’est pas une pipe: clades and phylogenetic nomenclature. J. Zool. Syst. Evol. Res. 2003;41:162–174. [Google Scholar]
- Richards TA, Cavalier-Smith T. Myosin domain evolution and the primary divergence of eukaryotes. Nature. 2005;436:1113–1118. doi: 10.1038/nature03949. [DOI] [PubMed] [Google Scholar]
- Roger AJ, Simpson AGB. Evolution: revisiitng the root of the eukaryote tree. Curr. Biol. 2009;19:R165–R167. doi: 10.1016/j.cub.2008.12.032. [DOI] [PubMed] [Google Scholar]
- Spiegel FW. A proposed phylogeny of the flagellated protostelids. Biosystems. 1991;25:113–120. doi: 10.1016/0303-2647(91)90017-f. [DOI] [PubMed] [Google Scholar]
- Spiegel FW, Lee SB, Rusk SA. Eumycetozoans and molecular systematics. Can. J. Bot. 1995;73:s738–s746. [Google Scholar]
- Stiller JW, Huang JL, Ding Q, Tian J, Goodwill C. Are algal genes in nonphotosynthetic protists evidence of historical plastid endosymbioses? BMC Genomics. 2009;10:484. doi: 10.1186/1471-2164-10-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens JJ. Missing data and the design of phylogenetic analyses. J. Biomed. Informatics. 2006;39:34–42. doi: 10.1016/j.jbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Wright M, Moisand A, Mir L. Structure of the flagellar apparatus of the swarm cells of Physarum polycephalum. Protoplasma. 1979;100:231–250. [Google Scholar]
- Zhan ZF, Xu KD, Warren A, Gong YC. Reconsideration of Phylogenetic Relationships of the Subclass Peritrichia (Ciliophora, Oligohymenophorea) Based on Small Subunit Ribosomal RNA Gene Sequences, with the Establishment of a New Subclass Mobilia Kahl, 1933. J. Euk. Microbiol. 2009;56:552–558. doi: 10.1111/j.1550-7408.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- Zwickl DJ, Hillis DM. Increased taxon sampling greatly reduces phylogenetic error. Syst. Biol. 2002;51:588–598. doi: 10.1080/10635150290102339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.