Abstract
Objectives. I examined age patterns of mortality differentials associated with body mass because the declining age effect observed in previous comparisons of cross-sectional age groups is susceptible to cohort and period distortions and because previous studies used time since baseline as time at risk, making the evaluation of age-specific mortality impossible.
Methods. I conducted a parametric survival analysis of data from the 1988–1994 National Health and Nutrition Examination Survey for 3 cohorts of American men and women born from 1901 through 1957 and observed from 1988 through 2006 under an age-period–cohort framework.
Results. Mortality differentials strengthened across cohorts but did not decline with age or change over the study period. Because excess overweight and obesity mortality increased from earlier cohorts to more recent ones, ignoring cohort differences led to a declining age pattern of excess mortality.
Conclusions. Cross-sectional age patterns of mortality differentials appear to be distorted by cohort differences. Age should be used as risk time to study age variations in associations between risk factors and time to event.
Widespread weight gain has led to epidemic proportions of excess body mass in the United States and elsewhere. Overweight and obesity (body mass index [BMI, defined as weight in kilograms divided by the square of height in meters] ≥ 25) are associated with a host of fatal and nonfatal conditions, such as cardiovascular diseases, cancer, diabetes, gallbladder diseases, osteoarthritis, and pulmonary diseases.1 Controversy remains over whether excess BMI is detrimental to survival in old age. It has been argued that age is associated with a decline in excess overweight and obese mortality2–5 and that an extra amount of fat is protective or at least brings no additional harm to old-age survival.3,6,7 Some researchers have proposed that weight guidelines should be adjusted for age to reflect the change over age in the mortality consequences of body mass,8,9 but others argue that weight guidelines should largely ignore analyses of older populations.10
Age variations in mortality differentials should reflect the varying importance of the risk factor for biological aging or physiological states. But it is not well understood why excess BMI should affect survival more or less as people grow older. Various hypotheses and speculations have been proposed, such as reduced physiological harms of body fat attributable to declining lypolytic activities11,12 and age-related measurement error in BMI,13 which does not distinguish between lean and fat mass and assumes that at a given body height, most of the variability in body weight is attributable to body fat. Mortality selection is another popular explanation. Survivors to old age may be selected for good health, perhaps more so in the heavier groups because of their higher early mortality.
Health conditions, however, are far from good for the overweight and obese elderly compared with the lean elderly.14–16 The high incidence and prevalence of chronic diseases and disabilities in old age have led public health experts to point out that both the quality and quantity of life should be valued12 and that excess weight could lead to a prolonged life lived in poor health. It is also recognized that mortality differentials, commonly measured in relative terms (that is, mortality ratios), may fail to adequately capture the death burden of excess BMI because adult mortality rates increase by age.2,4
Despite the controversy and discussion surrounding the age effect, methodological issues are yet to be resolved to obtain variations in the BMI–mortality relationship that can be appropriately related to age. Most previous work compared cross-sectional age groups that belonged to a multitude of birth cohorts at survey baseline and were followed up for mortality over a long period.2–5 These studies did not allow detection of age patterns that were independent of differences over birth cohort and time in the BMI–mortality association. In addition, because previous studies used time since baseline as time at risk (analysis time), younger participants at baseline might attain an age in the follow-up period that was greater than that of the baseline older groups. This age overlap among comparison groups has made it impossible to determine the age to which mortality rates or differentials pertain.
I used age as analysis time and applied an age-period–cohort framework to analyze age patterns of mortality differentials associated with BMI that were independent of period and cohort influences for American men and women born from 1901 through 1957 and observed from 1988 through 2006. Where appropriate, I further explored the cohort-versus-period sources of distortions observed in cross-sectional age patterns. I compared age patterns under an age-period–cohort specification against 3 other specifications: (1) for age and cohort only but not for period, (2) for age only, and (3) for none of the 3 time dimensions but for baseline age groups and time since baseline (to replicate previous studies).
Because the 3 quantities of age, period, and cohort are overidentified when measured in the same units,17 I grouped annual birth cohorts, thus imposing equality constraints within cohort groups to avoid the identification problem. This commonly used approach admittedly lacks theoretical underpinning and can be arbitrary. Because science has not yet produced an unambiguous way of estimating pure age effects,18 I tackled the problem from a different angle. Instead of attempting to establish the correct age patterns, I assessed which age patterns were more or less plausible, considering the observed cohort and period patterns, and I relied on existing empirical evidence on BMI-related health trends as well as statistical modeling to interpret cohort and period findings.
Reverse causation and confounding are long-recognized issues in BMI research.13 When selected members of the comparison groups die out, within-group compositions and mortality differentials could change along any of the 3 temporal dimensions. To reduce heterogeneities, I adjusted for a standard set of compositional factors.
METHODS
Data come from the 1988–1994 National Health and Nutrition Examination Survey (NHANES), conducted by the US National Center for Health Statistics.19 At baseline, the NHANES interviewed and examined a clustered and stratified probability sample of the US noninstitutionalized population. Anthropometric data (including measured body weight and height) were collected at a health examination, and respondents reported standard social, demographic, and behavioral information during the interview. Mortality was followed through December 2006 via linkage with the National Death Index.
Sample Selection and Variables
I selected the analysis sample by age at baseline. I excluded participants younger than 36 years because the covariate for weight loss was coded from a question about body weight 10 years ago that was only asked of respondents aged 36 years and older. I also excluded respondents aged 90 years and older because of top-coding at 90 years of age.
I imputed year of birth from age and approximated survey year at baseline. The NHANES data in the public domain do not provide information on either year of birth or year of survey but do supply age in months and survey phase (between October 1988 and October 1991 or between September 1991 and October 1994), so I used the midpoint (in century months) of each survey phase to approximate survey year. After the age exclusions, year of birth in the analysis sample ranged from 1901 to 1957. I stratified participants into 3 birth cohorts: 1901 to 1930, 1931 to 1940, and 1941 to 1957. A more refined classification with 10-year intervals revealed no substantial differences among those born from 1901 through 1930. Results were similar when 10-year cohorts started from the midpoint of each decade. The 1930s appeared to be the line of demarcation.
The third time dimension, period, was approximated by months since baseline. Results were basically the same under a slightly different specification that added up the midpoint of each survey phase and time since baseline.
I calculated BMI from baseline physical measures. I defined BMI groups according to World Health Organization guidelines1: underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5– < 25 kg/m2, reference), overweight (BMI = 25– < 30 kg/m2), moderately obese (BMI = 30– < 35 kg/m2), and severely obese (BMI ≥ 35 kg/m2). I distinguished 2 classes of obesity to allow for the shift to the right of the BMI distribution. I excluded underweight respondents because my analysis focused on excess BMI, and underweight mortality elevation has been largely attributed to manifest or occult diseases that lead to weight loss.20
After I excluded 56 additional participants who were pregnant at the time of the survey or had a missing BMI value, the analysis sample comprised 5218 men with 749 434 person-months and 2187 deaths, and 5790 women with 879 622 person-months and 1885 deaths. Table 1 presents sample descriptive statistics. The small number of deaths in the 2 more recent cohorts was attributable to low overall mortality at the younger ages and limited death exposure in some cohort–BMI groups. The NHANES was nationally representative, but the sample size was smaller than the convenience samples used previously.2–5
TABLE 1—
Sample Size, Person-Months, and Deaths, Unweighted: National Health and Nutrition Examination Survey, 1988–1994
| Women |
Men |
|||||
| Birth Cohort and BMI Statusa | No. | Person-Months | Deaths | No. | Person-Months | Deaths |
| Born in 1901–1930 | ||||||
| Normal weight | 904 | 112 824 | 564 | 895 | 93 052 | 684 |
| Overweight | 973 | 126 311 | 392 | 1105 | 130 720 | 750 |
| Moderate obesity | 480 | 65 464 | 265 | 395 | 51 458 | 244 |
| Severe obesity | 272 | 35 502 | 154 | 92 | 11 732 | 64 |
| Total | 2629 | 340 101 | 1564 | 2487 | 286 962 | 1742 |
| Born in 1931–1940 | ||||||
| Normal weight | 282 | 47 746 | 38 | 266 | 41 512 | 87 |
| Overweight | 330 | 55 514 | 58 | 389 | 64 835 | 82 |
| Moderate obesity | 212 | 34 405 | 43 | 194 | 31 647 | 44 |
| Severe obesity | 176 | 27 595 | 42 | 58 | 8898 | 17 |
| Total | 1000 | 165 260 | 181 | 907 | 146 892 | 230 |
| Born in 1941–1957 | ||||||
| Normal weight | 697 | 122 775 | 33 | 596 | 101 092 | 93 |
| Overweight | 653 | 113 017 | 45 | 770 | 135 292 | 65 |
| Moderate obesity | 441 | 75 637 | 32 | 321 | 55 880 | 31 |
| Severe obesity | 370 | 62 832 | 30 | 137 | 23 316 | 26 |
| Total | 2161 | 374 261 | 140 | 1824 | 315 580 | 215 |
| Total | 5790 | 879 622 | 1885 | 5218 | 749 434 | 2187 |
Note. BMI = body mass index. Analyses excluded participants who were underweight (BMI < 18.5 kg/m2) or were younger than 36 years at baseline.
Source. Data were drawn from the 1988–1994 National Health and Nutrition Examination Survey, with mortality through December 2006.19
Normal weight, BMI = 18.5 — < 25 kg/m2; overweight, BMI = 25 — < 30 kg/m2; moderate obesity, BMI = 30 — < 35 kg/m2; severe obesity, BMI ≥ 35 kg/m2.
Statistical Models
I modeled person-month records with the parametric Gompertz function. The model was characterized by an exponential increase of mortality over age a
where h(a) denoted age-specific mortality rates in the NHANES sample and β and γ denoted the scale and shape parameter of the mortality curve. The NHANES mortality rates on the logarithmic scale appeared to be linear over age (not shown), thus satisfying the parametric assumption rather well.
To examine age (a) variations in how mortality differed by BMI (W), and to assess whether period (T) and cohort (C) differences in the BMI–mortality association distorted the age patterns, I considered 3 models:
where the multiplication sign indicated interaction and the coefficients denoted vectors when the variables were of more than 2 levels.
Under equation 2, the BMI–mortality association was constant over age but differed across cohorts and periods (as respectively captured by the coefficients β4 and β5). Under equation 3, mortality differentials varied over age (as reflected in γ1) but were constrained to have no cohort or period variations. These 2 equations contrasted a constant against a varying age pattern, depending on whether they allowed for cohort and period differences. When equation 2 described the data better than did equation 3, I further compared equation 2 against equation 4, which allowed for cohort variations only. This comparison assessed the relative importance of period and cohort in the distorted age patterns.
I initially explored a more complete set of models (e.g., a model in which mortality differentials were allowed to vary over all 3 time dimensions) and found that my 3 final models were the simplest defensible ones. Because not all 3 models were nested, I based model comparison on the Akaike information criterion (AIC): a smaller AIC indicated a better model, but a difference smaller than 2 suggested a tie.21 When the AIC results were less clear-cut, I considered the change in parameter estimates.
To replicate previous studies and analyze differences between cross-sectional age groups, I fit a Cox model with time since baseline as analysis time and baseline age as strata. Interaction terms between BMI and the age strata captured whether BMI differences in mortality varied among baseline age groups. All analyses had the following baseline covariates: educational achievement, race/ethnicity, poverty-to-income ratio, marital status, smoking, weight loss in past 10 years, and lung diseases. Results did not differ substantially when I included heart problems, diabetes, and hypertension, which are likely sequelae of excess fat. Greater detail about the measurement and classification of the covariates and their bivariate associations with BMI is provided in Appendix A (available as a supplement to the online version of this article at http://www.ajph.org). I found substantial gender differences in the bivariate associations, so I separated all analyses by gender. Excess mortality was defined as relative risk (i.e., mortality ratio) minus 1.
I used Stata version 11.1 (StataCorp LP, College Station, TX) to implement both the parametric and the semiparametric survival analyses. I used the delta method to calculate the standard error of survey estimates. Except where otherwise noted, I used sample weights to represent the target population. Additional analysis that adjusted for survey-design effects (i.e., clustering and stratification) did not substantially change the results.
RESULTS
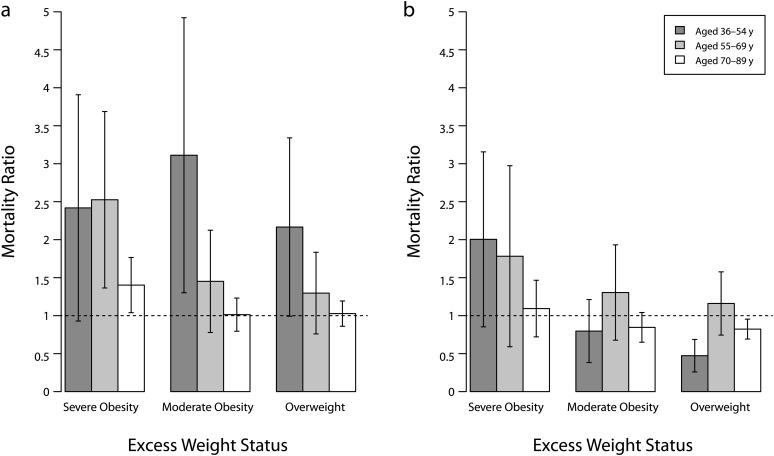
Figure 1 shows replication results. I compared overweight and obese mortality rates with normal-weight mortality across 3 baseline age groups (36–54, 55–69, and 70–89 years). Among women, excess mortality declined consistently from younger to older ages. Among participants younger than 55 years at baseline, mortality for obese and overweight participants was more than double the rate of mortality for normal-weight participants. This excess declined to nil or insignificance for persons aged 70 years and older. Among men, I observed no overweight or obese excess mortality, except among the severely obese, whose excess mortality was moderately statistically significant for the youngest group and declined for the older groups.
FIGURE 1—
Replication results, mortality ratios by cross-sectional age groups, estimates, and 95% confidence intervals among (a) women and (b) men: National Health and Nutrition Examination Survey, 1988–1994.
Note. BMI = body mass index. Estimates were based on Cox models. Covariates were educational achievement, race/ethnicity, poverty-to-income ratio, marital status, smoking, weight loss, and lung diseases. The analysis excluded underweight participants (BMI < 18.5 kg/m2) and participants younger than 36 years at baseline. Sample weights were used. The reference category was normal weight (BMI = 18.5 – < 25 kg/m2).
Source. Data were drawn from the 1988–1994 National Health and Nutrition Examination Survey, with mortality through December 2006.19
Overall, my results confirmed previous findings of declining excess mortality across baseline age groups.2–5 Because of the methodological issues in this type of analysis, the declining pattern could have been distorted by cohort or period differences and therefore had no bearing on whether the survival disadvantage of excess body mass varied over age.
Table 2 shows results from the 3 Gompertz models under the age-period–cohort framework. According to the AIC values, the 2 models with constant age patterns but variations across periods and cohorts (equation 2) or variations across cohorts only (equation 4) fit the data substantially better than did the model that assumed constant period and cohort patterns but age variations (equation 3). In light of the period and cohort differences observed in the data, constant age patterns were more plausible than varying age patterns, and cohort differences were responsible for the distortions.
TABLE 2—
Results for Selected Gompertz Models (Log Scale): National Health and Nutrition Examination Survey, 1988–1994
| Parameters | Equation 2,a Estimate (SE) | Equation 3,b Estimate (SE) | Equation 4,c Estimate (SE) |
| Women | |||
| Intercept | −15.139*** (0.442) | −15.946*** (0.457) | –15.342*** (0.422) |
| BMI statusd | |||
| Normal weight (Ref) | 1.000 | 1.000 | 1.000 |
| Overweight | 0.061 (0.149) | 1.777*** (0.608) | 0.009 (0.078) |
| Moderately obese | 0.006 (0.182) | 2.448*** (0.734) | 0.002 (0.104) |
| Severely obese | 0.559*** (0.213) | 2.364*** (0.717) | 0.397*** (0.123) |
| Age, mo | 0.009*** (0.000) | 0.010*** (0.000) | 0.001*** (0.000) |
| Birth cohort | |||
| C1: 1901–1930 (Ref) | 1.000 | 1.000 | |
| C2: 1931–1940 | −0.166 (0.245) | −0.273 (0.221) | |
| C3: 1941–1957 | 0.047 (0.320) | –0.139 (0.283) | |
| Time since baseline, mo | 0.002* (0.001) | ||
| Interactions | |||
| Age × overweight | −0.002*** (0.001) | ||
| Age × moderately obese | −0.002*** (0.001) | ||
| Age × severely obese | −0.002*** (0.001) | ||
| C2: overweight | 0.669** (0.273) | 0.654** (0.270) | |
| C2: moderately obese | 0.678** (0.323) | 0.655** (0.316) | |
| C2: severely obese | 0.673** (0.320) | 0.624** (0.317) | |
| C3: overweight | 0.761** (0.355) | 0.743** (0.352) | |
| C3: moderately obese | 1.153*** (0.381) | 1.127*** (0.379) | |
| C3: severely obese | 0.468 (0.413) | 0.407 (0.412) | |
| Period × overweight | −0.001 (0.001) | ||
| Period × moderately obese | −0.000 (0.002) | ||
| Period × severely obese | −0.002 (0.002) | ||
| Goodness of fit (AIC) | 331.740 | 335.197 | 330.361 |
| Men | |||
| Intercept | −14.240*** (0.441) | –13.724*** (0.445) | –14.307*** (0.439) |
| BMI statusa | |||
| Normal weight (Ref) | 1.000 | 1.000 | 1.000 |
| Overweight | −0.136 (0.137) | −1.042** (0.516) | −0.141* (0.078) |
| Moderately obese | −0.431** (0.206) | −0.604 (0.807) | −0.126 (0.109) |
| Severely obese | −0.037 (0.319) | 1.894* (0.976) | 0.131 (0.163) |
| Age, mo | 0.009*** (0.000) | 0.008*** (0.000) | 0.009*** (0.000) |
| Birth cohort | |||
| C1: 1901–1930 (Ref) | 1.000 | 1.000 | |
| C2: 1931–1940 | −0.108 (0.186) | −0.124 (0.182) | |
| C3: 1941–1957 | 0.373 (0.246) | 0.355 (0.229) | |
| Time since baseline, mo | −0.001 (0.001) | ||
| Interactions | |||
| Age × overweight | 0.001* (0.001) | ||
| Age × moderately obese | 0.001 (0.001) | ||
| Age × severely obese | –0.002* (0.001) | ||
| C2: overweight | −0.024 (0.241) | −0.021 (0.239) | |
| C2: moderately obese | 0.147 (0.298) | 0.196 (0.291) | |
| C2: severely obese | 0.339 (0.454) | 0.375 (0.434) | |
| C3: overweight | −0.684** (0.284) | −0.677** (0.278) | |
| C3: moderately obese | 0.007 (0.342) | 0.067 (0.334) | |
| C3: severely obese | 0.650* (0.372) | 0.687* (0.360) | |
| Period × overweight | 0.000 (0.001) | ||
| Period × moderately obese | 0.003 (0.002) | ||
| Period × severely obese | 0.002 (0.003) | ||
| Goodness of fit (AIC) | 854.795 | 865.539 | 852.659 |
Note. AIC = Akaike information criterion; BMI = body mass index; C = cohort. Analyses excluded participants who were underweight (BMI < 18.5) or were younger than 36 years at baseline. Covariates were educational achievement, race/ethnicity, poverty-to-income ratio, marital status, smoking, weight loss, and lung diseases. Sample weights were used.
Source. Data were drawn from the 1988–1994 National Health and Nutrition Examination Survey, with mortality through December 2006.19
h(a) = exp(β0 + β1 · W + β2 · C + β3 · T + β4 · W × C + β5 · W × T + γ0 · a).
h(a) = exp(β0 + β1 · W + γ0 · a + γ1 · W × a).
h(a) = exp(β0 + β1 · W + β2 · C + β3 · W × C + γ0 · a).
Normal weight, BMI = 18.5– < 25 kg/m2; overweight, BMI = 25– < 30 kg/m2; moderate obesity, BMI = 30– < 35 kg/m2; severe obesity, BMI ≥ 35 kg/m2.
*P < .1; **P < .05; ***P < .01.
The contribution of period differences was less clear-cut. Although the cohort-only model had the smallest AIC value of the 3 models, its AIC difference from the cohort-plus-period model was smaller than 2 in the sample of women, suggesting that the 2 models might fit the data equally well. The parameter estimates, however, favored the cohort-only model for both men and women, for 3 reasons.
First, most estimates of cohort differences in the mortality differentials were statistically significant, and they were all consistent across models. Second, none of the period estimates were significant (unsurprisingly, according to the AIC, a period-only model was worse than any of the 3 models shown), and contrary to expectation, the estimates for men and women were in the opposite directions. Finally, although estimates for the main effects of cohort and BMI status varied somewhat across equations 2 and 4, they were mostly insignificant. Two exceptions were the estimate for moderate obesity in men, which changed from a significant value (−0.431, equation 2) to an insignificant one (−0.126, equation 4), and the estimate for severe obesity in women, which declined from 0.559 to 0.397 but with no change in statistical significance. These BMI effect estimates pertained to the reference cohort but not to cohort differences. Notably, under equation 2 these 2 large estimates were counterbalanced by large but insignificant estimates for period change in the opposite direction, pointing to collinearity among the temporal parameters. Thus, arguably both the AIC values and the parameter estimates supported the simpler model, suggesting significant cohort but not period differences as the source of distorted age patterns.
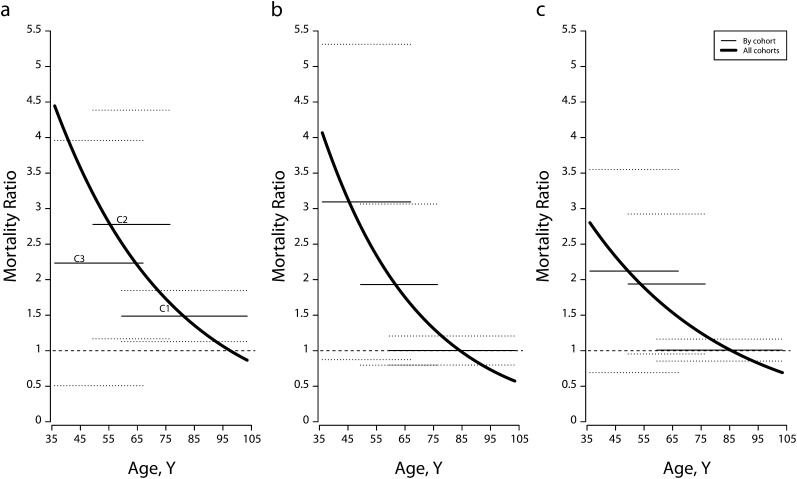
Figures 2 and 3 compare age-specific overweight and obese mortality rates with normal-weight rates under equation 3 (all cohorts combined) and equation 4 (separately by cohort). To make the data easier to read, I showed 95% confidence intervals only for the cohort-specific estimates. Among women, mortality differentials were trivial for participants born in 1930 or earlier, except for a 50% higher mortality among the severely obese. For the 2 later cohorts, excess mortality reached 100% or more for all 3 excess-BMI groups. Not all estimates were statistically significant at α = .05, but this was attributable to the small number of deaths (Table 1). Statistical uncertainties could be reduced by combining the 2 later cohorts (not shown). Relative mortality showed a declining age pattern when the cohort pattern was ignored.
FIGURE 2—
Age-specific mortality ratios, estimates, and 95% confidence intervals for women with (a) severe obesity, (b) moderate obesity, and (c) overweight, by birth cohort, versus all cohorts combined: National Health and Nutrition Examination Survey, 1988–1994.
Note. BMI = body mass index. Dotted lines represent 95% confidence intervals. Birth cohort C1 = 1901–1930, C2 = 1931–1940, and C3 = 1941–1957. Estimates were based on Gompertz models. Covariates were educational achievement, race/ethnicity, poverty—income ratio, marital status, smoking, weight loss, and lung diseases. The analysis excluded underweight participants (BMI < 18.5 kg/m2) and participants younger than 36 years at baseline. Sample weights were used. The reference category was normal weight (BMI = 18.5 – < 25 kg/m2).
Source. Data were drawn from the 1988–1994 National Health and Nutrition Examination Survey, with mortality through December 2006.19
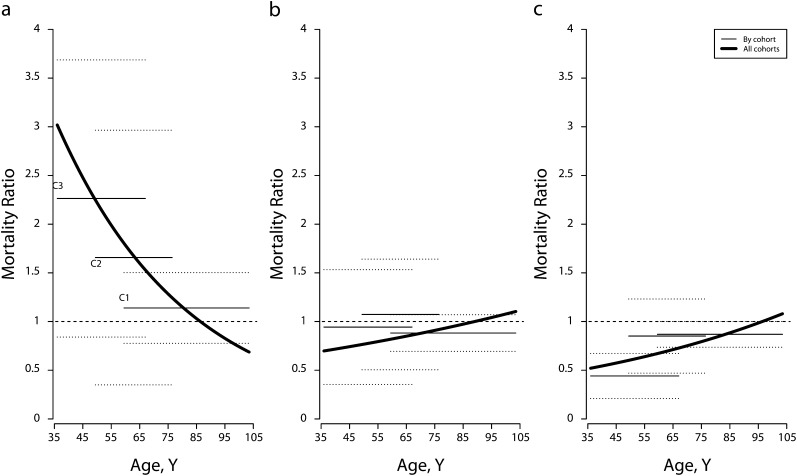
FIGURE 3—
Age-specific mortality ratios, estimates, and 95% confidence intervals for men with (a) severe obesity, (b) moderate obesity, and (c) overweight, by birth cohort, versus all cohorts combined: National Health and Nutrition Examination Survey, 1988–1994.
Note. BMI = body mass index. Dotted lines represent 95% confidence intervals. Birth cohort C1 = 1901–1930, C2 = 1931–1940, and C3 = 1941–1957. Estimates were based on Gompertz models. Covariates were educational achievement, race/ethnicity, poverty-to-income ratio, marital status, smoking, weight loss, and lung diseases. The analysis excluded underweight participants (BMI < 18.5 kg/m2) and participants younger than 36 years at baseline. Sample weights were used. The reference category was normal weight (BMI = 18.5 - < 25 kg/m2).
Source. Data were drawn from the 1988–1994 National Health and Nutrition Examination Survey, with mortality through December 2006.19
The direction and cohort pattern of mortality differences for men between severe obesity and normal weight were similar to results for women: the severely obese suffered excess mortality, and the disadvantage increased across cohorts. However, unlike for women, overweight or moderate obesity did not elevate mortality among men. Instead, overweight participants had the lowest mortality of all men, and this advantage increased substantially for the most recent cohort. The ways in which cohort differences among men distorted age patterns depended on BMI status. Overweight relative mortality became increasingly lower across cohorts, so it rose over age when all cohorts were combined. By contrast, cohort increment in the excess mortality of severe obesity, when ignored, led to a declining age pattern. Relative mortality for moderate obesity varied little by cohort, and the corresponding age distortions were trivial.
DISCUSSION
For 3 cohorts of American men and women born from 1901 through 1957, the long-term mortality consequences of body mass strengthened across cohorts but did not decline over age or change across the study period between 1988 and 2006. When my analyses ignored cohort differences, mortality differentials varied over age. Because excess mortality attributable to overweight and obesity increased from earlier to more recent cohorts, and because cohorts born earlier tended to be older in the study sample, failing to account for cohort increments led to a declining age pattern. A model that accommodated cohort differences but imposed a constant age pattern was more consistent with the data than one that ignored cohort differences but allowed for age variations.
My results are not directly comparable to previous research, which found that overweight and obese excess mortality declined over baseline age groups.2–5 My data replicated this finding. Such results have been interpreted as a declining effect of age on the survival disadvantages of excess body mass. Baseline age groups, however, belong to a multitude of birth cohorts, and their mortality is typically observed over a long period in BMI research. Mortality differentials have been expanding across cohorts. When I lumped all cohorts together, relative mortality took on a declining (or rising for overweight or moderately obese men) age pattern, accompanied by a substantial deterioration of model fit. These results indicated cohort influences on the declining age patterns observed in the cross-sectional samples.
Cohort effect is typically regarded as persistent influences from the past.22–24 Evidence of cohort increments in overweight or obese excess mortality is consistent with a growing body of evidence showing that excess fat in adolescence or early adulthood and weight gain over the life course have long-term implications for metabolic, cardiovascular, and mortality risks.13,25,26 I found that for women born after 1940, marked cohort increments in excess mortality occurred with overweight and moderate obesity, rather than severe obesity, and mortality became comparable across all 3 excess-BMI groups. An early and extended exposure to excess BMI appears to have taken a more aggravated death toll among the moderately fat groups.
Period influences include contemporaneous factors such as the adoption of new drugs and therapies to treat chronic diseases related to excess fat. Advances in health care may have reduced or even eliminated the negative health consequences of excess fat,27,28 but concerns remain about whether these advances are effective in providing a permanent cure.29,30 The divide between cohort and period perspectives underlies the ongoing debate about the impact of excess body mass on population health.27,31 I found that (1) according to the AIC statistic, the cohort-plus-period model fit the data as well as the cohort-only model, but (2) after accounting for significant and consistent cohort trends, estimated period trends in mortality differentials were all insignificant, in the negative direction for women and positive for men. Despite this lack of strong evidence, period influences cannot be ruled out, especially when major biomedical breakthroughs occur in the future.
In addition to debunking the cross-sectional age patterns and investigating the sources of distortions, my study supports consideration of an age-specific framework that has been neglected in medical research. Time since baseline is the standard analysis time, appropriately so because the predominant medical interest has been the comparison of on-study time across treatments that are administered at baseline. Despite a growing body of methodological and empirical work in favor of using age to analyze epidemiological survey data, the considerations have been primarily of estimation bias rather than substantive interpretation.27,32,33 One exception was a study that acknowledged the difficulty of estimating age-related quantities such as mean age at death under the time-on-study framework.34
When the research interest is age variations in the association between the risk factor and time to event, it is imperative to use age as analysis time because otherwise it is impossible to pinpoint the age of event when multiple birth cohorts and long-term follow-ups are involved. The empirical regularities in the age curve of human mortality35 provide an additional advantage of an age-specific analysis: that is, to allow for the estimation of parametric models (e.g., the Gompertz model) instead of semiparametric ones to improve statistical efficiency.
Telltale evidence of unobserved heterogeneities remained in the analysis. The overweight survival advantage that was extensively documented in previous studies (e.g., analyses based on NHANES data20,27) was limited to men only, and overweight women in more recent cohorts suffered substantial long-term excess mortality. Both the advantage (for men) and disadvantage (for women) strengthened for those born after 1940.
Although biological explanations such as gender differences in body composition36 are plausible, I found substantial gender and cohort differences in the association between BMI and a series of socioeconomic, demographic, and behavioral factors (Appendix A, available as a supplement to the online version of this article at http://www.ajph.org, provides greater detail). A higher BMI was associated with less education and more poverty among women. Among men, however, lower socioeconomic status and unhealthy behaviors and conditions were no less or even more prevalent among the lean, and in the most recent cohort, the percentage of college graduates was highest among the overweight. Despite the statistical adjustment, residual confounding could have been severe in the results for men and possibly for women in earlier cohorts. Further research is needed to trace the dynamic social and behavioral mechanisms that influence individual BMI status.
Acknowledgments
This article was based on one chapter of a doctoral thesis that the author completed at the Center for Demography and Ecology, Department of Sociology, University of Wisconsin–Madison.
An earlier version of this article was presented at the 2009 Australian Association of Gerontology National Conference in Canberra and the 2011 Population Association of America Annual Meeting in Washington, DC.
I thank Michel Guillot, John A. Logan, Robert M. Hauser, Zhen Zeng, Rick Nordheim, Alberto Palloni, and 3 anonymous reviewers for their helpful comments and suggestions.
Human Participant Protection
No institutional review board approval was needed because this study used publicly available anonymous data.
References
- 1.World Health Organization Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity. Geneva, Switzerland: World Health Organization; 2000 [PubMed] [Google Scholar]
- 2.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338(1):1–7 [DOI] [PubMed] [Google Scholar]
- 3.Bender R, Jöckel K-H, Trautner C, Spraul M, Berger M. Effect of age on excess mortality in obesity. JAMA. 1999;281(16):1498–1504 [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med. 1999;341(15):1097–1105 [DOI] [PubMed] [Google Scholar]
- 5.Park HS, Song Y-M, Cho S-I. Obesity has a greater impact on cardiovascular mortality in younger men than in older men among non-smoking Koreans. Int J Epidemiol. 2006;35(1):181–187 [DOI] [PubMed] [Google Scholar]
- 6.Andres R, Elahi D, Tobin JD, Muller DC, Brant L. Impact of age on weight goals. Ann Intern Med. 1985;103(6 pt 2):1030–1033 [DOI] [PubMed] [Google Scholar]
- 7.Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc. 2001;49(7):968–979 [DOI] [PubMed] [Google Scholar]
- 8.Heiat A. Impact of age on definition of standards for ideal weight. Prev Cardiol. 2003;6(2):104–107 [DOI] [PubMed] [Google Scholar]
- 9.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161(9):1194–1203 [DOI] [PubMed] [Google Scholar]
- 10.Hu FB. Obesity and mortality. : Hu FB, Obesity Epidemiology. New York, NY: Oxford University Press; 2008:216–231 [Google Scholar]
- 11.Elia M. Obesity in the elderly. Obesity Res. 2001;9(suppl 4):s244S–s248 [DOI] [PubMed] [Google Scholar]
- 12.Seidell JC, Visscher TL. Body weight and weight change and their health implications for the elderly. Eur J Clin Nutr. 2000;54(suppl 3):S33–S39 [DOI] [PubMed] [Google Scholar]
- 13.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341(6):427–434 [DOI] [PubMed] [Google Scholar]
- 14.Himes CL. Obesity, disease, and functional limitation in later life. Demography. 2000;37(1):73–82 [PubMed] [Google Scholar]
- 15.Lang IA, Llewellyn DJ, Alexander K, Melzer D. Obesity, physical function, and mortality in older adults. J Am Geriatr Soc. 2008;56(8):1474–1478 [DOI] [PubMed] [Google Scholar]
- 16.Launer LJ, Harris T, Rumpel C, Madans J. Body mass index, weight change, and risk of mobility disability in middle-aged and older women: the epidemiologic follow-up study of NHANES I. JAMA. 1994;271(14):1093–1098 [PubMed] [Google Scholar]
- 17.Mason WM, Smith HL. Age-period-cohort analysis and the study of deaths from pulmonary tuberculosis. : Mason WM, Fienberg SE, Cohort Analysis in Social Research: Beyond the Identification Problem. New York, NY: Springer; 1985:151–227 [Google Scholar]
- 18.Preston SH, Wang H. Sex mortality differences in the United States: the role of cohort smoking patterns. Demography. 2006;43(4):631–646 [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Vital Health Stat 1. 1994;(32):1–407 [PubMed] [Google Scholar]
- 20.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298(17):2028–2037 [DOI] [PubMed] [Google Scholar]
- 21.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd ed New York, NY: Springer; 2002 [Google Scholar]
- 22.Ryder NB. The cohort as a concept in the study of social change. Am Sociol Rev. 1965;30(6):843–861 [PubMed] [Google Scholar]
- 23.Hobcraft J, Menken J, Preston S. Age, period, and cohort effects in demography: a review. Popul Index. 1982;48(1):4–43 [PubMed] [Google Scholar]
- 24.Guillot M. Period versus cohort life expectancy. : Rogers RG, Crimmins EM, International Handbook of Adult Mortality. New York, NY: Springer; 2011 [Google Scholar]
- 25.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327(19):1350–1355 [DOI] [PubMed] [Google Scholar]
- 27.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867 [DOI] [PubMed] [Google Scholar]
- 28.Gregg EW, Cheng YJ, Cadwell BLet al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293(15):1868–1874 [DOI] [PubMed] [Google Scholar]
- 29.Cannon CP. Balancing the benefits of statins versus a new risk—diabetes. Lancet. 2010;375(9716):700–701 [DOI] [PubMed] [Google Scholar]
- 30.Hu FB. Overweight and increased cardiovascular mortality: no French paradox. Hypertension. 2005;46(4):645–646 [DOI] [PubMed] [Google Scholar]
- 31.Olshansky SJ, Passaro DJ, Hershow RCet al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–1145 [DOI] [PubMed] [Google Scholar]
- 32.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80 [DOI] [PubMed] [Google Scholar]
- 33.Thiébaut ACM, Bénichou J. Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study. Stat Med. 2004;23(24):3803–3820 [DOI] [PubMed] [Google Scholar]
- 34.Lamarca R, Alonso J, Gómez G, Muñoz A. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53(5):M337–M343 [DOI] [PubMed] [Google Scholar]
- 35.Preston SH, Heuveline P, Guillot M. Demography: Measuring and Modeling Population Processes. Malden, MA: Blackwell; 2001 [Google Scholar]
- 36.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143(3):228–239 [DOI] [PubMed] [Google Scholar]