Abstract
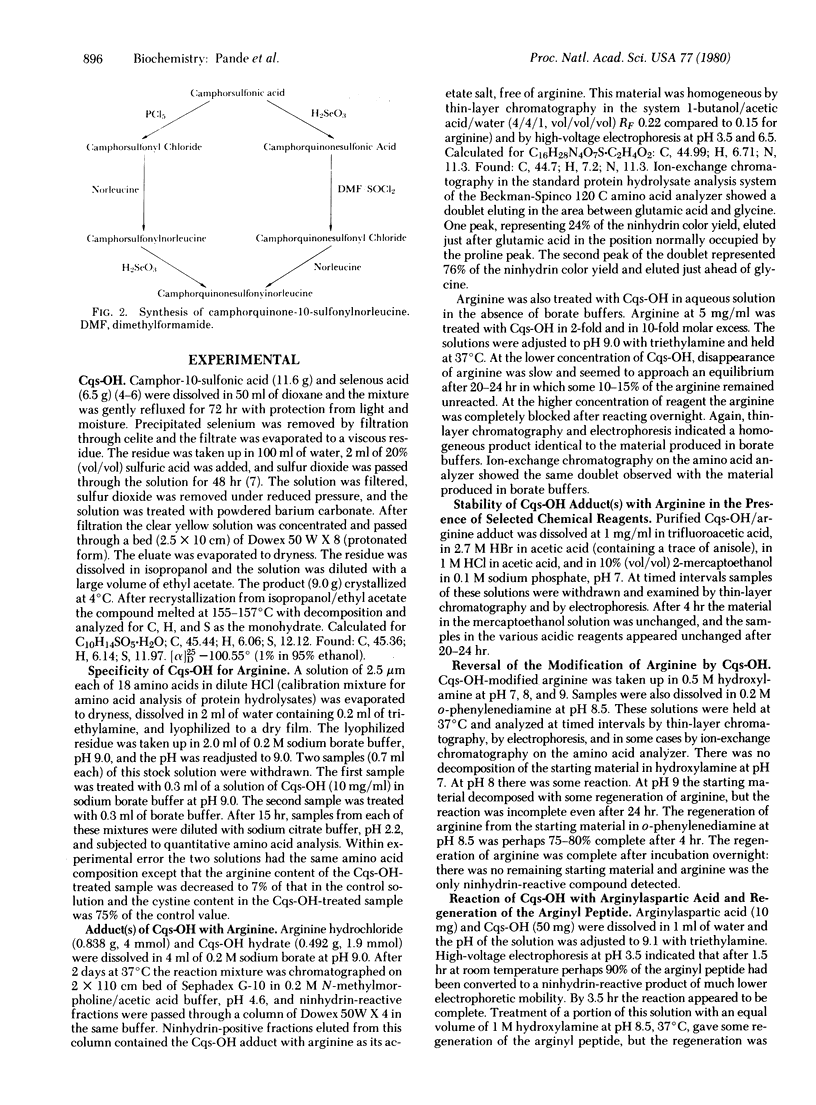
Camphorquinone-10-sulfonic acid hydrate was prepared by the action of selenous acid on camphor-10-sulfonic acid. Camphorquinone-10-sulfonylnorleucine was prepared either from the sulfonic acid via the sulfonyl chloride or by selenous acid oxidation of camphor-10-sulfonylnorleucine. These reagents are useful for specific, reversible modification of the guanidino groups of arginine residues. Camphorquinonesulfonic acid is a crystalline water-soluble reagent that is especially suitable for use with small arginine-containing molecules, because the sulfonic acid group of the reagent is a convenient handle for analytical and preparative separation of products. Camphorquinonesulfonylnorleucine is more useful for work with large polypeptides and proteins, because hydrolysates of modified proteins may be analyzed for norleucine to determine the extent of arginine modification. The adducts of the camphorquinone derivatives with the guanidino group are stable to 0.5 M hydroxylamine solutions at pH 7, the recommended conditions for cleavage of the corresponding cyclohexanedione adducts. At pH 8-9 the adducts of the camphorquinone derivatives with the guanidino group are cleaved by o-phenylenediamine. The modification and regeneration of arginine, of the dipeptide arginylaspartic acid, of ribonuclease S-peptide, and of soybean trypsin inhibitor are presented as demonstrations of the use of the reagents. The use of camphorquinonesulfonyl chloride to prepare polymers containing arginine-specific ligands is discussed.
Keywords: protein modification, protein semisynthesis, ribonuclease S-peptide, soybean trypsin inhibitor
Full text
PDF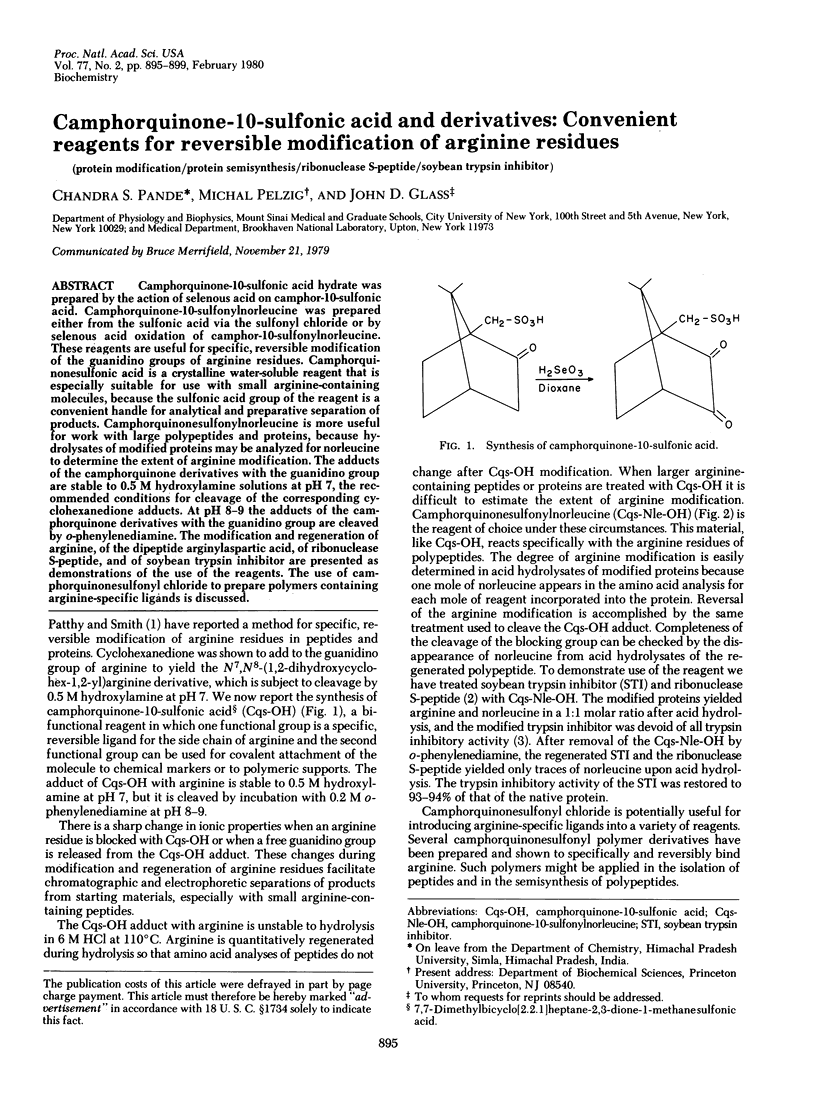



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Glass J. D., Pelzig M. Benzyloxycarbonylarginine nitrophenyl ester salts: 1-hydroxybenzotriazole catalyzed acylations of amines. Int J Pept Protein Res. 1978 Aug;12(2):75–80. doi: 10.1111/j.1399-3011.1978.tb02870.x. [DOI] [PubMed] [Google Scholar]
- Glass J., Pelzig M. Enzymes as reagents in peptide synthesis: enzyme-labile protection for carboxyl groups. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2739–2741. doi: 10.1073/pnas.74.7.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. D., Shaw E. Thiobenzyl benzyloxycarbonyl-L-lysinate, substrate for a sensitive colorimetric assay for trypsin-like enzymes. Anal Biochem. 1979 Mar;93(2):223–226. doi: 10.1016/s0003-2697(79)80141-4. [DOI] [PubMed] [Google Scholar]
- Liu W. H., Feinstein G., Osuga D. T., Haynes R., Feeney R. E. Modification of arginines in trypsin inhibitors by 1,2-cyclohexanedione. Biochemistry. 1968 Aug;7(8):2886–2892. doi: 10.1021/bi00848a027. [DOI] [PubMed] [Google Scholar]
- MOYER J. H., PEVEY K., HEIDER C. H., KINROSS-WRIGHT V. A comparative study of four tranquilizing agents, phenobarbital, and inert placebo. Geriatrics. 1958 Mar;13(3):153–170. [PubMed] [Google Scholar]
- Meyers C., Glass J. D. Enzymes as reagents in peptide synthesis: enzymatic removal of amine protecting groups. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2193–2196. doi: 10.1073/pnas.72.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Reversible modification of arginine residues. Application to sequence studies by restriction of tryptic hydrolysis to lysine residues. J Biol Chem. 1975 Jan 25;250(2):557–564. [PubMed] [Google Scholar]
- RICHARDS F. M., VITHAYATHIL P. J. The preparation of subtilisn-modified ribonuclease and the separation of the peptide and protein components. J Biol Chem. 1959 Jun;234(6):1459–1465. [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]



