Abstract
2-Alkynoic fatty acids display antimycobacterial, antifungal, and pesticidal activities but their antiprotozoal activity has received little attention. In this work we synthesized the 2-octadecynoic acid (2-ODA), 2-hexadecynoic acid (2-HDA), and 2-tetradecynoic acid (2-TDA) and show that 2-ODA is the best inhibitor of the Leishmania donovani DNA topoisomerase IB enzyme (LdTopIB) with an EC50 = 5.3 ± 0.7 μM. The potency of LdTopIB inhibition follows the trend 2-ODA> 2-HDA> 2-TDA, indicating that the effectiveness of inhibition depends on the fatty acid carbon chain length. All of the studied 2-alkynoic fatty acids were less potent inhibitors of the human topoisomerase IB enzyme (hTopIB) as compared to LdTopIB. 2-ODA also displayed in vitro activity against Leishmania donovani (IC50 = 11.0 μM), but it was less effective against other protozoa, Trypanosoma cruzi (IC50 = 48.1 μM) and T. brucei rhodesiense (IC50 = 64.5 μM). The antiprotozoal activity of the 2-alkynoic fatty acids, in general, followed the trend 2-ODA> 2-HDA> 2-TDA. The experimental information gathered so far indicates that 2-ODA is a promising antileishmanial compound.
Keywords: Acetylenic fatty acids, Leishmania donovani, 2-octadecynoic acid, topoisomerase IB
Acetylenic fatty acids have entertained both medicinal chemists and natural products chemists for the diversity of biological activities that they have displayed.1,2 Among these fatty acids the octadecynoic acids are worth mentioning. Earlier studies identified the 5-octadecynoic acid (5-ODA) as a natural product from the root of the plant Ximena americana with pesticidal activity as demonstrated by the inhibition of the hatching of the pod-sucking bug Clavigralla tomentosicollis eggs.3 On the other hand, the roots of Pentagonia gigantifolia yielded the acid 6-octadecynoic acid (6-ODA), which displayed potent antifungal activity against a series of fluconazole resistant Candida albicans strains with IC50 values between 0.45–0.65 μg/mL.4 Among a series of 2-alkynoic fatty acids the 2-octadecynoic acid (2-ODA) and its metabolites displayed the best antimycobacterial activity against Mycobacterium smegmatis and Mycobacterium bovis BCG by inhibiting fatty acid biosynthetic pathways of considerable importance for mycobacteria.5 Interesting to mention is that by placing the unsaturation at the other extreme of the acyl chain, the acid 17-octadecynoic acid (17-ODA) is obtained, which is a well-known suicide inhibitor of the leukotriene B4 ω-oxidase (IC50 < 5 μM) as well as a selective inhibitor of the renal CYP450 ω-hydroxylase.6 All of these findings attest to the biological potential of the octadecynoic acids as antiinfective agents and enzyme inhibitors, but no data on the inhibition of key enzymes in Leishmania parasites by these acids has been reported.
Several other 2-alkynoic fatty acids show antiprotozoal activity and inhibit protozoal enzymes. For example, our team studied the antiprotozoal activity of 2-hexadecynoic acid (2-HDA) and found that it effectively inhibited plasmodial FAS-II enzymes (IC50s between 1.5 and 13.9 μM) and arrests erythrocytic and liver stage Plasmodium infections.7 In addition, we showed that 2-HDA displays antiprotozoal activity against L. donovani amastigotes (IC50 = 17.8 μM) but no studies on key L. donovani enzymes amenable for therapeutic intervention were performed. These initial results motivated us to study 2-TDA, 2-HDA and 2-ODA as antiprotozoal compounds so as to determine the antiprotozoal activity of the acids as a function of carbon chain length. Therefore, in this work we synthesized 2-TDA, 2-HDA, and 2-ODA and determined their growth inhibitory activities against L. donovani, T. cruzi, and T. b. rhodesiense in vitro. The best antiprotozoal results were obtained against L. donovani and therefore, we studied the inhibition of the Ld TopIB enzyme by 2-TDA, 2-HDA, and 2-ODA and compared it to hTopIB.
DNA topoisomerases have been the target of many studies since early successes with the camptothecins and similar structural analogs.8 In particular, type I DNA topoisomerases are a well-recognized target for cancer therapy. The trypanosomal and leishmania type IB DNA topoisomerases differ significantly from the homologous mammalian structures since they are phylogenetically unique and possess an anomalous dimeric structure.9,10 These enzymes have been recognized as excellent targets for the development of antiparasitic drugs. However, no clear structure activity relationships (SAR) exist for the interaction of drugs, in particular fatty acids, against these type IB topoisomerases. Our series of 2-alkynoic fatty acids give us an excellent tool to gain better insights into the structural requirements needed for effective LdTopIB inhibition by fatty acids, in particular if these activities can be correlated to the toxicity against L. donovani. The results of this study indicate that C18 is an effective carbon chain length among the 2-alkynoic acids for inhibiting LdTopIB, which happens to correlate with the toxicities observed against L. donovani axenic amastigotes.
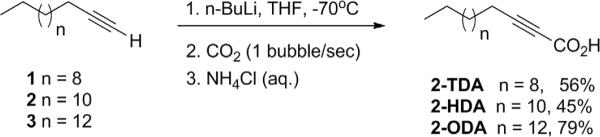
The synthesis of 2-TDA, 2-HDA, and 2-ODA followed an already published procedure7 wherein commercially available 1-tridecyne, 1-pentadecyne, or 1-heptadecyne was reacted with n-BuLi in THF at −70°C followed by quenching with CO2 and final protonation with NH4Cl (Fig. 1). The yields ranged from 45–79%. The purity of the synthesized compounds was determined to be > 95% by capillary GC-MS and 13C NMR. The spectral data of the synthesized 2-alkynoic fatty acids were in agreement with those previously reported.5,7
Figure 1.
Synthesis of 2-TDA, 2-HDA, and 2-ODA with the corresponding yields.
The antiprotozoal activities of the synthesized 2-alkynoic fatty acids were studied against Leishmania donovani (axenic amastigotes), Trypanosoma brucei rhodesiense (bloodstream forms), and Trypanosoma cruzi (intracellular amastigotes in L6 rat skeletal myoblasts) as previously described.7 As shown in Table 1, among the 2-alkynoic fatty acids 2-ODA displayed the best antiprotozoal activity against all the studied protozoa with IC50 values ranging between 11.0μ64.5 μM, followed by 2-HDA (IC50's between 17.8–83.6 μM) and finally 2-TDA (IC50's between 24.7–255.4 μM). Therefore, the 2-acetylenic fatty acids were effective in killing the protozoa by following the order 2-ODA> 2-HDA > 2-TDA. Leishmania donovani amastigotes were the most susceptible to the 2-alkynoic fatty acids (IC50's between 11.0–24.7 μM), but the test compounds were not as effective against T. cruzi (IC50's between 62.4–80.0 μM) and T. brucei rhodesiense (IC50's between 64.5–255.4 μM). Overall, 2-ODA showed the broadest spectrum of antiprotozoal activity, but the most effective effect was observed against L. donovani.
Table 1.
Antiprotozoal activities of the 2-alkynoic fatty acids. The IC50 values are in μM.
| Compounds | Trypanosoma brucei rhodesiense | Trypanosoma cruzi | Leishmania donovani | L6 cell cytotoxicity |
|---|---|---|---|---|
| 2-TDA | 255.4 | 62.4 | 24.7 | 40.9 |
| 2-HDA | 83.6 | 80.0 | 17.8 | 340.3 |
| 2-ODA | 64.5 | 48.1 | 11.0 | 20.0 |
| Standard | 0.0075a | 1.47b | 0.49c | 0.02d |
The values represent the average of at least two independent assays performed in duplicates.
Reference compounds are:
Melarsoprol
Benznidazole
Miltefosine
Podophyllotoxin.
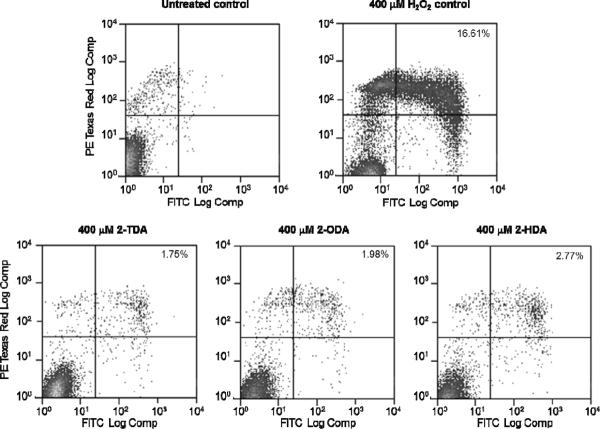
Aimed at exploring a possible mechanism responsible for the antileishmanial activity displayed by the 2-alkynoic fatty acids apoptosis was probed in Leishmania infantum (2-HDA displayed an IC50 of 14.9 μM against L. infantum promastigotes) by detecting the translocation of phosphatidylserine (PS) to the cell surface with the Annexin-FITC reagent. To test for apoptosis in Leishmania is quite reasonable since it has been reported that Leishmania amastigotes can fake its own death by exposing PS on its surface and gain access to macrophages.11 This PS translocation results in the inhibition of NO production and the induction of TGF-β secretion and IL-10 synthesis.11 In our experiment (Fig. 2) L. infantum promastigotes were treated with 2-TDA, 2-HDA, and 2-ODA at concentrations ranging from 100–400 μM. At the highest concentration of 400 μM (Fig. 2) no significant concentration of apoptotic cells were observed for neither of the acetylenic fatty acids tested, but a positive test for hydrogen peroxide as the control was observed.12 This experiment demonstrates that apoptosis is not the main mechanism by which the 2-alkynoic acids kill the Leishmania promastigotes.
Figure 2.
Apoptosis analysis by staining with Annexin-V-FITC and propidium iodide. Untreated and H2O2 treated cell were used as negative and positive controls, respectively. Top right quadrant shows the percent of late apoptotic cells. Apoptosis was detected by translocation of phosphatidyl serine (PS) to the cell surface with the annexin V-FITC reagent (BD Pharmigen). Fraction of Annexin V-positive cells was measured with CellQuest software (BD Biosciences, San Jose, CA). To estimate the number of apoptotic cells L. infantum promastigotes was treated with 400 μM compounds for 24 h, harvest, wash twice with PBS, and resuspended in 1×106 cells/ml. Cells were fluorescently labeled by addition of 20 μl of binding buffer, 5 μl of Annexin V-FITC and 5 μl of propidium iodide. After incubation at room temperature in the dark for 15 min, cells were applied to flow cytometry analysis. A minimum of 10,000 cells in the gated region was analyzed using a BD FACS Calibur Flow Cytometer. Results were interpreted by the percentage of total cells appearing in each quadrant. The experiment is representative of three independent trials.
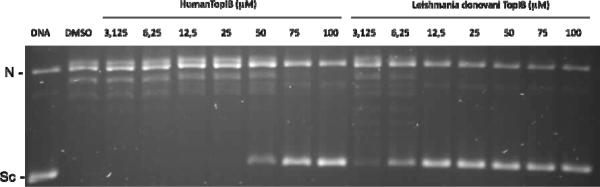
Other mechanisms of toxicity for 2-TDA, 2-HDA, and 2-ODA in L. donovani were examined by concentrating on the inhibition of the Leishmania donovani topoisomerase IB enzyme (LdTopIB) (Table 2, Fig. 3). One of the most recent contributions against protozoan-caused infectious diseases takes advantage of the structural differences between the protozoan and the host TopIB, since the unusual heterodimeric TopIB of kinetoplastid parasites, such as LdTopIB, can be used for the development of new compounds targeting only the parasite TopIB without interfering with the monomeric TopIB of the human host.13 For this reason, the inhibition of LdTopIB by the 2-alkynoic fatty acids was examined and compared to hTopIB. As expected, 2-ODA was the most efficient inhibitor with an EC50 = 5.3 ± 0.7 μM followed by 2-HDA with an EC50 = 28.7 ± 1.3 μM (Fig. 3). The effectiveness of inhibition followed the order 2-ODA> 2-HDA> 2-TDA. This trend correlates quite well with the toxicity displayed by the 2-alkynoic acids towards the L. donovani amastigotes.
Table 2.
Inhibition of the relaxation activities of the LdTopIB and hTopIB enzymes by the studied fatty acids and their toxicities against murine macrophages (μM).
| Compounds | LdTopIB EC50 | hTopIB EC50 | Murine macrophages BALB/c IC50 |
|---|---|---|---|
| 2-TDA | 67.8 ± 1.1 | > 100 | > 100 |
| 2-HDA | 28.7 ± 1.3 | > 100 | > 100 |
| 2-ODA | 5.3 ± 0.7 | 51.9 ± 1.0 | > 100 |
| CPT | 0.67 ± 0.08 | 2.0 ± 1.0 | 0.62 ± 0.13 |
Figure 3.
Comparison of the inhibition of the relaxation activity of human TopIB (left) and recombinant LdTopIB (right) by 2-ODA. One unit of recombinant LdTopIB was assayed in a plasmid DNA relaxation assay for 30 min at 37°C in the presence of 3.125–100 μM 2-ODA. Reaction products were resolved in agarose gel and subsequently visualized by ethidium bromide staining. The relative position of the negatively supercoiled DNA substrate is indicated by Sc, N is the nicked DNA, whereas the ladder of relaxed DNA topoisomer bands is shown in between. Reactions were stopped with a mixture of 1% SDS and 6.1 μg of proteinase K. Lane 1 contains 0.5 μg of pSK plasmid DNA and lane 2 DMSO.
In the latter experiment the inhibition of 2-ODA towards hTopIB was compared to LdTopIB and the results are also shown in Table 2 and Figure 3. While 2-ODA was able to inhibit LdTopIB at 5.3 μM, it was less effective against hTopIB (EC50 = 51.9 μM). In fact, neither 2-TDA nor 2-HDA was inhibitory towards hTopIB at 100 μM. These results clearly reveal that it will be possible to preferentially interfere with LdTopIB without inhibiting the human enzyme, a finding that could have therapeutic value. It is evident that LdTopIB is more sensitive to fatty acid inhibition than hTopIB.
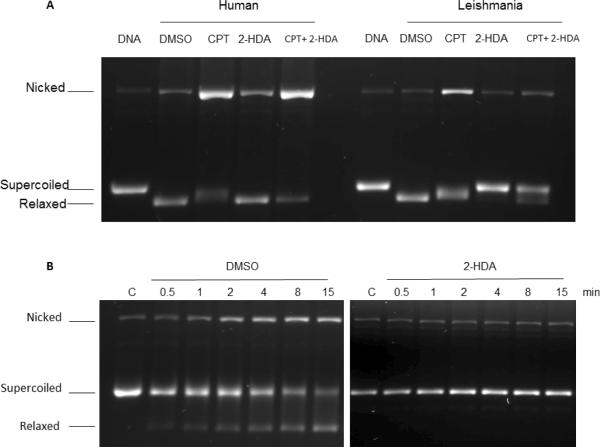
The next study contemplated if the 2-alkynoic fatty acids inhibited hTopIB and LdTopIB with a similar or different mechanism as camptothecin (CPT), a well-known topoisomerase I inhibitor.8 Although 2-HDA is a bit less inhibitory than 2-ODA towards LdTopIB, the availability of compound prompted us to choose 2-HDA as the model fatty acid to study the fatty acid-CPT mechanism. From Figure 4 it is evident that 2-HDA inhibits the catalytic activity of LdTopIB at 100 μM concentration (DNA is supercoiled in the presence of the acid) but there is no inhibition of hTopIB since the DNA substrate is totally relaxed at the same 2-HDA concentration. However, from Figure 4A (lane 5 under human) it seems that 2-HDA enhances the stabilization of cleavable complexes between CPT and hTopIB and prevents the formation of the corresponding cleavable complex made between CPT and LdTopIB (Fig. 4A, lane 5 under Leishmania). In order to further explore the mechanism of 2-HDA inhibition two parallel experiments were then performed. In a control experiment LdTopIB was first reacted with the supercoiled plasmid DNA and CPT in DMSO for 15 min at 4 °C and in another experiment LdTopIB was pre-incubated (15 min) first with 2-HDA (100 μM) followed by the addition of 100 μM CPT and the experiment was run for the same amount of time as the control. The left panel in Figure 4B shows that CPT inhibited LdTopIB in a reversible way when it was pre-incubated with DMSO (control), and after 4 min the supercoiled band was less intense and the relaxed DNA band became stronger. In addition, the nicked DNA band in Figure 4B (left) grew in intensity due to the stabilization of the ternary cleavage complexes (poisoning effect of CPT). The right panel of Figure 4B shows an assay where a pre-incubation of 2-HDA and LdTopIB was performed before the addition of DNA and CPT. Two interesting findings were obtained: i) there was no relaxation of the DNA at any extent, since only the supercoiled band was present when the gel was run, and ii) the nicked band did not grow in intensity with respect to the control lane, so CPT was unable to stabilize the cleavable complexes. If the enzyme were to cut the DNA substrate, CPT could interact with the DNA-protein complexes and thus stabilize them. Therefore, we can hypothesize that 2-HDA inhibits the LdTopIB-mediated DNA relaxation by a complete different mechanism as CPT, and prevents the formation of cleavable complexes avoiding the ability of CPT to stabilize enzyme-DNA complexes. This is consistent with the findings of Castelli and co-workers where they analysed the effect of conjugated eicosapentaenoic acid (cEPA) on the homologous human enzyme catalytic cycle.14 In particular, pre-incubation of cEPA with hTopIB before addition of DNA did not permit the stabilization of the cleavable complex by CPT and, in addition, cleavage inhibition was enhanced.14 Additional experiments with a suicide substrate, where the enzyme is not able to carry out the relegation step, revealed an identical relegation rate with or without the presence of cEPA. This is in contrast to what is normally observed with CPT, which strongly reduces the topoisomerase IB relegation rate.14 Taken as a whole, the most plausible explanation in our system is for the 2-alkynoic fatty acids to be interacting with LdTopIB by binding in a region close to the topoisomerase active site and either inhibiting the enzyme binding to DNA or blocking the cleavage reaction step.
Figure 4.
Supercoiled plasmid DNA TopIB-mediated cleavage assay comparing the inhibition and poisoning of recombinant hTopIB (A left) and LdTopIB (A right) by 2-HDA and CPT. Both enzymes were assayed in the presence of DMSO (drug vehicle), 100 μM CPT (positive control for cleavage stabilization), 100 μM 2-HDA and a mix of 100 μM 2-HDA/CPT. Samples were incubated for 4 min at 25 °C, stopped with 1% SDS and digested for one extra hour at 37°C in the presence of 1 mg/ml proteinase K. DNA was extracted with one volume of phenol-chloroform and samples were run on a 1% agarose gel containing ethidium bromide to a final concentration of 40 μg/ml in order to separate supercoiled and relaxed DNA. The bottom figure (B left panel) shows that a pre-incubation (15 min at 4 °C) with DMSO had no influence on the CPT inhibiting/poisoning mechanism, as the nicked DNA band increased its relative intensity with respect to the control, at different time intervals. When LdTopIB was pre-incubated with a 2-HDA excess (100 μM) previous to the addition of 0.5 μg of pSK DNA the enzymatic activity was fully inhibited by 2-HDA and CPT poisoning effect did not take place (B right panel). After the time-course assay samples were extracted and run as previously described in 4A. The nicked band corresponds to the stabilized cleavage complexes. The results are representative of three independent trials.
Toxicity of the 2-alkynoic fatty acids was determined against L6 cells, a primary cell line from rat skeletal myoblasts. As shown in Table 1, 2-ODA was the most toxic of the three 2-alkynoic fatty acids towards L6 cells (IC50 = 20 μM). Surprisingly, 2-HDA displayed negligible toxicity towards L6 cells (IC50 = 340.3 μM), which makes it a more therapeutically valuable compound. While 2-HDA seems to be an outlier in Table 1 we also determined that palmitic acid displays an IC50 of 209 μM against these L6 cells. This marked difference between the toxicity of fatty acids towards L6 cells does not seem to be novel. For example, oleate and palmitate display different effects with respect to mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells.15 Selective toxicity of the 2-alkynoic acids was also studied against BALB/c murine macrophages (Table 2). In this system, none of the 2-alkynoic fatty acids displayed significant toxicity, i.e., in all three examples the IC50 value was > 100 μM. This result indicates that the 2-alkynoic fatty acids display different toxicity towards different cell lines. The difference in toxicity between BALB/c and L6 cells for 2-ODA could also lie in the fact that we used a primary cell line for the latter.
The results presented herein indicate that among the studied 2-alkynoic fatty acids 2-ODA is the most toxic against L. donovani amastigotes with a mechanism of action that could involve, although not exclusively, the inhibition of the LdTopIB enzyme. As to the reasons why 2-ODA is a better inhibitor than 2-HDA or 2-TDA we can only speculate at this point since there is no complete crystal structure for LdTopIB available, only partial structures of regions similar to hTopIB or computational simulations of the whole enzyme. However, the increased affinity of 2-ODA could be simply due to an increase number of van der Waals interactions with LdTopIB by the extra methylene groups in the molecule. More research is evidently needed in order to elucidate the subtle differences in the interaction of fatty acids with LdTopIB vs. hTopIB. However, as mentioned above, one possible mechanism of inhibition is for the 2-alkynoic fatty acids to interact with LdTopIB by binding in a region close to the topoisomerase active site and just blocking the cleavage reaction, as it was demonstrated in the interaction of cEPA with hTopIB.14 In any instance, 2-ODA or 2-HDA stand out as useful fatty acids that could be used in drug formulations to treat leishmaniasis.
Acknowledgments
The project described was supported by Award Number SC1GM084708 from the National Institutes of General Medical Sciences of the NIH. M. Cartagena thanks the UPR RISE program for a graduate fellowship. We thank Gabriel Cintrón for technical assistance. This research was also supported in part by Ministerio de Ciencia e Innovation (grant AGL2010-16078/GAN), by Junta de Castilla y León (grant Gr-238) and Instituto de Salud Carlos III (grant PI09/0448 and the Tropical Diseases Network RICET) from Ministerio de Salud y Consumo of the Spanish Kingdom. Prada CF is a pre-doctoral fellow granted by Junta de Castilla y León (ESF; European Social Founding).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gershon H, Shanks L. Can. J. Microbiol. 1978;24:593. doi: 10.1139/m78-096. [DOI] [PubMed] [Google Scholar]
- 2.Li X-C, Jacob MR, Kahn SI, Ashfaq MK, Babu KS, Agarwal AK, ElSohly HN, Manly SP, Clark AM. Antimicrob. Agents Chemother. 2008;52:2442. doi: 10.1128/AAC.01297-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fatope MO, Adoum OA, Takeda Y. J. Agric. Food Chem. 2000;48:1872. doi: 10.1021/jf990550k. [DOI] [PubMed] [Google Scholar]
- 4.Li X-C, Jacob MR, ElSohly HN, Nagle DG, Smillie TJ, Walker LA, Clark AM. J. Nat. Prod. 2003;66:1132. doi: 10.1021/np030196r. [DOI] [PubMed] [Google Scholar]
- 5.Morbidoni HR, Vilchèze C, Kremer L, Bittman R, Sacchettini JC, Jacobs WR., Jr. Chem. Biol. 2006;13:297. doi: 10.1016/j.chembiol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Shak S, Reich NO, Goldstein IM, Ortiz de Montellano PR. J. Biol. Chem. 1985;260:13023. [PubMed] [Google Scholar]
- 7.Tasdemir D, Sanabria D, Lauinger IL, Tarun A, Herman R, Perozzo R, Zloh M, Kappe SH, Brun R, Carballeira NM. Bioorg. Med. Chem. 2010;18:7475. doi: 10.1016/j.bmc.2010.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pommier Y. Nat. Rev. Cancer. 2006;6:789. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 9.Das A, Mandal C, Dasgupta A, Sengupta T, Majumder HK. Nucleic Acid Res. 2002;30:794. doi: 10.1093/nar/30.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodley AL, Chakraborty AK, Xie S, Burri C, Shapiro TA. Proc. Natl. Acad. Sci. USA. 2003;100:794. doi: 10.1073/pnas.1330762100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Freitas Balanco JM, Costa Moreira MF, Bonomo A, Torres Bozza P, Amarante-Mendes G, Pirmez C, Barcinski MA. Curr. Biol. 2001;11:1870. doi: 10.1016/s0960-9822(01)00563-2. [DOI] [PubMed] [Google Scholar]
- 12.Das M, Mukherjee SB, Shaha C. J. Cell Sci. 2001;114:2461. doi: 10.1242/jcs.114.13.2461. [DOI] [PubMed] [Google Scholar]
- 13.Reguera RM, Redondo CM, Gutierrez de Prado R, Pérez-Pertejo Y, Balaña-Fouce R. Biochim. Biophys. Acta. 2006;1759:117. doi: 10.1016/j.bbaexp.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Castelli S, Campagna A, Vassallo O, Tesauro C, Fiorani P, Tagliatesta P, Oteri F, Falconi M, Majumder HK, Desideri A. Arch. Biochem. Biophys. 2009;486:103. doi: 10.1016/j.abb.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Yuzefovych L, Wilson G, Rachek L. Am. J. Physiol. Endocrinol. Metab. 2010;299:E1096. doi: 10.1152/ajpendo.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]