Abstract
The fatty acids (±)-2-methoxy-6Z-heptadecenoic acid (1), (±)-2-methoxy-6-heptadecynoic acid (2) and (±)-2-methoxyheptadecanoic acid (3) were synthesized and their inhibitory activity against the Leishmania DNA topoisomerase IB enzyme (LdTopIB) determined. Acids 1 and 2 were synthesized from 4-bromo-1-pentanol, the former in ten steps and in 7% overall yield, while the latter in seven steps and in 14% overall yield. Acid 3 was prepared in six steps and in 42% yield from 1-hexadecanol. Acids 1–3 inhibited the LdTopIB enzyme following the order 2 > 1 ⪢ 3, with 2 displaying an EC50 = 16.6 ± 1.1 μM and 3 not inhibiting the enzyme. Acid 1 preferentially inhibited the LdTopIB enzyme over the human TopIB enzyme. Unsaturation seems to be a prerequisite for effective inhibition, rationalized in terms of weak intermolecular interactions between the active site of LdTopIB and either the double or triple bonds of the fatty acids. Toxicity towards Leishmania donovani promastigotes was also investigated resulting in the same order 2 > 1 > 3, with 2 displaying an EC50 = 74.0 ± 17.1 μM. Our results indicate that α-methoxylation decreases the toxicity of C17:1 fatty acids towards L. donovani promastigotes, but improves their selectivity index.
Keywords: Leishmania donovani, methoxylated fatty acids, synthesis, topoisomerase IB
INTRODUCTION
Visceral leishmaniasis continues to be a serious tropical disease with around 500,000 cases reported each year, mainly in third world countries such as India, Bangladesh, Indonesia, and Sudan [1]. The causative agent is Leishmania donovani, injected by a sand fly into a human in the form of promastigotes, which eventually invade the spleen and liver as amastigotes resulting in anaemia and fever and ultimately in death if left untreated [2]. Several therapeutic venues are presently available, including pentavalent antimonials such as meglumine antimoniate or oral chemotherapeutic drugs such as miltefosine. A main problem with many of these drugs is emerging resistance by the parasite and some can display considerable toxicity [3–4].
Some fatty acids have been reported to display antileishmanial activity, but earlier reports are contradictory. For example, among a series of C4–C18 fatty acids it was reported that the C18 fatty acids were the most toxic to L. donovani promastigotes with the unsaturated fatty acids being more toxic than their saturated analogs [5]. In particular, oleic acid displayed a MIC of 0.09 μmol/mL against L. donovani, while n-octadecanoic acid only showed a MIC of 1.39 μmol/mL. On the other hand, in an earlier work, n-decanoic acid, n-dodecanoic acid, and n-hexadecanoic acid, at 100 μg/mL, inhibited the motility of L. donovani promastigotes, but oleic acid was reported to have no effect [6].
Our research team has been actively studying fatty acids as agents to treat parasitic diseases. In an effort to further clarify the structural characteristics needed for a fatty acid to be effective against L. donovani promastigotes our team synthesized and tested the natural occurring (from the plant Sommera sabiceoides) fatty acids 6-heptadecynoic acid and 6-icosynoic acid as well as its olefinic analogs [7]. We found that these Δ6 acetylenic fatty acids displayed good antiprotozoal activity towards L. donovani promastigotes (EC50's = 1–6 μg/mL) and that the corresponding saturated fatty acids n-heptadecanoic acid and n-eicosanoic acid were not effective at all [7]. In addition, we also established that these unsaturated fatty acids were reasonably good inhibitors (EC50's = 36–49 μM) of the leishmania DNA topoisomerase IB enzyme (LdTopIB) a likely intracellular target for these fatty acids [7].
LdTopIB is a potential drug target for visceral leishmaniasis since it is different from other Topo IB in the sense that it is phylogenetically unique and has an anomalous dimeric structure [8]. When the sequence of the human topoisomerase IB enzyme (hTopIB) and both subunits of the LdTopIB enzyme are compared we find that the degree of homogeneity of the large subunit of LdTopIB with the hTopIB is low (45%) and even less (29%) when the corresponding regions of the hTopIB are compared with the small subunit of LdTopIB [8]. This comparison is important since it is exactly in these not conserved regions that we find the areas more sensitive to drugs. This difference makes the LdTopIB enzyme an attractive target for drug intervention, in particular if a compound can preferentially interact with LdTopIB over hTopIB. Therefore, our aim in this study was to synthesize and test against LdTopIB the 2-methoxylated analogs of the 6-heptadecynoic and 6Z-heptadecenoic acids previously studied [7]. The additional 2-methoxylated functionality was chosen inspired by the possibility for the α-methoxy group to favorably interact with the enzyme via hydrogen bonding as well as by the naturally occurring (±)-2-methoxy-6Z-heptadecenoic acid (1), an acid that we identified in the sponge Calyx podatypa and with the potential to display some interesting biological properties [9]. Based on our previous findings with the 6-heptadecynoic and 6Z-heptadecenoic acids, we expected the corresponding α-methoxylated fatty acids to be good inhibitors of LdTopIB as well as to display toxicity towards L. donovani promastigotes.
RESULTS AND DISCUSSION
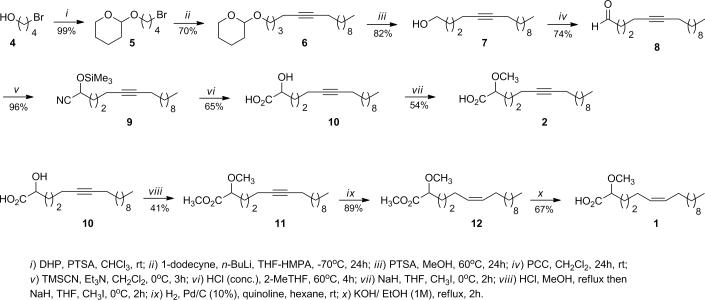
The synthesis of the (±)-2-methoxy-6-heptadecynoic acid (2) started with commercially available 4-bromo-1-butanol (4), which was protected with 2,3-dihydro-2H-pyran (DHP) using catalytic amounts of PTSA in chloroform affording the 2-(4-bromobutyl)-tetrahydro-2H- pyran (5) in a 99% yield (Scheme 1). In the subsequent step commercially available 1-dodecyne was successfully coupled with 5 in the presence of n-BuLi in THF-HMPA at −70°C which resulted in the tetrahydropyranyl protected alkynol 6 in a 70% yield. The deprotection of 6 was achieved with PTSA in methanol at 60°C which yielded 5-hexadecynol (7) in an 82% yield. Alcohol 7 was oxidized using PCC in CH2Cl2 affording 5-hexadecynal (8) in a 74% yield. The aldehyde was then reacted with trimethylsilyl cyanide (TMSCN) in dichloromethane and catalytic amounts of triethylamine (Et3N) at 0°C resulting in the desired 2-(trimethylsilyloxy)-6-heptadecynenitrile (9) in a 96% yield, which was then converted into the (±)-2-hydroxy-6-heptadecynoic acid (10) in a 65% yield by acid hydrolysis of nitrile 9 in 2-methyltetrahydrofuran (2-MeTHF) as solvent. In the final step the desired acid 2 [10] was obtained in a 54% yield by methylation of 10 with sodium hydride (NaH) and methyliodide (CH3I) in THF as solvent (Scheme 1). This synthesis was achieved in seven steps and in 14% overall yield
Scheme 1.
Synthesis of (±)-2-methoxy-6-heptadecynoic acid (2) and the (±)-2-methoxy-6Z-heptadecenoic acid (1).
For the synthesis of the (±)-2-methoxy-6Z-heptadecenoic acid (1) the same synthetic route outlined in Scheme 1 was followed using the (±)-2-hydroxy-6-heptadecynoic acid (10) as the starting material (Scheme 1). The synthesis continued with the esterification of 10 using HCl/MeOH under reflux resulting in the desired methyl (±)-2-hydroxy-6-heptadecynoate which was subsequently reacted with NaH and CH3I in dry THF to obtain methyl (±)-2-methoxy-6-heptadecynoate (11) in a combined 41% yield. Methyl ester 11 was hydrogenated under Lindlar conditions in dry hexane affording methyl (±)-2-methoxy-6Z-heptadecenoate (12) in an 89% yield. The desired acid 1 [11] was obtained in a 67% yield through the saponification of methyl ester 12 using KOH/EtOH (1M) under reflux (Scheme 1). This represents the first synthesis for the naturally occurring (±)-2-methoxy-6Z-heptadecenoic acid (1), which was achieved in ten steps and in a 7% overall yield.
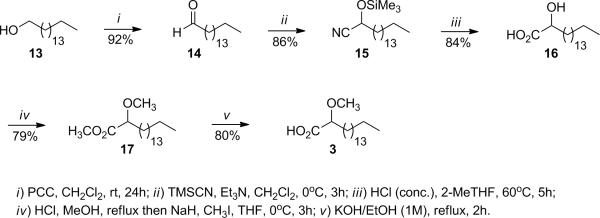
The synthesis of the (±)-2-methoxyheptadecanoic acid (3) started with the oxidation of commercially available 1-hexadecanol (13) with pyridinium dichromate (PCC) in CH2Cl2, which afforded hexadecanal (14) in a 92% yield (Scheme 2). Hexadecanal was then reacted with trimethylsilyl cyanide in CH2Cl2 using catalytic amounts of Et3N at 0°C affording the 2-(trimethylsilyloxy)heptadecanitrile (15) in an 86% yield. The nitrile 15 was then converted into the (±)-2-hydroxyheptadecanoic acid (16) in an 84% yield by acid hydrolysis of 15 in 2-MeTHF as solvent. The methyl (±)-2-methoxyheptadecanoate (17) was obtained in a combined 79% yield by first preparing the methyl ester of acid 16 with HCl/MeOH and then methylating the hydroxy group with NaH and CH3I in dry THF as solvent. The desired acid 3 [12] was finally obtained in an 80% yield by the saponification of 17 with KOH/EtOH (1M) under reflux. The synthesis took six steps from 1-hexadecanol and a total overall yield of 42%.
Scheme 2.
Synthesis of the (±)-2-methoxyheptadecanoic acid (3).
Aimed at examining the effect of α-methoxylation on the inhibitory potential of these acetylenic fatty acids the new α-methoxylated fatty acids 1–3 were tested against LdTopIB following our previously published procedure [7]. We were in a good position to assess the effect of a triple bond versus a double bond on the inhibitory activity since the number of carbon atoms remained constant in our chosen examples. As can be seen from Table 1 the (±)-2-methoxy-6-heptadecynoic acid (2) was the most efficient inhibitor with an EC50 = 16.6 ± 1.1 μM (Fig. 1). The effectiveness of inhibition followed the order 2 > 1 > 3, where the (±)-2-methoxy-6Z-heptadecenoic acid (1) also displayed considerable inhibition of the enzyme with an EC50 = 41.3 ± 5.6 μM and the (±)-2-methoxyheptadecanoic acid (3) did not inhibit LdTopIB (EC50 > 1000 μM). Since the chain length (C17) and unsaturation position at C-6 remained constant among our chosen examples, we can conclude that acids 1–2 were more effective inhibitors of LdTopIB than either 6Z-heptadecenoic acid or 6-heptadecynoic acid. Therefore, α-methoxylation seems to be a viable way of increasing the inhibitory potential of C17 fatty acids towards LdTopIB.
Table 1.
Inhibition of the relaxation activities of the LdTopIB enzyme by the studied fatty acids.
| Fatty acid | EC50 (μM) |
|---|---|
| (±)-2-OMe-6-heptadecynoic (2) | 16.6 ± 1.1 |
| 6-heptadecynoic | 71.7 ± 3.8 |
| (±)-2-OMe-6Z-heptadecenoic (1) | 41.3 ± 5.6 |
| 6Z-heptadecenoic | 80.4 ± 9.3 |
| (±)-2-OMe-heptadecanoic (3) | >1000 |
| n-heptadecanoic | >1000 |
Fig. 1.
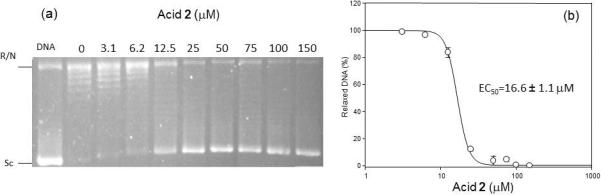
Inhibition of the relaxation activity of recombinant LdTopIB by acid 2 (a) and the corresponding plot of the relaxation activity (%) of the LdTopIB enzyme versus concentration (μM) of the acetylenic fatty acid 2 (b). One unit of recombinant LdTopI was assayed in a plasmid DNA relaxation assay for 30 min at 37°C in the presence of 3.1−150 μM acid 2. Reaction products were resolved in agarose gel and subsequently visualized by ethidium bromide staining. The relative position of the negatively supercoiled DNA substrate is indicated by Sc, N is the nicked DNA, whereas the ladder of relaxed DNA topoisomer bands is in between. Reactions were stopped with a mixture of 1% SDS and 6.1 μg of proteinase K. Lane 1 contains 0.2 μg of pHOT plasmid DNA.
In a separate experiment we also compared the inhibition of 1 towards LdTopIB versus hTopIB and the results are shown in Fig. 2. Acid 1 was able to inhibit LdTopIB at the listed concentration, but it was not effective against hTopIB at the same concentrations. These results indicate that it will be possible to preferentially interfere with LdTopIB without inhibiting the human enzyme, a finding that could have therapeutic value. The latter seems to be a general finding for fatty acids, i.e, in all of the fatty acids that we have tested so far (unpublished data), we have found that LdTopIB is more sensitive to inhibition by fatty acids than hTopIB.
Fig. 2.
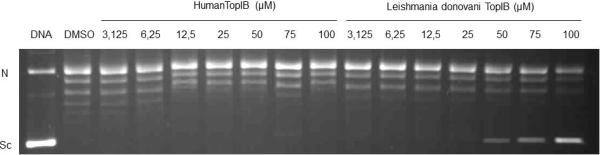
Comparison of the inhibition of the relaxation activity of human TopIB (left) and recombinant LdTopIB by acid 1. One unit of recombinant LdTopIB was assayed in a plasmid DNA relaxation assay for 30 min at 37°C in the presence of 3.125−100 μM acid 1. Reaction products were resolved in agarose gel and subsequently visualized by ethidium bromide staining. The relative position of the negatively supercoiled DNA substrate is indicated by Sc, N is the nicked DNA, whereas the ladder of relaxed DNA topoisomer bands is shown in between. Reactions were stopped with a mixture of 1% SDS and 6.1 μg of proteinase K. Lane 1 contains 0.2 μg of pHOT plasmid DNA and lane 2 DMSO.
Based on the fact that the inhibition of LdTopIB followed the order 2 > 1 ⪢ 3 one can argue that the inhibition decreases in the order: triple bond ≥ double bond ⪢ single bond. Since we do not expect a big difference between the geometries of acids 2 and 3, and we consider the fact that a fatty acid with a cis cyclopropane group does not inhibit LdTopIB [13], other factors, such as intermolecular cation-π interactions, might be significant near the binding sites of the fatty acids with LdTopIB. One could hypothesize that the ammonium groups (R-NH3+ or R2C=NH2+) in amino acids such as lysine (K) and/or arginine (R), known to be present in the active sites of these TopIB enzymes [14], might interact with the double or triple bonds in these fatty acids. These forces are quite significant, in the proximity of 10 kcal/mol, as previous computational studies have shown [15–16].
In order to study these cation-π interactions further with modern computational techniques, the σ⋯π interactions between NH4+ and C2H2/C2H4/C2H6 were investigated by means of ab initio methods using the Gaussian 09 program [17]. All the geometries were fully optimized at the level of MP2/aug-cc-pVTZ [18], and CCSD(T)/aug-cc-pVTZ [19–21] single-point energy computations were carried out to refine the energy data. The zero-point energy (ZPE) and basis set superposition error (BSSE) corrections were also considered at the MP2/aug-cc-pVTZ level of theory. Natural bond orbital (NBO) analysis [22] was used to evaluate the charge transfer between NH4+ and C2Hx. Both the NH4+⋯C2H2 and NH4+⋯C2H4 complexes are of Cs symmetry, while NH4+⋯C2H6 has C1 symmetry (Fig. 3). The distances between the H pointing to C2Hx (x = 2, 4, 6) in NH4+ and the center of C-C bond (DH-O) in the NH4+⋯C2H2, NH4+⋯C2H4,and NH4+⋯C2H6 complexes are 2.078, 2.099, and 2.274 Å, respectively. The N-H bond (DN-H) of NH4+ pointing to C2Hx (x = 2, 4, 6) is elongated from 1.023 Å in the free NH4+ structure to 1.043, 1.046, and 1.031 Å in the corresponding complexes (Fig. 3, Table 2). Among these three complexes, the interaction between NH4+ and C2H6 is the weakest (−5.27 kcal/mol), and those between NH4+ and C2Hx (x = 2, 4) are comparable (−9.95 and −9.99 kcal/mol, respectively), with NH+4⋯C2H4 having a slightly stronger binding energy. The electrostatic interactions likely dictate the stabilities of these complexes, the charge transfers (Δq) (0.043 e, 0.055 e, and 0.015 e, respectively) well correlate with the binding energies for NH4+⋯C2Hx (x = 2, 4, 6). These findings could help explain why acids 1 and 2 inhibit LdTopIB, while acid 3, as well as all saturated fatty acids tested so far, do not inhibit the enzyme.
Fig. 3.
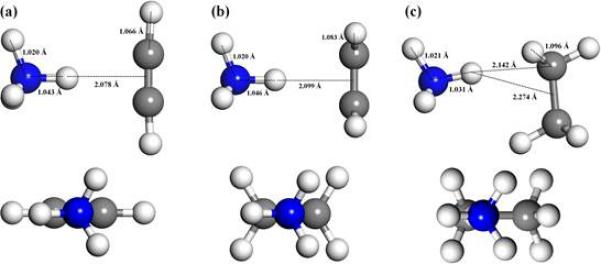
The lowest-energy structures of NH4+…C2Hx [x = 2(a), 4(b), 6(c)] at MP2/aug-cc-pVTZ level of theory.
Table 2.
The main structural parameters, the charge transfer between NH4+ and C2Hx, and binding energies of optimized NH4+⋯C2Hx (x = 2, 4, 6) complexes.a
| NH4+⋯C2H2 | NH4+⋯C2H4 | NH4+⋯C2H6 | |
|---|---|---|---|
| DN-H (Å) | 1.043 | 1.046 | 1.031 |
| DH-O (Å) | 2.078 | 2.099 | 2.274 (2.142) |
| Δq (e) | 0.043 | 0.055 | 0.015 |
| Eb (kcal/mol) | −9.95 | −9.99 | −5.27 |
DH-O is the distance between H of NH4+ pointing to C2Hx (x = 2, 4, 6) and the center of C-C, the data in parentheses denotes the distance between H of NH4+ and its closer C atom. Eb was obtained at CCSD(T)/aug cc-pVTZ//MP2/aug-cc-pVTZ level of theory, including the MP2-estimated ZPE and BSSE corrections.
The mode of inhibition of 1–2 against LdTopIB could very well be similar to the one reported for conjugated eicosapentaenoic acid (cEPA) with hTopIB [14]. In this study it was concluded that cEPA essentially binds to a region close to the active site of the enzyme and inhibits DNA cleavage by blocking the area, but it does not prevent DNA from binding. In fact, in this computational model it was shown that cEPA binds in close proximity to K443, K587, and N722, and prevents the catalytic Y723 from performing a nucleophilic substitution on the DNA phosphate [14].
Based on the enzymatic studies we further explored the antiprotozoal activity of the 2-methoxylated fatty acids towards Leishmania donovani promastigotes following our already published procedure [7] and the results are shown in Table 3. Among the studied 2-methoxylated fatty acids the (±)-2-methoxy-6-heptadecynoic acid (2) was the most toxic fatty acid towards L. donovani promastigotes with an EC50 = 74.0 ± 17.1 μM. When compared to the 6-heptadecynoic acid (EC50 = 25.1 ± 1.4 μM) it became evident that 2-methoxylation somewhat decreased the toxicity of 6-heptadecynoic acid towards L. donovani. However, the 6-heptadecynoic acid displays considerable toxicity towards murine macrophages, resulting in a low therapeutic index of 0.85 (Table 3). On the other hand, acid 2 did not display toxicity towards murine macrophages resulting in an excellent therapeutic index (> 700). These results indicate that α-methoxylation decreases the toxicity of C17 fatty acids towards L. donovani promastigotes, but improves their selectivity index when compared to murine macrophages, thus making acid 2 the most viable drug candidate among the studied fatty acids.
Table 3.
Antileishmanial activity of the studied fatty acids (μM).
| Fatty acid | L. donovani promastigotes (EC50) | Murine macrophages (IC50) | Therapeutic index (IC50/EC50) |
|---|---|---|---|
| (±)-2-OMe-6-heptadecynoic (2) | 74.0 ± 17.1 | >700 | >32 |
| 6-heptadecynoic | 25.1 ± 1.4 | 21.3 ± 0.9 | 0.85 |
| (±)-2-OMe-6Z-heptadecenoic (1) | 404.1 ± 11.9 | 483.9 ± 15.8 | 1.2 |
| 6Z-heptadecenoic | >100 | 44.4 ± 1.6 | n/a |
| (±)-2-OMe-heptadecanoic (3) | >1000 | >1000 | n/a |
| n-heptadecanoic | >100 | 52.6 ± 7.5 | n/a |
| miltefosine | 0.8 ± 0.1 | 1.9 ± 0.4 | 2.5 |
Interesting were the results with either the (±)-2-methoxy-6Z-heptadecenoic acid (1) or the 6Z-heptadecenoic acid, which were not effective against L. donovani promastigotes despite the fact that they were inhibitors of LdTopIB. Acid 1 presented some antileishmanial activity towards L. donovani promastigotes with an EC50 = 404.1 ± 11.9 μM (Table 3). These results indicate that a triple bond is preferred over a double bond in these C17 fatty acids in order to display antiprotozoal activity. This observation was further confirmed by the finding that both (±)-2-methoxyheptadecanoic acid (3) and n-heptadecanoic acid were not effective at all against the parasite. However, there seems to be other mechanisms of toxicity, besides LdTopIB inhibition, responsible for the toxicity of 1–2 towards L. donovani promastigotes. In any instance, the antiprotozoal studies with acid 2 correlate with the LdTopIB inhibitory studies, which indicate that acid 2 is the best overall candidate among the studied compounds.
CONCLUSIONS
Based on the LdTopIB inhibitory studies and the antileishmanial studies with L. donovani promastigotes, the novel fatty acid (±)-2-methoxy-6-heptadecynoic acid (2) was identified as a viable candidate against leishmaniasis based on its high inhibition of the LdTopIB enzyme and its high therapeutic index. It was found that the combination of α-methoxylation and a C-6 triple bond enhances the inhibition of C17 fatty acids towards the LdTopIB enzyme.
ACKNOWLEDGEMENTS
The project described was supported by Award Number SC1GM084708 from the National Institutes of General Medical Sciences of the NIH and by NSF Grant (EPS-1010094). M. Cartagena thanks the UPR RISE program for a graduate fellowship. We thank Dr. Fred Strobel (Emory University) for the high resolution mass spectral data. This research was also supported in part by Ministerio de Ciencia e Innovation (grant AGL2010-16078/GAN), by Junta de Castilla y León (grant Gr-238) and Instituto de Salud Carlos III (grant PI09/0448 and the Tropical Diseases Network RICET) from Ministerio de Salud y Consumo of the Spanish Kingdom.
REFERENCES AND NOTES
- 1.Tiuman TS, Santos AO, Ueda-Nakamura T, Filho BP, Nakamura CV. Int. J. Infect. Dis. 2011;15:525. doi: 10.1016/j.ijid.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Sacks DL, Perkin PV. Am J. Trop. Med. Hyg. 1985;34:456. doi: 10.4269/ajtmh.1985.34.456. [DOI] [PubMed] [Google Scholar]
- 3.Glasser JS, Murray CK. Am. J. Trop. Med. Hyg. 2011;84:566. doi: 10.4269/ajtmh.2011.10-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vélez ID, Colmenares LM, Muñoz CA. Rev. Inst. Med. Trop. Sao Paulo. 2009;51:231. doi: 10.1590/s0036-46652009000400011. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri G, Ghoshal K, Banerjee AB. Indian J. Med. Res. 1986;84:361. [PubMed] [Google Scholar]
- 6.Cunningham LV, Kazan BH, Kuwahara SS. J. Gen. Microbiol. 1972;70:491. doi: 10.1099/00221287-70-3-491. [DOI] [PubMed] [Google Scholar]
- 7.Carballeira NM, Cartagena MM, Fernández Prada C, Fernández-Rubio C, Balaña-Fouce R. Lipids. 2009;44:953. doi: 10.1007/s11745-009-3345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villa H, Otero-Marcos AR, Reguera RM, Balaña-Fouce R, García-Estrada C, Pérez-Pertejo Y, Tekwani BL, Myler PJ, Stuart KD, Bjornsti MA, Ordóñez D. J. Biol. Chem. 2003;278:3521. doi: 10.1074/jbc.M203991200. [DOI] [PubMed] [Google Scholar]
- 9.Carballeira NM, Pagán M. J. Nat. Prod. 2000;63:666. doi: 10.1021/np990529d. [DOI] [PubMed] [Google Scholar]
- 10.Spectral data for the (±)-2-methoxy-6-heptadecynoic acid (2). IR (neat) Vmax: 3365, 2926, 2855, 1734, 1457, 1380, 1119 cm−1; 1H-NMR (CDCl3, 500 MHz) δ (ppm): 3.83 (1H, t, J = 4.6 Hz), 3.44 (3H, s, -OCH3), 2.20 (2H, t, J = 7.0 Hz), 2.12 (2H, t, J = 6.9 Hz), 1.62 (2H, q, J = 7.2 Hz), 1.25 (18H, brs, -CH2−), 0.87 (3H, t, J = 6.9 Hz, -CH3); 13CNMR (CDCl3, 125 MHz) δ (ppm): 176.97 (C-1), 81.07 (C-7), 79.72 (C-2), 79.02 (C-6), 58.24 (C-2'), 31.88 (C-15), 31.36 (C-13), 29.57 (C-12), 29.52 (C-14), 29.30 (C-9), 29.14 (C-11), 28.88 (C-3), 24.44 (C-16), 22.66 (C-4), 18.71 (C-5), 18.45 (C-8), 14.09 (C-17). HRMS Calcd for C18H32O3 [M+H]+ 297.2424, found 297.2423.
- 11.Spectral data for the (±)-2-methoxy-6Z-heptadecenoic acid (1). IR (neat) Vmax: 3330, 3005, 2928, 2856, 1720, 1650, 1458, 1380, 969, 778 cm−1; 1H-NMR (CDCl3, 500 MHz) δ (ppm): 5.35 (2H, m), 3.78 (1H, t, J = 6.7 Hz), 3.42 (3H, s, -OCH3), 2.01 (4H, m), 1.76 (2H, m), 1.23 (18H, brs, -CH2−), 0.85 (3H, t, J = 7.0 Hz, -CH3); 13C-NMR (CDCl3, 125 MHz) δ (ppm): 176.87 (C-1), 131.26 (C-7), 129.04 (C-6), 80.46 (C-2), 58.28 (C-2'), 31.88 (C-15), 31.81 (C-3, C-9), 29.67 (C-10), 29.61 (C-11), 29.53 (C-12), 29.41 (C-13), 29.31 (C-14), 27.23 (C-8), 26.71 (C-5), 24.83 (C-4), 22.67 (C-16), 14.13 (C-17). HRMS Calcd for C18H35O3 [M+H]+ 299.2583.
- 12.Spectral data for the (±)-2-methoxyheptadecanoic acid (3). IR (neat) Vmax: 3420, 2922, 2852, 1716, 1457, 1381, 1090 cm−1; 1H-NMR (CDCl3, 500 MHz) δ (ppm): 3.79 (1H, t, J = 5.0 Hz), 3.46 (3H, s, −OCH3), 1.52 (2H, m), 1.28 (26H, brs, −CH2−), 0.87 (3H, t, J = 6.7 Hz, −CH3); 13C-NMR (CDCl3, 125 MHz) δ (ppm): 175.89 (C-1), 80.27 (C-2), 58.34 (C-2'), 32.22 (C-15), 31.93 (C-3), 29.70 (C-12, C-13), 29.67 (C-10, C-11), 29.63 (C-9), 29.55 (C-8), 29.42 (C-7), 29.37 (C-6), 29.32 (C-5), 29.08 (C-14), 24.82 (C-4), 22.70 (C-16), 14.13 (C-17).
- 13.Carballeira NM, Montano N, Reguera RM, Balaña-Fouce R. Tetrahedron Lett. 2010;51:6153. doi: 10.1016/j.tetlet.2010.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castelli S, Campagna A, Vassallo O, Tesauro C, Fiorani P, Tagliatesta P, Oteri F, Falconi M, Majumder HK, Desideri A. Arch. Biochem. Biophys. 2009;486:103. doi: 10.1016/j.abb.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Deakyne CA, Meot-Ner M. J. Am. Chem. Soc. 1985;107:474. [Google Scholar]
- 16.Ma JC, Dougherty DA. Chem. Rev. 1997;97:1303. doi: 10.1021/cr9603744. [DOI] [PubMed] [Google Scholar]
- 17.Gaussian 09. Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian, Inc.; Wallingford CT: 2009. [Google Scholar]
- 18.Möller C, Plesset MS. Phys. Rev. 1934;46:618. [Google Scholar]
- 19.Purvis GD, Bartlett RJ. J. Chem. Phys. 1982;76:1910. [Google Scholar]
- 20.Raghavachari K, Trucks GW, Pople JA, Head-Gordon M. Chem. Phys. Lett. 1989;157:479. [Google Scholar]
- 21.Watts JD, Gauss J, Bartlett RJ. J. Chem. Phys. 1993;98:8718. [Google Scholar]
- 22.NBO Version 3.1. Glendening ED, Reed AE, Carpenter JE, Weinhold F.