Abstract
Objectives. We studied the effect of antiretroviral therapy (ART) on the quality of life (QOL) of Cubans with HIV/AIDS.
Methods. We conducted a cross-sectional study including administration of the Medical Outcomes Study–HIV Health Survey Questionnaire to a representative sample of the 1592 Cubans receiving ART in 2004. For univariate analyses, we compared mean HIV scale scores. We used logistic regression models to estimate the association between role function and year of diagnosis, between pain and sex, and between health transition and region of diagnosis, with adjustment for demographics, ART regimen, and clinical status.
Results. There were 354 participants (73 women, 281 men). Scores for all functional activities showed means higher than 80 out of 100. Pain interfered more in women than in men (73.2 vs 81.9; P = .01). When HIV diagnosis occurred after 2001, the probability of experiencing difficulties performing work (odds ratio [OR] = 4.42; 95% CI = 1.83, 10.73) and pain (OR = 1.70; 95% CI = 1.01, 2.88) increased compared with earlier diagnosis. People treated with indinavir showed a greater perception of general health (58.9 vs 52.4; P = .045) and greater health improvement (78.6 vs 67.8; P = .002).
Conclusions. Although Cubans receiving ART are maintaining a high QOL, we observed significant differences by sex and time of diagnosis. QOL assessment can serve as a health outcome and may allow identification of QOL reductions potentially related to ART side effects.
In the Caribbean region, which is characterized by the highest prevalence of HIV outside of sub-Saharan Africa, AIDS is one of the main causes of adult death.1 Cuba has an estimated adult prevalence of HIV of 0.1%—the lowest in the Caribbean and the rest of the Americas—despite a rising HIV incidence.1 Transmission occurs fundamentally among men who have sexual intercourse with other men.2 Cuba is a country with a high development index and a low proportion of people below certain deprivation threshold levels in each of the dimensions of the high development index, as measured by the human poverty index.3
Between 1986 and 1994, life in AIDS sanatoria was mandatory for all Cubans diagnosed with HIV—a contentious policy that generated multiple debates.4–7 Sanatoria were originated to provide medical and psychological care and to train people to live with HIV and to cope with the impact of the diagnosis.8,9 Whereas some authors argue that the quarantine contributed to the slow growth of the epidemic in Cuba,2,10 other studies have associated its low-level transmission with high condom use and an intensive policy of HIV testing, counseling, contact tracing, and active follow-up of all people diagnosed with HIV.2 Because of the human and social cost of quarantine, an outpatient care system was initiated in 199411 with the aim of reintroducing people with HIV back into society. By the end of 2008, 74% of those diagnosed with HIV in Cuba received ambulatory care and 26% either lived in sanatoria—now called Centers for Comprehensive Care for People with HIV/AIDS—or were staying there temporarily while they received training on how to live with HIV.12 This training, which is also provided to those in ambulatory care, consists mostly on how to eat a healthy diet, maintain good personal hygiene, keep medical appointments, complete examinations, adhere to treatment, avoid substance use, and prevent HIV transmission and reinfection. To ensure appropriate nutrition, people with HIV are entitled to additional food rations.11
Until 1996, some patients received antiretroviral (ARV) monotherapy or dual therapy and, between 1996 and 2001, a small number of patients received triple antiretroviral therapy (ART), mostly through donations. Since 2001, after the Cuban government started to produce generic ARVs,11 ART became the standard regimen, free of cost to the patient.2,11 Nationally produced ARVs included zidovudine (AZT), lamivudine (3TC), stavudine (d4T), indinavir (IDV), didanosine (DDI), and nevirapine (NVP). A greater number of therapeutic combinations was introduced in 2003, when a grant from the Global Fund to Fight AIDS, Tuberculosis, and Malaria (GFATM) allowed the purchase of additional ARVs. In 2003, Cuba achieved universal access to ART11 for all those who met clinical eligibility criteria; that is, HIV infection with a CD4 count less than 350 cells per cubic millimeter with or without opportunistic infections; an AIDS-defining illness such as lymphoma, tuberculosis, or Kaposi’s sarcoma independent of viral load; or a viral load of at least 55 000 copies per milliliter. As of May 2009, 73.4% of patients receiving ART were treated with generic medications manufactured in Cuba, 0.8% with ARVs purchased through the GFATM, and 25.8% with a mix of Cuban generics and ARVs purchased through the GFATM.12
In Cuba, the use of ART has proven to be effective, improving immunologic parameters, increasing survival, and diminishing the occurrence of opportunistic infections and AIDS-related mortality11,13—even though development of drug resistance and treatment failure, associated with nonadherence to ART, have been documented.14,15 Now that HIV infection is a chronic illness, quality of life (QOL) assessment can serve as a health outcome and may also allow clinicians and other health workers to identify any reductions in QOL potentially related to short- and long-term side effects of ART. Because nonadherence can be potentially related to reductions in QOL linked with side effects,16 its resulting increased viral load may also have a public health impact in terms of increasing the likelihood of transmission of HIV.17 Although the impact of ART on QOL was explored in a qualitative study among pregnant women in Cuba,18 this article presents the first quantitative study of the effect of the provision of ART on QOL of a nationally representative sample of people living with HIV/AIDS in Cuba.
METHODS
We conducted a cross-sectional study of the Cuban population with HIV/AIDS receiving ART that included, for the first time in Cuba, the administration of the Spanish-language version of the Medical Outcomes Study (MOS)–HIV Health Survey Questionnaire19—adapted from the version used in Mexico,20 to which we replaced 1 word with another of equivalent meaning. This questionnaire, aimed at people living with HIV, defines 11 health domains: general health perceptions, physical function, role function, social function, cognitive function, pain, mental health, energy/fatigue, health distress, QOL, and health transition. Table 1 describes each of these domains. It has been administered to more than 20 000 people with HIV to measure QOL, as reported in descriptive studies and clinical essays all over the world, demonstrating its reliability and validity.20,23–28
TABLE 1—
Domains Included in the Medical Outcomes Study–HIV Health Survey Questionnaire
| Domain | Definition | Number of Items |
| General health perceptions | Individual items in the scale ask patients to report on their general health, resistance to illnesses, and health outlook. | 5 |
| Physical functioning | This dimension consists of items that assess a range of severe and minor physical limitations. These items represent different levels and kinds of limitations including lifting heavy objects or participating in strenuous sports; walking uphill or climbing a few flights of stairs; bending, lifting, or stooping; and walking a short distance. Limitations in self-care activities are measured with a single item assessing the ability to eat, dress, bathe, or use the toilet by oneself. | 6 |
| Role functioning | Items are used to assess the impact of patients’ health on their ability to perform on the job, around the house, or in school. Patients are asked if their health keeps them from working at a job, doing work around the house, or going to school. The scale also asks if patients are unable to do certain kinds of work, housework, or schoolwork because of their health. | 2 |
| Pain | The survey assesses both the intensity of bodily pain and the degree of interference with normal activities because of pain. | 2 |
| Social functioning | This subscale asks patients to assess the extent to which their health in the past 4 weeks has limited their social activities. | 1 |
| Mental health | One or more items from each of the 4 major mental health dimensions (anxiety, depression, loss of behavioral/emotional control, and psychological well-being) are included in the scale. | 5 |
| Energy/fatigue | This scale measures differences in vitality. | 4 |
| Health distress | This dimension assesses the degree to which patients are discouraged and afraid because of their health problems. The scale also asks patients about the amount of time in the past 4 weeks that they have felt despair and weighed down by health problems. | 4 |
| Cognitive functioning | This dimension measures the degree of difficulty patients have experienced in the past 4 weeks with respect to their cognitive abilities. Patients are asked to assess how much of the time they have had difficulty reasoning or solving problems, been forgetful, and have had difficulty in remaining attentive and concentrating on activities. | 4 |
| Quality of life | In this dimension, patients are asked to assess the quality of their life during the past 4 weeks. The response categories range from very well, could hardly be better, to very bad, could hardly be worse. | 1 |
| Health transition | This question asks patients about the amount of change in their physical and emotional health over a 4-week period and has been found to provide useful information about actual changes in health status during the period before the administration of the survey. | 1 |
Study Population
On the basis of inclusion criteria—aged 18 years and older, diagnosed with HIV/AIDS, and receiving ART—our study population size in August 2004 was 1592 people (300 women and 1292 men).12 Using EpiInfo version 6.04a (Centers for Disease Control and Prevention, Atlanta, GA), we estimated a sample size of 309 to achieve a 95% confidence interval (CI) with 5% α level. We increased this value by 30% to account for those who would not consent to participate or who could not be reached. We randomly selected 400 people with SIDATRAT version 2.1 (Pedro Kourí Institute of Tropical Medicine [IPK], Havana, Cuba), a confidential electronic database that includes demographic and clinical information of all people diagnosed with HIV in Cuba since its first case in 1986 and until their death or loss to follow-up.29 We performed a simple random sample from this database and data extracted included sex, year of diagnosis, age at the time of diagnosis, and region where the person was diagnosed. Provinces were grouped in 4 regions: Havana City, Western Cuba, Central Cuba, and Eastern Cuba. This was based on the geographical variation of first-line ART regimens and on the potential difference in quality of care provided in different parts of the country. Year of diagnosis was grouped into 3 periods based on the availability of ART: (1) before July 1996, when no effective therapy was available for HIV; (2) between July 1996 and May 2001, when ART was known but with limited access in Cuba; and (3) from June 2001, when ART became available in Cuba, until August 2004, when we selected the sample. Age at time of diagnosis was categorized as follows: younger than 18 years, 18 to 29 years, 30 to 39 years, and 40 years or older. This was based on the local implications of an HIV diagnosis as it relates to QOL for different age groups.
The study period was from July 2005 to September 2007. People who met the inclusion criteria were invited to participate during their follow-up visits at the Hospital of the IPK—the national reference center for AIDS treatment in Cuba. The study was approved by the Ethics Committees of the IPK and Harvard Medical School. All study participants provided written informed consent, granted access to their clinical history, and were interviewed in an open-ended life history format (under analysis) in addition to the oral administration of the MOS-HIV questionnaire. Participants also provided demographic data (self-identified skin color, educational level, marital status, self-identified sexual practice) and from their clinical history were obtained: AIDS classification, number of years under treatment, used ART combinations, treatment changes, use of indinavir, CD4 count, and viral load.
Interviewers read each question and the choice of answers to each study participant and recorded by hand all the answers; all questions were answered. The completion of the questionnaire took 10 to 15 minutes per person. Items and scales were scored following the MOS-HIV Health Survey Scoring Guidelines.21 Final scores ranged from 0 to 100, with a higher value indicating better health or less pain. The questionnaire demonstrated good internal consistency (Cronbach’s α = 0.89) in this context. For the MOS-HIV subscales, Cronbach’s α ranged from 0.64 to 0.88. All of the subscales with greater than 1 item demonstrated Cronbach’s α estimates above 0.70, except for the “role functioning” subscale (0.64); however, this subscale only has 2 items. For convergent validity, correlations between specific items and the respective subscales ranged from 0.36 to 0.95, with only 1 item below 0.65 for all of the subscales. In terms of discriminant validity, correlations between subscales and items not within the respective subscale ranged from 0.06 to 0.63 with only 3 items for all of the subscales with correlations 0.60 or greater.
Analyses
We analyzed scores by the 4 categorical variables and additional demographic and ART data with comparisons of means. We performed univariate analyses for the MOS-HIV subscales and overall scale with t-tests. We estimated the association between role function and year of diagnosis, between pain and sex, and between transition of health and region of diagnosis by using logistic regression models predicting the lowest tertile for each scale as the outcome. To adjust for possible confounders, we included demographic variables, ART regimen, and clinical status in the multivariate models. Because of the significant number of outcome variables (10 MOS-HIV subscales and 1 overall scale), we report only outcomes that demonstrated associations to allow for parsimony in the presentation of results. We used the change-in-estimate modeling strategy described by Greenland30 to determine inclusion of variables in multivariate models. First we estimated the univariate association (odds ratio [OR]) between the outcome (e.g., role functioning) and the primary predictor (date of diagnosis). We included potentially confounding variables, such as sex, region, and skin color among others as an additional (third) variable in the model (Table 2). If the OR estimate of the association between the outcome and primary predictor changed by 10% or more, then this confounding variable was included in the final model. This process was iterative for all potentially confounding variables and served as the basis for later models. We calculated ORs and their respective 95% CIs based on these logistic regression models.
TABLE 2—
Descriptive Characteristics of Study Participants (n = 354), Medical Outcomes Study–HIV Health Survey Questionnaire: Cuba, 2005–2007
| Characteristics | No. (%) |
| Sex | |
| Female | 73 (20.6) |
| Male | 281 (79.4) |
| Region | |
| Havana City | 208 (58.8) |
| Western Cuba | 37 (10.5) |
| Central Cuba | 65 (18.4) |
| Eastern Cuba | 44 (12.4) |
| Skin color | |
| White | 297 (83.9) |
| Mixed | 19 (5.4) |
| Black | 38 (10.7) |
| Marital status | |
| Married | 17 (4.8) |
| Single | 337 (95.2) |
| Sexual practice | |
| Homosexual or bisexual | 247 (69.8) |
| Heterosexual | 107 (30.2) |
| Educational level | |
| Unknown | 14 (4) |
| Illiterate | 1 (0.3) |
| Primary education | 26 (7.3) |
| High-school diploma | 126 (35.6) |
| Technical degree | 31 (8.8) |
| College incomplete | 121 (34.2) |
| College degree | 35 (9.9) |
| Date of HIV diagnosis | |
| Before July 1, 1996 | 80 (22.6) |
| Between July 1, 1996, and May 31, 2001 | 181 (51.1) |
| From June 1, 2001 onward | 93 (26.3) |
| Age at HIV diagnosis, y | |
| < 18 | 13 (3.7) |
| 18–29 | 180 (50.8) |
| 30–39 | 112 (31.6) |
| ≥ 40 | 49 (13.8) |
| Determination of AIDS diagnosis | |
| CD4 < 200 cells/mm3 | 297 (83.9) |
| Opportunistic infection | 57 (16.1) |
| Years living with AIDS | |
| ≤ 1 y | 187 (52.8) |
| > 1 y and ≤ 5 y | 99 (28) |
| > 5 y | 68 (19.2) |
| Years from HIV diagnosis to treatment | |
| ≤ 1 y | 79 (22.3) |
| > 1 y and ≤ 5 y | 158 (44.6) |
| > 5 y | 117 (33.1) |
| Most frequent starting combination therapies | |
| 021—IDV–DDI–d4T | 21 (5.9) |
| 022—IDV–DDI–AZT | 13 (3.7) |
| 038—IDV–3TC–d4T | 87 (24.6) |
| 039—IDV–3TC–AZT | 50 (14.1) |
| 056—NVP–3TC–d4T | 56 (15.8) |
| 060—3TC–AZT | 35 (9.9) |
| 095—NVP–3TC–AZT | 29 (8.2) |
| Most frequent current combination therapies | |
| 021—IDV–DDI–d4T | 9 (2.5) |
| 022—IDV–DDI–AZT | 3 (0.8) |
| 038—IDV—3TC–d4T | 40 (11.3) |
| 039—IDV–3TC–AZT | 17 (4.8) |
| 048—EFV–3TC–AZT | 11 (3.1) |
| 055—NEL–3TC–d4T | 11 (3.1) |
| 056—NVP–3TC–d4T | 69 (19.5) |
| 095—NVP–3TC–AZT | 67 (18.9) |
| 113—EFV–3TC–d4T | 12 (3.4) |
| 304—KAL–3TC–AZT | 7 (2) |
| Presence of IDV in current combination therapy | |
| No IDV | 279 (78.8) |
| With IDV | 75 (21.2) |
| Change to IDV in their combination therapy | |
| Never IDV | 143 (40.4) |
| No IDV before—IDV later | 28 (7.9) |
| IDV before—no IDV later | 136 (38.4) |
| Always IDV | 47 (13.3) |
| Level of CD4 cell count after first year of treatment | |
| < 200 cells/mm3 | 130 (36.7) |
| ≥ 200 cells/mm3 | 224 (63.3) |
Note. 3TC = lamivudine; AZT = zidovudine; d4T = stavudine; DDI = didanosine; EFV = efavirenz ; IDV = indinavir; KAL = lopinavir/ritonavir; NEL = nelfinavir; NVP = nevirapine.
Statistical analysis was guided by the following hypotheses:
people with HIV diagnosis after ART had been made available in Cuba in 2001 would show a higher QOL,
younger age at diagnosis would demonstrate a higher QOL,
the Havana City region would have higher QOL compared with other regions of Cuba,
men would demonstrate higher QOL compared with women, and
indinavir as part of the ART regimen would be associated with higher QOL, despite the occurrence of side effects, possibly because of drug efficacy.
RESULTS
Of the 400 people selected, 357 (89.3%) were reached and invited to participate in the study. A total of 354 (73 women and 281 men) agreed to participate (22.2% of the study population), 3 refused (1 woman and 2 men), and 43 (10.8%) could not be reached because they had missed or rescheduled their clinical appointment at the IPK. Eighty (22.6%) participants were diagnosed before July 1996, 181 (51.1%) between July 1996 and May 2001, and 93 (26.3%) from June 2001. Table 2 shows descriptive characteristics of study participants disaggregated by demographics, those related to HIV diagnosis and those related to ARV treatment. Table 3 shows the mean and standard deviation for each variable disaggregated by the 4 categorical values defined earlier. Although we did not include the role of late diagnosis as a category for sample selection, we observed a different distribution in the percentage of people presenting with AIDS at the time of their first HIV test when we compared diagnostic groups: there were 0.0% of people among those diagnosed before July 1996, 6.1% among those diagnosed between July 1996 and May 2001, and 19.4% among those diagnosed on or after June 2001.
TABLE 3—
Medical Outcomes Study–HIV Health Survey Scale Scores: Cuba 2005–2007
| Variable | Overall, Mean (SD) | Physical, Mean (SD) | Role, Mean (SD) | Social, Mean (SD) | Cognitive, Mean (SD) | Pain, Mean (SD) | Mental, Mean (SD) | Vitality, Mean (SD) | Distress, Mean (SD) | Quality, Mean (SD) | Transition, Mean (SD) |
| Total | 53.79 (25.02) | 87.52 (18.37) | 83.33 (31.56) | 88.98 (23.98) | 80.27 (21.77) | 80.07 (25.48) | 70.50 (23.68) | 75.01 (22.30) | 79.01 (27.07) | 65.61 (24.36) | 70.13 (26.53) |
| Sex | |||||||||||
| Female | 54.52 (26.35) | 85.73 (19.22) | 85.62 (30.60) | 87.67 (26.59) | 77.12 (23.44) | 73.21 (28.32)* | 67.01 (23.47) | 73.56 (24.85) | 75.75 (28.59) | 64.04 (24.65) | 68.49 (28.57) |
| Male | 53.59 (24.70) | 87.99 (18.14) | 82.74 (31.84) | 89.32 (23.30) | 81.09 (21.28) | 81.85 (24.43)* | 71.40 (23.69) | 75.39 (21.62) | 79.86 (26.66) | 66.01 (24.31) | 70.55 (26.01) |
| Region | |||||||||||
| Havana City | 51.59 (25.22) | 86.42 (19.61) | 80.29 (33.64) | 86.73 (26.32) | 81.30 (21.17) | 78.26 (26.74) | 69.10 (23.42) | 73.56 (22.60) | 77.72 (27.39) | 63.58 (23.67) | 67.67 (26.76)a |
| Western Cuba | 59.59 (23.73) | 87.16 (19.40) | 87.84 (29.83) | 89.73 (23.86) | 81.49 (22.42) | 83.78 (22.62) | 70.16 (24.90) | 74.59 (22.28) | 77.97 (27.95) | 70.95 (25.35) | 79.73 (26.90)a |
| Central Cuba | 54.08 (25.51) | 89.23 (16.39) | 88.46 (26.17) | 92.31 (17.92) | 78.46 (21.40) | 82.74 (22.99) | 75.32 (23.46) | 77.92 (22.27) | 78.62 (27.39) | 68.85 (25.78) | 74.23 (26.13)a |
| Eastern Cuba | 58.86 (23.60) | 90.53 (13.52) | 86.36 (29.26) | 94.09 (19.09) | 77.05 (24.69) | 81.57 (25.13) | 70.27 (24.04) | 77.95 (20.92) | 86.59 (23.84) | 65.91 (24.17) | 67.61 (23.86)a |
| Date of HIV diagnosis | |||||||||||
| Before July 1, 1996 | 57.06 (24.24) | 88.96 (16.92) | 90.63 (25.28)b | 92.75 (19.68) | 83.75 (19.75) | 82.36 (21.63) | 73.95 (22.34) | 79.25 (21.05) | 82.44 (23.89) | 69.38 (26.08) | 70.31 (28.15) |
| Between July 1, 1996, and May 31, 2001 | 52.18 (26.19) | 87.62 (17.89) | 82.60 (32.29)b | 87.62 (25.94) | 80.58 (22.20) | 80.85 (26.74) | 69.68 (24.46) | 72.65 (23.48) | 78.34 (28.12) | 64.09 (24.62) | 69.89 (25.78) |
| From June 1, 2001 onward | 54.09 (23.24) | 86.11 (20.46) | 78.49 (34.10)b | 88.39 (23.28) | 76.67 (22.26) | 76.58 (25.93) | 69.12 (23.20) | 75.97 (20.51) | 77.37 (27.59) | 65.32 (22.13) | 70.43 (26.82) |
| Age at HIV diagnosis, y | |||||||||||
| < 18 | 59.62 (23.85) | 92.95 (08.23) | 88.46 (21.93) | 93.85 (15.02) | 79.23 (20.29) | 88.89 (19.77) | 76.00 (23.15) | 86.54 (15.99) | 74.62 (32.30) | 67.31 (23.68) | 73.08 (31.39) |
| 18–29 | 56.28 (24.90) | 88.84 (18.79) | 81.39 (34.26) | 89.33 (23.96) | 80.31 (22.32) | 81.73 (24.23) | 70.96 (22.30) | 76.06 (22.07) | 79.83 (26.79) | 65.69 (23.93) | 71.11 (26.27) |
| 30–39 | 50.13 (25.47) | 86.01 (18.15) | 84.82 (29.89) | 86.96 (25.57) | 78.79 (23.23) | 76.88 (28.62) | 68.79 (26.22) | 72.41 (23.61) | 76.12 (29.30) | 65.63 (26.24) | 68.75 (27.57) |
| ≥ 40 | 51.43 (24.04) | 84.69 (18.89) | 85.71 (27.00) | 91.02 (22.38) | 83.78 (16.06) | 78.91 (23.16) | 71.27 (22.96) | 74.08 (20.73) | 83.78 (20.45) | 64.80 (22.20) | 68.88 (24.22) |
Western vs Havana P = .012; Western vs Eastern P = .035.
Before July 1, 1996, vs after June 1, 2001; P = .01.
*P = .01.
Functional Activity Scales
All scales reflecting functional activities showed a mean greater than 80: 87.5 for physical, 83.3 for role, 88.9 for social, and 80.3 for cognitive. The lowest value among the 11 MOS-HIV scales was the perception of QOL, with a mean of 65.6. Among people with a CD4 count less than 200 cells per cubic millimeter at the time of survey, social function scored lower compared with those with a CD4 count greater than or equal to 200 cells per cubic millimeter (85.2 vs 91.1; P = .026); there were no statistically significant differences between those with a CD4 count less than 200 cells per cubic millimeter and those with greater than or equal to 200 cells per cubic millimeter for physical, role, and cognitive functioning. People whose CD4 counts had remained less than 200 cells per cubic millimeter after at least 1 year of treatment reported a lower perception of their general health (50.1 vs 55.9; P = .033) and lower social functioning (85.2 vs 91.2; P = .025).
Table 3 demonstrates that there were not many significant differences across subscales when we examined variation by sex, region, date of diagnosis, and age at diagnosis, with a few exceptions. Women reported a lower score on the pain subscale, reflecting higher levels of pain compared with men (73.2 vs 81.9; P = .01); when we analyzed sex by time of diagnosis, however, the differences were only significant when we compared men and women diagnosed before July 1996 (73.12 vs 88.21; P = .002). Health transition (the change in physical and emotional health over the previous 4 weeks) scored a mean of 70.1, with a better score among participants diagnosed in the Western region than among those diagnosed in Havana (79.7 vs 67.7; P = .012) and in the Eastern region (79.7 vs 67.6; P = .035). In addition, those who were diagnosed between 1986 and 1996 demonstrated better role functioning compared with those diagnosed between 2001 and 2004 (90.6 vs 78.5; P = .01).
Although age at diagnosis did not demonstrate an association with QOL based on the 4 age categories shown in Table 3 (< 18, 18–29, 30–39, ≥ 40), scores were higher among people diagnosed before age 30 years than among those diagnosed at age 30 years or older (56.5 vs 50.5; P = .025). In addition, people whose treatment included the protease inhibitor indinavir showed a greater perception of general health (58.9 vs 52.4; P = .045) and a greater health transition or health improvement (78.6 vs 67.8; P = .002) than those who received regimens not containing indinavir—despite the association of indinavir with adverse side effects. People who changed treatment combination but stayed with indinavir showed a better health transition than those who did not use it (81.9 vs 67.0; P = .007).
Pain
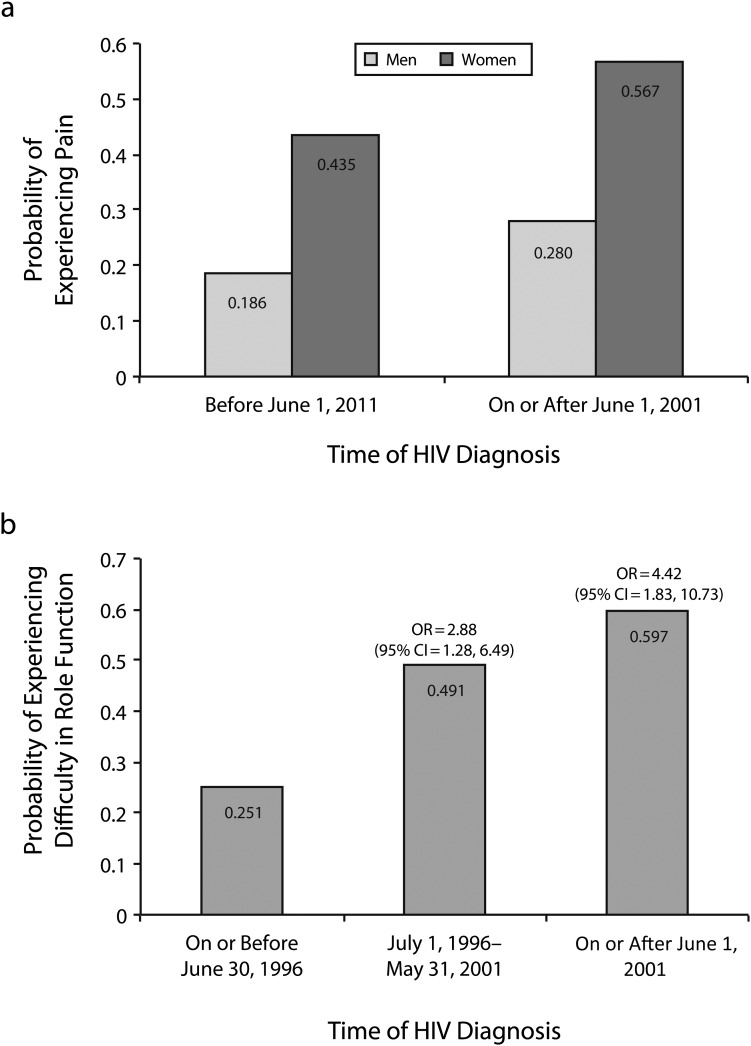
Pain interfered more in women’s lives than in men’s (81.9 vs 73.2; P = .01). Men diagnosed after 2001 reported more pain (77.3 vs 83.6; P = .048) and lower cognitive functioning (76.8 vs 82.8; P = .034) than men diagnosed before 2001, but we observed no significant differences in women by diagnostic group. However, Figure 1 (panel 1) shows a logistic regression model in which the probability of experiencing pain among women and men increased when HIV diagnosis occurred on or after June 2001 (OR = 1.70; 95% CI = 1.01, 2.88); in both diagnostic groups, women had a higher probability of experiencing pain (OR = 3.36; 95% CI = 1.26, 8.92).
FIGURE 1—
Predicted probability of experiencing pain by sex and time of HIV diagnosis and association of experiencing difficulty in role function and time of HIV diagnosis.
Role Function Activity Scales
People diagnosed from June 2001 onward reported more difficulty in role function (performing work, study, or housework activities) compared with people diagnosed before July 1996 (78.4 vs 90.6; P = .010), as much in women as in men. There were no statistically significant differences when we compared these 2 groups against those diagnosed between July 1996 and May 2001. Figure 1 (panel 2) shows a logistic regression model in which the probability of experiencing difficulties in role function increased when, compared with people diagnosed before July 1996, HIV diagnosis occurred between July 1996 and May 2001(OR = 2.88; 95% CI = 1.28, 6.49) and from June 2001 onward (OR = 4.42; 95% CI = 1.83, 10.73). Results of the final multivariate logistic regression analyses that demonstrated significant findings after we controlled for confounding variables are shown in Table 4. Multivariate analyses for QOL scales other than role function, pain, and health transition demonstrated null findings and were not included in Table 4. In addition, indinavir use was also not significantly associated with any of the QOL scales after we controlled for confounding variables. All of the other primary predictors hypothesized in the research questions to be associated with the QOL scales (sex, region, date of diagnosis, and age of diagnosis) demonstrated statistically significant findings and are included in Table 4.
TABLE 4—
Multivariate Logistic Regression Analyses: Medical Outcomes Study–HIV Health Survey, Cuba, 2005–2007
| Variable | Role Function, OR (95% CI) | Pain, OR (95% CI) | Health Transition, OR (95% CI) |
| Sex | |||
| Female | … | 3.36* (1.26, 8.92) | … |
| Male (Ref) | … | 1.00 | … |
| Region | |||
| Havana City (Ref) | … | 1.00 | 1.00 |
| Western Cuba | … | 1.59 (0.74, 3.46) | 0.67 (0.39, 1.49) |
| Central Cuba | … | 1.25 (0.45, 3.45) | 0.39* (0.16, 0.97) |
| Eastern Cuba | … | 1.24 (0.51, 3.01) | 0.59 (0.27, 1.30) |
| Date of HIV diagnosis | |||
| Before July 1, 1996 (Ref) | 1.00 | … | … |
| Between July 1, 1996, and May 31, 2001 | 2.88* (1.28, 6.49) | … | … |
| From June 1, 2001 onward | 4.42* (1.83, 10.73) | … | … |
| Date of HIV diagnosis before or after 2001 | |||
| Before 2001 (Ref) | … | 1.00 | … |
| On or after 2001 | … | 1.70* (1.01, 2.88) | … |
| Age at HIV diagnosis, y | |||
| < 18 (Ref) | 1.00 | 1.00 | … |
| 18–29 | 1.26 (0.26, 5.99) | 0.31 (0.07, 1.36) | … |
| 30–39 | 1.21 (0.57, 2.60) | 0.73 (0.37, 1.44) | … |
| ≥ 40 | 0.94 (0.42, 2.12) | 0.90 (0.44, 1.85) | … |
| Skin color | |||
| White (Ref) | 1.00 | … | … |
| Mixed | 0.54 (0.25, 1.17) | … | … |
| Black | 0.54 (0.12, 2.32) | … | … |
| Cause of AIDS classification | |||
| CD4 < 200 cells/mm3 (Ref) | 1.00 | … | … |
| Opportunistic infection | 0.51 (0.26, 1.02) | … | … |
| Sexual practice | |||
| Homosexual or bisexual (Ref) | … | 1.00 | … |
| Heterosexual | … | 1.34 (0.54, 3.34) | … |
| Years from HIV diagnosis to treatment | |||
| ≤ 1 y (Ref) | … | … | 1.00 |
| > 1 y and ≤ 5 y | … | … | 0.86 (0.47, 1.55) |
| > 5 y | … | … | 1.48 (0.90, 2.44) |
Note. CI = confidence interval; OR = odds ratio.
*P ≤ .05.
DISCUSSION
This study shows that Cubans receiving ART are maintaining a high QOL. On average, the scores were as high as or higher than all of the countries for which data are available on all 11 dimensions and overall scale measured by the MOS-HIV: Mexico,20 Spain,26 Italy,23 the United States,27 Thailand,24 and Singapore.25 Although it is beyond the scope of this article to compare the responses to the HIV epidemic in all of these countries, the relatively high QOL in Cuba could be explained by the combined impact of the training sessions on how to live with HIV, other measures of social support provided to people with HIV, easy access to treatment and close clinical monitoring of HIV disease, and an overall support of basic socioeconomic needs provided by the Cuban government to its population18—all of which occurs in a context of a high development index and low poverty levels.3
However, we observed several significant differences in the study. Although we had hypothesized that people diagnosed with HIV before ART became available in Cuba would experience a worse QOL than those diagnosed later, the former reported less pain and more cognitive functioning than the latter. One might expect that they would be in better physical health than those diagnosed before 2001 because their HIV disease would not have progressed as much. One possible explanation for this finding would be that people who were diagnosed after May 2001 and who were already receiving ART by August 2004 may have been diagnosed at a later stage in their HIV disease than those diagnosed earlier. This may be related to social or economic factors that can delay access to care as well as a broader group of patients accessing care because of the significant increase in efficacy of ART compared with previous regimens to treat HIV. Therefore, if those diagnosed after 2001 were more ill on average compared with those diagnosed earlier, then this may explain in part the lower QOL scores. An alternative explanation is that those who were diagnosed earlier and were more ill may have died in the earlier age cohort. In addition, it is plausible that people who were diagnosed after May 2001 also have difficulty with adhering to medication, following a healthy diet, keeping a job, and other factors that may affect their QOL. As both women and men diagnosed after 2001 reported more difficulty working, it also seems plausible that, as ART alleviates some of the physical symptoms of AIDS, the side effects of treatment and daily pill taking are a greater burden for those who have had access to ART since they became clinically eligible—whereas those who were diagnosed before 2001 may have taken treatment less for granted and may better tolerate both side effects and the decrease in number of pills that has taken place with new ARVs.
Women reported experiencing more pain than did men. Actually, several studies have shown differences between women and men in the way they experience and cope with pain and in their health-seeking behavior.31–36 Although, except for pain, the differences in our study were slight and not statistically significant, women scored lower than men on 9 of the 11 variables measured by the MOS-HIV and slightly higher on overall perceptions of health and perceptions of role function. The lower scores could be reflective of the greater social vulnerability of women with HIV compared with men with HIV, differences in reporting, or different clinical response to ART between women and men. The higher score in perceptions of role function in women may reflect the fact that women often cannot compromise their family caregiving responsibilities.37
The incidence of HIV infection is rising in Cuba in both men and women.2 This study indicates that women are not enjoying the same health-related QOL as are men. Furthermore, men and women diagnosed more recently have lower perceptions of QOL than those diagnosed earlier, despite presumably being younger and at a less advanced stage of HIV disease. Women and men who have been diagnosed after 2001 are likely to be more representative of those who will initiate ART in the future, and future research should focus on trying to understand and change this discrepancy. Achieving higher QOL for people living with HIV is an important goal in and of itself that also has implications for the efficacy of secondary prevention of HIV.
Study participants receiving indinavir demonstrated higher QOL in the present study. Other findings have shown similar results—those who reduced the dosage of indinavir demonstrated a decrease in QOL compared with those who did not change their dose.38 A number of studies have also found significant differences regarding QOL and the use of protease inhibitors39–41 more generally, supporting both a positive and negative relationship with QOL.
We observed that study participants who had lived with HIV for a longer period of time also experienced a greater vitality and QOL. This may be indicative of the fact that these patients have learned how to better cope with HIV and have developed strategies to better deal with problems associated with their disease. QOL has been associated with a patient’s clinical status, specifically with CD4 count and viral load, and is considered a practical tool for monitoring patients’ health status.42 Consistent with our findings, one study found significant differences with regard to physical functioning, roles, energy level, and perception of health status among patients having a CD4 count of less than 200 cells per cubic millimeter compared with those who have higher CD4 cell counts, and efforts to improve patients’ CD4 count have been associated with the improvement of QOL.43 People who have AIDS have been reported to have lower scores in all areas compared with those who have not yet advanced to AIDS,39 which reaffirms the need for rigorous efforts to prevent progression of HIV to AIDS.
Study Strengths and Limitations
There are a number of limitations of the present study. The cross-sectional design limits our ability to clearly determine the direction of the associations analyzed in this study. Although a longitudinal design may allow us to make inferences on the temporal relationships that were examined, resources were not available to follow up this cohort of individuals over time. In addition, 5 of the 10 QOL subscales demonstrated ceiling effects (physical, role functioning, social functioning, cognitive functioning, and pain), reducing the capacity to compare these results with findings from other studies.
Our study is limited in that it is cross-sectional. It is highly likely that the provision of ART has improved the QOL of Cuban people with HIV/AIDS; however, we do not have MOS-HIV scores from patients before they initiated ART. In addition, we did not account for coinfections, such as hepatitis B or C, which may also affect both the efficacy of therapy and the QOL of patients.
A strength of the study is its sample size, as it includes 22.2% of the total population of people in Cuba receiving ART. By selecting the sample for statistical representation along sex, year of diagnosis, age at diagnosis, and region, we have increased the likelihood that our sample is representative of the Cuban population receiving ART. To our knowledge, it is the first quantitative study on quality of life of people with HIV conducted with a representative sample of the national population of a low- or middle-income country.
Conclusions
This study supports the idea that QOL assessment can serve as a health outcome and may allow identification of quality of life reductions potentially related to ART side effects.
Acknowledgments
The study was funded through research grants made to A. Castro by the Wilbur Marvin Fund at the David Rockefeller Center for Latin American Studies at Harvard University, Bridge Award of the Minority Faculty Development Program at Harvard Medical School’s Office for Faculty Development and Diversity, Harvard University’s William F. Milton Fund, Atlantic Philanthropies (grant 14217), and the Ford Foundation (grants 1055-0735 and 1085-0193, made to the Cuban Studies Program at the David Rockefeller Center for Latin American Studies at Harvard University).
The authors are grateful to all of the people with HIV/AIDS in Cuba who participated in the study as well as to the Pedro Kourí Institute of Tropical Medicine and its many employees who facilitated the study, the Cuban Ministry of Public Health, GPSIDA (AIDS Prevention Group in Cuba), the Cuban Centers for HIV/AIDS Comprehensive Care, the Cuban National and Provincial Centers for HIV/AIDS Prevention, HIVOS Holland, Oxfam’s Cuban Program, and Gail Reed of Medical Education Cooperation With Cuba. Jorge Campos-Díaz and Yasmin Khawja contributed to data collection and Kristin McPhillips contributed to the literature review. David Bangsberg provided invaluable comments to the article.
Note. Funders played no role in study design or data analysis. The study complied with all laws and regulations governing academic exchanges between institutions from Cuba and the United States.
Human Participant Protection
The study was approved by the ethics committees of the Pedro Kourí Institute of Tropical Medicine in Havana and Harvard Medical School in Boston. All study participants provided written informed consent.
References
- 1.2008 Report on the Global AIDS Epidemic. Geneva, Switzerland: Joint United Nations Program on HIV/AIDS; 2008 [Google Scholar]
- 2.de Arazoza H, Joanes J, Lounes Ret al. The HIV/AIDS epidemic in Cuba: description and tentative explanation of its low HIV prevalence. BMC Infect Dis. 2007;7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Human Development Report 2009. Overcoming Barriers: Human Mobility and Development. New York, NY: United Nations Development Program; 2009 [Google Scholar]
- 4.Santana S, Faas L, Wald K. Human immunodeficiency virus in Cuba: the public health response of a Third World country. Int J Health Serv. 1991;21(3):511–537 [DOI] [PubMed] [Google Scholar]
- 5.Bayer R, Healton C. Controlling AIDS in Cuba. The logic of quarantine. N Engl J Med. 1989;320(15):1022–1024 [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Stable EJ. Cuba’s response to the HIV epidemic. Am J Public Health. 1991;81(5):563–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheper-Hughes N. AIDS, public health, and human rights in Cuba. Lancet. 1993;342(8877):965–967 [DOI] [PubMed] [Google Scholar]
- 8.Aragonés López C, Campos Díaz JR, Sánchez Valdés L, Pérez Ávila JL. Grupos de Prevención del SIDA (GPSIDA): 15 años de trabajo sostenido en la prevención del VIH/sida [AIDS Prevention Group: 15 years of sustained efforts for the prevention of HIV/AIDS]. Rev Cubana Med Trop. 2007;59(3). [PubMed] [Google Scholar]
- 9.Burr C. Assessing Cuba’s approach to contain AIDS and HIV. Lancet. 1997;350(9078):647. [DOI] [PubMed] [Google Scholar]
- 10.Granich R, Jacobs B, Mermin J, Pont A. Cuba’s national AIDS program. The first decade. West J Med. 1995;163(2):139–144 [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez J, Pérez D, González Iet al. Approaches to the Management of HIV/AIDS in Cuba. Geneva, Switzerland: World Health Organization; 2004 [Google Scholar]
- 12.Ministry of Public Health Surveillance System, National Program on HIV/AIDS. Havana, Cuba: Ministerio de Salud Pública; 2009 [Google Scholar]
- 13.Pérez J, Aragonés C, Pérez D, Abreu D. Results of the antiretroviral treatment in Cuba 2001–2007. Abstract CDB0242. Presented at XVII International AIDS Conference; Mexico City, Mexico; August 3–8, 2008.
- 14.Pérez L, Álvarez LP, Carmona Ret al. Genotypic resistance to antiretroviral drugs in patients infected with several HIV type 1 genetic forms in Cuba. AIDS Res Hum Retroviruses. 2007;23(3):407–414 [DOI] [PubMed] [Google Scholar]
- 15.Pérez L, Thomson MM, Bleda MJet al. HIV type 1 molecular epidemiology in Cuba: high genetic diversity, frequent mosaicism, and recent expansion of BG intersubtype recombinant forms. AIDS Res Hum Retroviruses. 2006;22(8):724–733 [DOI] [PubMed] [Google Scholar]
- 16.Protopopescu C, Raffi F, Roux Pet al. Factors associated with non-adherence to long-term highly active antiretroviral therapy: a 10 year follow-up analysis with correction for the bias induced by missing data. J Antimicrob Chemother. 2009;64(3):599–606 [DOI] [PubMed] [Google Scholar]
- 17.Cohen MS, Chen YQ, McCauley Met al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro A, Khawja Y, González-Núñez I. Sexuality, reproduction, and HIV in women: the impact of antiretroviral therapy in elective pregnancies in Cuba. AIDS. 2007;21(suppl 5):S49–S54 [DOI] [PubMed] [Google Scholar]
- 19.Wu AW, Rubin HR, Mathews WCet al. A health status questionnaire using 30 items from the Medical Outcomes Study. Preliminary validation in persons with early HIV infection. Med Care. 1991;29(8):786–798 [DOI] [PubMed] [Google Scholar]
- 20.Peña de León E, Aguilar Gaytán SS, Suárez Mendoza AA, Reyes Terán G. A validation of the MOS-HIV quality of life measure in HIV-infected patients in Mexico [in Spanish]. Rev Panam Salud Publica. 2007;21(5):313–319 [DOI] [PubMed] [Google Scholar]
- 21.Wu AW. MOS-HIV Health Survey Scoring Guidelines. Boston, MA: Medical Outcomes Trust; 1997 [Google Scholar]
- 22.Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV). Qual Life Res. 1997;6(6):481–493 [DOI] [PubMed] [Google Scholar]
- 23.Dorz S, Lazzarini L, Cattelan Aet al. Evaluation of adherence to antiretroviral therapy in Italian HIV patients. AIDS Patient Care STDS. 2003;17(1):33–41 [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa M, Natpratan C. Quality of life among people living with HIV/AIDS in northern Thailand: MOS-HIV Health Survey. Qual Life Res. 2004;13(3):601–610 [DOI] [PubMed] [Google Scholar]
- 25.Paton NI, Chapman CA, Chan SPet al. Validation of the Medical Outcomes Study HIV Health Survey as a measure of quality of life in HIV-infected patients in Singapore. Int J STD AIDS. 2002;13(7):456–461 [DOI] [PubMed] [Google Scholar]
- 26.Ruíz Pérez I, Rodríguez Baño J, López Ruz MAet al. Health-related quality of life of patients with HIV: impact of sociodemographic, clinical and psychosocial factors. Qual Life Res. 2005;14(5):1301–1310 [DOI] [PubMed] [Google Scholar]
- 27.Shalit P, True A, Thommes JA. Quality of life and tolerability after administration of enfuvirtide with a thin-walled needle: QUALITE Study. HIV Clin Trials. 2007;8(1):24–35 [DOI] [PubMed] [Google Scholar]
- 28.Taylor TN, Dolezal C, Tross S, Holmes WC. Reliability and validity of two HIV/AIDS-specific quality of life instruments adapted for use in HIV-positive Zimbabweans. AIDS Care. 2009;21(5):598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aragonés López C, Campos Díaz JR, Orta M, Pérez Ávila J. Sidatrat: a web based information system to support antiretroviral therapy in Cuba. Abstract CDB0241. Presented at XVII International AIDS Conference; Mexico City, Mexico; August 3–8, 2008. Available at: http://ias-2005.org/Abstracts/A200714058.aspx. Accessed March 11, 2011. [PubMed]
- 30.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breitbart W, McDonald MV, Rosenfeld Bet al. Pain in ambulatory AIDS patients. I: Pain characteristics and medical correlates. Pain. 1996;68(2–3):315–321 [DOI] [PubMed] [Google Scholar]
- 32.Dobalian A, Tsao JC, Duncan RP. Pain and the use of outpatient services among persons with HIV: results from a nationally representative survey. Med Care. 2004;42(2):129–138 [DOI] [PubMed] [Google Scholar]
- 33.Gaughan DM, Hughes MD, Seage GR, IIIet al. The prevalence of pain in pediatric human immunodeficiency virus/acquired immunodeficiency syndrome as reported by participants in the Pediatric Late Outcomes Study (PACTG 219). Pediatrics. 2002;109(6):1144–1152 [DOI] [PubMed] [Google Scholar]
- 34.Hewitt DJ, McDonald M, Portenoy RK, Rosenfeld B, Passik S, Breitbart W. Pain syndromes and etiologies in ambulatory AIDS patients. Pain. 1997;70(2–3):117–123 [DOI] [PubMed] [Google Scholar]
- 35.Norval DA. Symptoms and sites of pain experienced by AIDS patients. S Afr Med J. 2004;94(6):450–454 [PubMed] [Google Scholar]
- 36.Rotheram-Borus MJ. Variations in perceived pain associated with emotional distress and social identity in AIDS. AIDS Patient Care STDS. 2000;14(12):659–665 [DOI] [PubMed] [Google Scholar]
- 37.Davidson AJ, Bertram SL, Lezotte DCet al. Comparison of health status, socioeconomic characteristics, and knowledge and use of HIV-related resources between HIV-infected women and men. Med Care. 1998;36(12):1676–1684 [DOI] [PubMed] [Google Scholar]
- 38.Badía X, Podzamczer D, Moral Iet al. Health-related quality of life in HIV patients switching to twice-daily indinavir/ritonavir regimen or continuing with three-times-daily indinavir-based therapy. Antivir Ther. 2004;9(6):979–985 [PubMed] [Google Scholar]
- 39.Ruiz-Pérez I, Olry de Labry-Lima A, López-Ruz MAet al. Clinical status, adherence to HAART and quality of life in HIV-infected patients receiving antiretroviral treatment [in Spanish]. Enferm Infecc Microbiol Clin. 2005;23(10):581–585 [DOI] [PubMed] [Google Scholar]
- 40.Fumaz CR, Tuldra A, Ferrer MJet al. Quality of life, emotional status, and adherence of HIV-1-infected patients treated with efavirenz versus protease inhibitor-containing regimens. J Acquir Immune Defic Syndr. 2002;29(3):244–253 [DOI] [PubMed] [Google Scholar]
- 41.Barreiro P, Soriano V, Blanco F, Casimiro C, de la Cruz JJ, González-Lahoz J. Risks and benefits of replacing protease inhibitors by nevirapine in HIV-infected subjects under long-term successful triple combination therapy. AIDS. 2000;14(7):807–812 [DOI] [PubMed] [Google Scholar]
- 42.Delate T, Coons SJ. The use of 2 health-related quality-of-life measures in a sample of persons infected with human immunodeficiency virus. Clin Infect Dis. 2001;32(3):E47–E52 [DOI] [PubMed] [Google Scholar]
- 43.Gill CJ, Griffith JL, Jacobson D, Skinner S, Gorbach SL, Wilson IB. Relationship of HIV viral loads, CD4 counts, and HAART use to health-related quality of life. J Acquir Immune Defic Syndr. 2002;30(5):485–492 [DOI] [PubMed] [Google Scholar]