Abstract
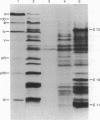
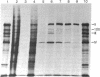
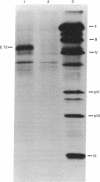
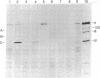
Adenovirus type 2 mRNAs were injected via glass capillaries into Vero cells, a line of African green monkey kidney cells permissive for adenovirus growth. Polypeptides synthesized after injection were labeled with 35S-labeled amino acids, precipitated with antiviral sera, and analyzed by polyacrylamide gel electrophoresis. Both early and late viral mRNAs give rise to authentic polypeptides. The in vivo translation of mRNAs can be measured as late as 24 hr after injection. The ability to analyze the protein products of microinjected mRNAs directly should greatly extend the applications of the procedure. Vero cells injected with early mRNA from adenovirus type 2 support the growth of adenovirus-associated virus, a defective virus that is dependent on adenovirus helper functions. This result demonstrates that a measurable biological activity, the ability to overcome the defectiveness of adenovirus-associated virus, resides in early adenovirus mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J. F., Lewis J. B., Anderson C. W., Gesteland R. F. Enhanced differential synthesis of proteins in a mammalian cell-free system by addition of polyamines. J Biol Chem. 1975 Jul 25;250(14):5688–5695. [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Berridge M. V., Lane C. D. Translation of Xenopus liver messenger RNA in Xenopus oocytes: vitellogenin synthesis and conversion to yolk platelet proteins. Cell. 1976 Jun;8(2):283–297. doi: 10.1016/0092-8674(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Laughlin C. A., de la Maza L. M., Myers M. Adeno-associated virus autointerference. Virology. 1979 Jan 30;92(2):449–462. doi: 10.1016/0042-6822(79)90149-1. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Rose J. A. Transcription in vivo of a defective parvovirus: sedimentation and electrophoretic analysis of RNA synthesized by adenovirus-associated virus and its helper adenovirus. Virology. 1974 Sep;61(1):182–199. doi: 10.1016/0042-6822(74)90253-0. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Effect of cycloheximide on RNA metabolism early in productive infection with adenovirus 2. J Virol. 1974 Jul;14(1):26–32. doi: 10.1128/jvi.14.1.26-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Callahan R., Westphal H. Cell-free synthesis of adenovirus coat proteins. J Biol Chem. 1974 Oct 10;249(19):6331–6338. [PubMed] [Google Scholar]
- Eron L. Post-transcriptional restriction of human adenovirus expression in monkey cells. J Virol. 1975 May;15(5):1256–1261. doi: 10.1128/jvi.15.5.1256-1261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Sharp P. A. Adenovirus transcription. V. Quantitation of viral RNA sequences in adenovirus 2-infected and transformed cells. J Mol Biol. 1976 Sep 25;106(3):749–774. doi: 10.1016/0022-2836(76)90263-1. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Microinjection of early SV40 DNA fragments and T antigen. Methods Enzymol. 1980;65(1):816–825. doi: 10.1016/s0076-6879(80)65076-9. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Regulatory mechanism of simian virus 40 gene expression in permissive and in nonpermissive cells. J Virol. 1976 Mar;17(3):854–858. doi: 10.1128/jvi.17.3.854-858.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Shiroki K., Shimojo H. Complementation of adeno-associated virus growth with temperature-sensitive mutants of human adenovirus types 12 and 5. J Gen Virol. 1975 Nov;29(2):239–242. doi: 10.1099/0022-1317-29-2-239. [DOI] [PubMed] [Google Scholar]
- Handa H., Shiroki K., Shimojo H. Helper factor(s) for growth of adeno-associated virus in cells transformed by adenovirus 12. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4508–4510. doi: 10.1073/pnas.74.10.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter M. L., Lewis J. B. Adenovirus type 2 early proteins synthesized in vitro and in vivo: identification in infected cells of the 38,000- to 50,000- molecular-weight protein encoded by the left end of the adenovirus type 2 genome. J Virol. 1978 Jun;26(3):736–749. doi: 10.1128/jvi.26.3.736-749.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kitchingman G. R., Lai S. P., Westphal H. Loop structures in hybrids of early RNA and the separated strands of adenovirus DNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4392–4395. doi: 10.1073/pnas.74.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor H. D., Carrier S., Jordan L. Complementation of adeno-associated satellite virus (AAV) by temperature-sensitive mutants of adenovirus type 31. J Gen Virol. 1977 Jun;35(3):545–553. doi: 10.1099/0022-1317-35-3-545. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maness P. F., Edelman G. M. Assay for early cytoplasmic effects of the src gene product of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2750–2754. doi: 10.1073/pnas.75.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Neuwald P. D., Lai S. P., Maizel J. V., Jr, Westphal H. Electron microscopy of late adenovirus type 2 mRNA hybridized to double-stranded viral DNA. J Virol. 1977 Mar;21(3):1010–1018. doi: 10.1128/jvi.21.3.1010-1018.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald P. D., Meyer J., Maizel J. V., Jr, Westpahl H. Early gene expression of adenovirus type 2: R-loop mapping of mRNA and time course of viral DNA, mRNA, and protein synthesis. J Virol. 1977 Mar;21(3):1019–1030. doi: 10.1128/jvi.21.3.1019-1030.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E. Groups of adenovirus type 2 mRNA's derived from a large primary transcript: probable nuclear origin and possible common 3' ends. J Virol. 1978 Mar;25(3):811–823. doi: 10.1128/jvi.25.3.811-823.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Maizel J. V., Jr, Inman J. K., Shatkin A. J. Structural proteins of adenovirus-associated viruses. J Virol. 1971 Nov;8(5):766–770. doi: 10.1128/jvi.8.5.766-770.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborio J. L., Oberg B. In vivo and in vitro synthesis of adenovirus type 2 early proteins. J Virol. 1976 Mar;17(3):865–875. doi: 10.1128/jvi.17.3.865-875.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G., Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G. Microinjection studies of duck globin messenger RNA translation in human and avian cells. Cell. 1976 Dec;9(4 Pt 2):725–732. doi: 10.1016/0092-8674(76)90136-7. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Ginsberg H. S., Rose J. A. DNA-minus temperature-sensitive mutants of adenovirus type 5 help adenovirus-associated virus replication. J Virol. 1975 Jan;17(1):140–148. doi: 10.1128/jvi.17.1.140-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal J., Craig E. A., Raskas H. J. Sequence relationships between adenovirus 2 early RNA and viral RNA size classes synthesized at 18 hours after infection. J Virol. 1975 Jan;15(1):137–144. doi: 10.1128/jvi.15.1.137-144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal H. In vitro translation of adenovirus messenger RNA. Curr Top Microbiol Immunol. 1976;73:125–139. doi: 10.1007/978-3-642-66306-2_4. [DOI] [PubMed] [Google Scholar]
- Westphal H., Lai S. P. Quantitative electron microscopy of early adenovirus RNA. J Mol Biol. 1977 Nov 5;116(3):525–548. doi: 10.1016/0022-2836(77)90082-1. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]