Abstract
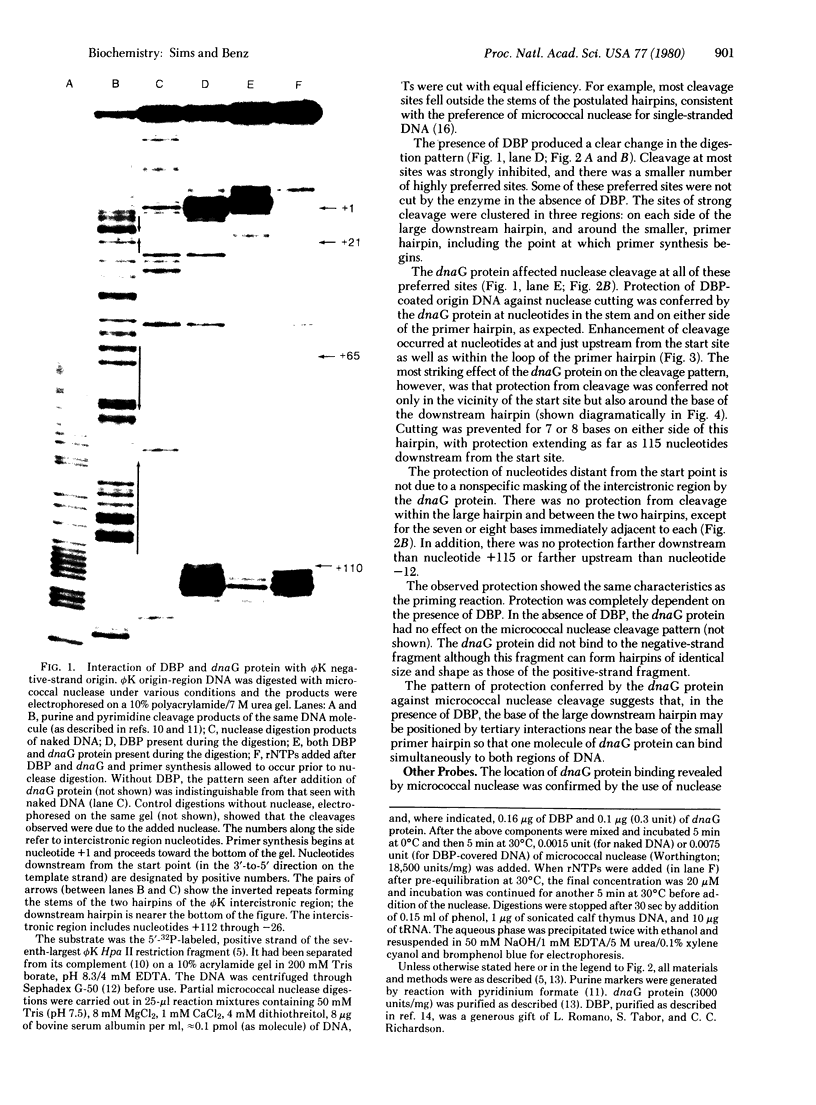
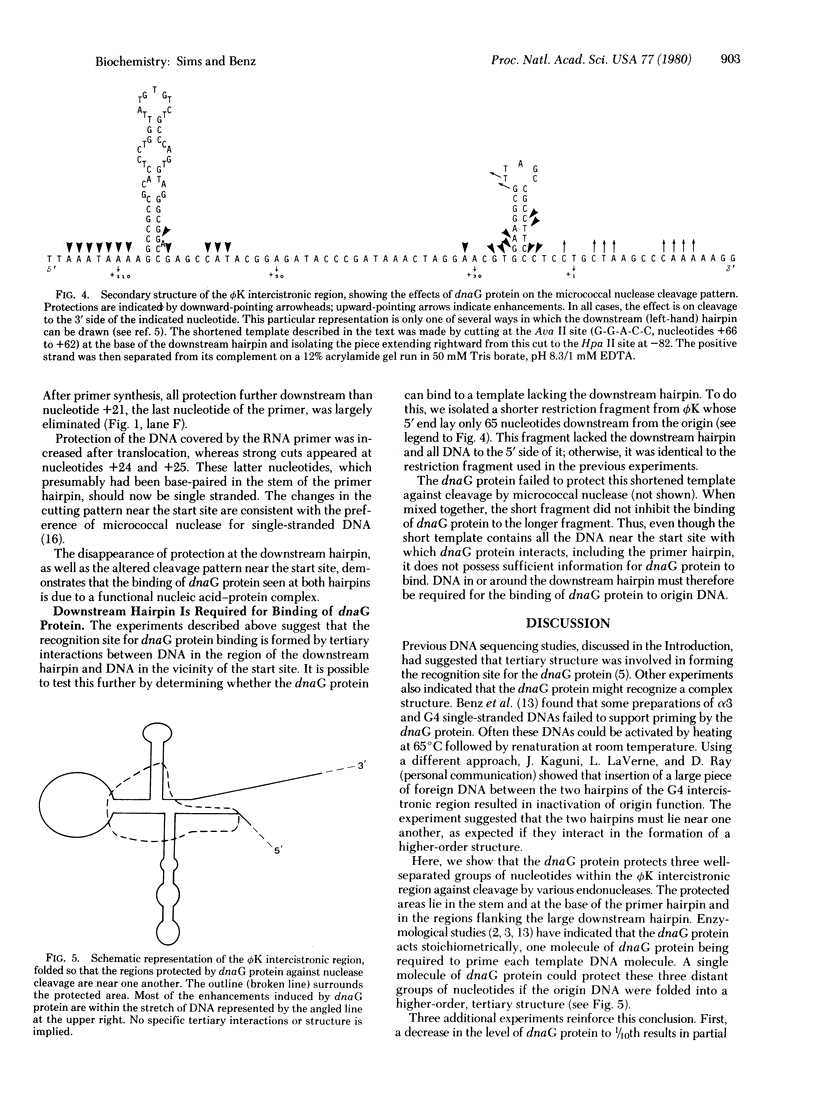
The dnaG protein of Escherichia coli initiates DNA replication by synthesizing primer oligonucleotides for elongation by DNA polymerae. The experiments reported here probe the nature of the nucleic acid element recognized by the dnaG protein. Three well-separated groups of nucleotides within the negative-strand origin of the single-stranded phage phi K are protected by the dnaG protein against nuclease digestion. DNA as far as 115 bases from the start site of primer synthesis is involved in binding of the dnaG protein to the replication origin. One molecule of dnaG protein could protect all of these nucleotides if the DNA were folded into a higher-order tertiary structure. Protection of the phi K origin by dnaG protein requires DNA binding protein, and it does not occur if the group of protected nucleotides most distant from the start site is removed from the template. There is no binding of dnaG protein to the complementary strand of the phi K origin-region DNA. The observed protection of the positive strand is due to a functional nucleic acid-protein complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER M., HEPPEL L. A., HURWITZ J. The purification and properties of micrococcal nuclease. J Biol Chem. 1961 Nov;236:3014–3019. [PubMed] [Google Scholar]
- Arai K., Kornberg A. A general priming system employing only dnaB protein and primase for DNA replication. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4308–4312. doi: 10.1073/pnas.76.9.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Fiddes J. C., Barrell B. G., Godson G. N. Nucleotide sequences of the separate origins of synthesis of bacteriophage G4 viral and complementary DNA strands. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1081–1085. doi: 10.1073/pnas.75.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. D., Meyer B. J., Ptashne M. Interactions between DNA-bound repressors govern regulation by the lambda phage repressor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5061–5065. doi: 10.1073/pnas.76.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- RUSHIZKY G. W., KNIGHT C. A., ROBERTS W. K., DEKKER C. A. Studies on the mechanism of action of micrococcal nuclease. II. Degradation of ribonucleic acid from tobacco mosaic virus. Biochim Biophys Acta. 1962 May 14;55:674–682. doi: 10.1016/0006-3002(62)90845-4. [DOI] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Sims J., Capon D., Dressler D. dnaG (primase)-dependent origins of DNA replication. Nucleotide sequences of the negative strand initiation sites of bacteriophages St-1, phi K, and alpha 3. J Biol Chem. 1979 Dec 25;254(24):12615–12628. [PubMed] [Google Scholar]
- Sims J., Dressler D. Site-specific initiation of a DNA fragment: nucleotide sequence of the bacteriophage G4 negative-strand initiation site. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3094–3098. doi: 10.1073/pnas.75.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- Wickner S. DNA or RNA priming of bacteriophage G4 DNA synthesis by Escherichia coli dnaG protein. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2815–2819. doi: 10.1073/pnas.74.7.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechel K., Bouché J. P., Kornberg A. Replication of phage G4. A novel and simple system for the initiation of deoxyribonucleic acid synthesis. J Biol Chem. 1975 Jun 25;250(12):4684–4689. [PubMed] [Google Scholar]