Abstract
Objectives. We investigated whether abused and neglected children are at risk for negative physical health outcomes in adulthood.
Methods. Using a prospective cohort design, we matched children (aged 0–11 years) with documented cases of physical and sexual abuse and neglect from a US Midwestern county during 1967 through 1971 with nonmaltreated children. Both groups completed a medical status examination (measured health outcomes and blood tests) and interview during 2003 through 2005 (mean age = 41.2 years).
Results. After adjusting for age, gender, and race, child maltreatment predicted above normal hemoglobin, lower albumin levels, poor peak airflow, and vision problems in adulthood. Physical abuse predicted malnutrition, albumin, blood urea nitrogen, and hemoglobin A1C. Neglect predicted hemoglobin A1C, albumin, poor peak airflow, and oral health and vision problems, Sexual abuse predicted hepatitis C and oral health problems. Additional controls for childhood socioeconomic status, adult socioeconomic status, unhealthy behaviors, smoking, and mental health problems play varying roles in attenuating or intensifying these relationships.
Conclusions. Child abuse and neglect affect long-term health status—increasing risk for diabetes, lung disease, malnutrition, and vision problems—and support the need for early health care prevention.
A developing scientific consensus indicates that the “origins of adult disease are often found among developmental and biological disruptions occurring during the early years of life.”1(p2252) In the United States, an estimated 3.3 million children were referred to child protection service agencies for suspected maltreatment in 2009 and about 700 000 were determined to be victims.2 Child maltreatment has been related to numerous physical health conditions, including infectious diseases, pain, hypertension, diabetes, asthma, heart disease, inflammation, obesity, and poor general health.3–11 Researchers have also found that patients who report histories of abuse have high rates of primary care visits12,13 and high annual health care costs.14,15 However, the existing literature relies heavily on cross-sectional designs that cannot demonstrate that childhood adversities cause particular outcomes but only that childhood adversities are associated with certain outcomes.16 Additionally, reliance on retrospective self-reports of childhood abuse is problematic because of problems with forgetting, reconstruction of memory, and inconsistencies in reports over time,17–24 introducing considerable ambiguity into the meaning of the associations reported in these studies. A review of studies relating childhood trauma and physical disorders among adults in the United States demonstrated that future research needs to include “objectively measured biological data using a longitudinal design.”25(p509)
This article presents findings from the first prospective study of documented cases of childhood physical and sexual abuse and neglect and matched controls who were followed up and administered a medical status examination (physical tests and blood collection) in middle adulthood. A variety of outcomes from this project have been described in previous articles.26–29 We sought to determine whether children with documented histories of abuse and neglect are at increased risk for negative physical health outcomes in adulthood compared with a group of nonabused and nonneglected matched controls and to compare these maltreated children and controls in adulthood to a US sample on comparable health indicators.30
This study offers several advantages. First, unlike most cross-sectional studies of childhood maltreatment, we used a prospective matched cohort design, thereby providing an appropriate comparison group and assessment of the correct temporal sequence of events. Second, we used measured health outcomes through a physical examination and the results of blood tests, rather than self-reports of medical problems. Third, we traced development beyond adolescence and young adulthood to middle adulthood. Fourth, the large heterogeneous sample included men and women and was varied in terms of race/ethnicity. Fifth, we examined risk for negative health consequences in victims of 3 different types of childhood maltreatment: physical abuse, sexual abuse, and neglect. Sixth, we used unambiguous definitions of child abuse and neglect. Finally, we used documented cases of childhood maltreatment to minimize potential problems with reliance on retrospective self-reports.
METHODS
We collected data as part of a large prospective cohort design study31,32 in which abused and neglected children were matched with nonabused and nonneglected children and followed into adulthood. Because of the matching procedure, the participants were assumed to differ only in the risk factor, that is, having experienced childhood sexual or physical abuse or neglect. Because it is not possible to assign participants randomly to groups, the assumption of equivalency for the groups was an approximation. The control group may also differ from the abused and neglected individuals on other variables nested with abuse or neglect. For complete details of the study design and participant selection criteria, see Widom.33
The original sample of abused and neglected children (n = 908) comprised all substantiated cases of childhood physical and sexual abuse and neglect processed from 1967 to 1971 in the county juvenile (family) or adult criminal courts of a Midwestern metropolitan area. Cases of abuse and neglect were restricted to children aged 11 years or younger at the time of the incident.
A control group of children without documented histories of childhood abuse or neglect (n = 667) was matched with the abuse and neglect group on age, gender, race/ethnicity, and approximate family social class during the same period. This matching was important because it is theoretically plausible that any relationship between child abuse and neglect and subsequent outcomes is confounded with or explained by social class differences.34–39 The matching procedure we used was derived from a broad definition of social class that included neighborhoods the children were reared in and schools they attended.40 The control group established the base rates of health outcomes expected in a sample of adults from comparable circumstances who did not come to the court’s attention in childhood as victims of abuse or neglect.
We matched children who were younger than school age at the time of the abuse or neglect with children of the same gender, race, date of birth (±1 week), and hospital of birth using county birth record information. For children of school age, we used records of more than 100 elementary schools for the same period to find matches with children of the same gender, race, date of birth (±6 months), class in elementary school during the years 1967 through 1971, and home address, preferably within a 5-block radius of the abused or neglected child. Overall, matches were found for 74% of the abused and neglected children. Nonmatches occurred for numerous reasons. For birth records, nonmatches occurred in situations in which the abused or neglected child was born outside the county or state or in which date of birth information was missing. For school records, nonmatches occurred because of lack of adequate identifying information for the abused and neglected children or because the elementary school had closed during the past 20 years and class registers were unavailable. We reanalyzed earlier findings using only matched pairs, and the results did not change with the smaller sample size.26,34
In the initial phase of the study, we compared the abused and neglected children to the matched comparison group on juvenile and adult criminal arrest records.34 A second phase involved tracking, locating, and interviewing both groups during 1989–1995, approximately 22 years after the abuse or neglect (n = 1196). We conducted subsequent follow-up interviews in 2000 through 2002 and again in 2003 through 2005. We used information collected during 2003 through 2005 in the medical status examination (n = 807), which included physical tests, blood collection through venipuncture, a comprehensive health interview, and other questionnaires and assessment instruments.
Although there was attrition associated with death, refusals, and our inability to locate individuals over the various waves of the study, the composition of the sample at the 4 time points has remained about the same (data available as a supplement to the online version of this article at http://www.ajph.org). The abuse and neglect group represented 56% to 58% at each period; non-Hispanic Whites constituted 62% to 66%; and males constituted 48% to 51% of the samples. There were no significant differences across the samples on these variables or in mean age across the 4 phases of the study.
Because of racial/ethnic differences in health status41,42 and because the sample was composed primarily of non-Hispanic Whites and non-Hispanic Blacks, we excluded participants of other ethnic backgrounds (6.6% of the sample) from these analyses, resulting in 754 participants. Of these, 634 consented to physical examinations and 598 to blood tests. The current sample was on average aged 41.2 years (range = 32.0–49.0), 52.9% women, 63.4% non-Hispanic White, and 36.6% non-Hispanic Black (using self-reports). The sample was skewed toward the lower end of the socioeconomic spectrum: 60.0% completed high school, 54.9% held unskilled or semiskilled jobs, and only 13.7% held semiprofessional or professional jobs.43
Procedures
Interviewers were blind to the purpose of the study and to the inclusion of an abuse and neglect group. Participants were also blind to the purpose of the study and were told that they had been selected to participate as part of a large group of individuals who grew up in that area during the late 1960s and early 1970s.
Measures
Child abuse and neglect. We assessed childhood physical and sexual abuse and neglect through review of official records processed during 1967 through 1971. Physical abuse cases included injuries such as bruises, welts, burns, abrasions, lacerations, wounds, cuts, bone and skull fractures, and other evidence of physical injury. Sexual abuse cases ranged from relatively nonspecific charges of “assault and battery with intent to gratify sexual desires” to more specific charges of “fondling or touching in an obscene manner,” sodomy, incest, and rape. Neglect cases reflected a judgment that the parents’ deficiencies in child care were beyond those found acceptable by community and professional standards at the time and represented extreme failure to provide adequate food, clothing, shelter, and medical attention to children.
Health outcomes. A licensed registered nurse performed the medical status examination in the participant’s home or another quiet location of the participant’s choice. We designed the examination to be sensitive to the major disease processes occurring in participants aged 40 years from lower socioeconomic backgrounds, some of whom were homeless, living in shelters, or without access to medical care. Data collection consisted of 2 visits: (1) medical status examination, interview, and administration of the Mantoux test for tuberculosis; and (2) interview and reading of the tuberculosis test (48–72 hours later). Each participant was provided with a copy of the measured results and mailed a written interpretation of all test results, along with recommendations for consultation with a health professional, if indicated. A licensed physician reviewed these letters and recommendations.
Nurses collected 45 milliliters of blood for all the medical screens and assays. Nurses observed universal precautions during all draws, using standard venipuncture procedures, through a single venipuncture using a small gauge butterfly needle and multiple draw adapter. Blood was wrapped and shipped overnight to University Hospital, Newark, New Jersey. Nurses also labeled and completed a chain-of-custody form for each person’s sample and placed it in the transport box with the tubes.
The University Hospital Pathology Laboratory conducted routine blood tests. Nurses assessed measured health outcomes (blood pressure, height, weight, resting heart rate, and airway resistance, as well as brief eye, hearing, and oral health examinations and the Mantoux test). Table 1 shows tests, health outcomes, and diagnostic criteria. Nurses took 2 blood pressure measurements with the participant seated using an automatic blood pressure monitor and appropriate-sized cuff and averaged the measurements, assessed weight using a digital scale,44 measured height using a standard procedure with the participant standing erect against a closed door or wall, obtained resting heart rate through wrist pulse measurement, and assessed pulmonary airway resistance using a peak airflow meter. The nurses also asked participants about their health status using standardized questions from the National Health and Nutrition Examination Survey.45 We coded blood test results as within (0) or outside (1) “acceptable range,” according to hospital norms. We used continuous measures for albumin and blood urea nitrogen to better capture variation across groups.
TABLE 1—
Tests, Health Outcomes, and Diagnostic Criteria: Adult Health Outcomes for Abused and Neglected Children and Matched Controls, Midwestern United States, 2003–2005
| Health Outcomes | Description | Measure | Control Group, Mean (SD) | Abuse and Neglect Group, Mean (SD) |
| Kidney and liver disease | g/dL; indicates nutritional status, including protein deficiency and liver function | Albumin | 4.34 (0.34) | 4.27 (0.30) |
| Kidney disease | mg/dL | BUN | 13.62 (4.14) | 13.82 (4.50) |
| Heart disease | ≥ 0.1 mg/L is above normal (coded “2”) and < 0.1 mg/L is normal (coded “1”) | CRP | 3.62 (14.43) | 3.45 (11.89) |
| Poor glycemic control (diabetes) | ≥ 6.0% is above normal (coded “1”); < 6.0% is normal (coded “0”) | HbA1C | 5.45 (0.67) | 5.53 (0.98) |
| Anemia | For women, hemoglobin < 12.0 g/dL or hematocrit < 37.0% coded “1”; higher values coded “0”; for men, hemoglobin < 14.0 g/dL or hematocrit < 42.0% coded “1”; higher values coded “0” | Women: Hemoglobin | 12.97 (1.65) | 13.44 (1.31) |
| Hematocrit | 38.78 (4.70) | 40.45 (3.84) | ||
| Men: Hemoglobin | 15.22 (1.11) | 15.05 (0.85) | ||
| Hematocrit | 45.14 (3.60) | 44.80 (2.97) | ||
| Malnutrition | BMI ≤ 18.4 kg/m2 or lymphocyte count < 171.8 (low quartile) or albumin < 3 g/dL | BMI | 29.48 (7.13) | 30.07 (8.10) |
| WBC | 7.27 (2.59) | 7.21 (2.54) | ||
| Lymphocytes | 34.99 (13.24) | 33.88 (11.43) | ||
| WBC*lymphocytes | 240.11 (108.33) | 233.70 (89.21) | ||
| Albumin | 4.34 (0.34) | 4.27 (0.30) | ||
| Hepatitis C | Positive coded “1”; negative coded “0.” | Hepatitis C antibody | ||
| HIV | Positive on both the EIA and WB tests coded “1”; not positive coded “0” | EIA, WB | ||
| Syphilis | Positive on both the RPR test and FTA test coded “1”; negative coded “0” | RPR, FTA | ||
| Lung disease | Height and gender were used to categorize the highest of 3 readings (L/min) from the full range peak airflow meter (Respironics model no. HS755), low coded “1”; normal or high coded “0” | Peak airflow | 445.75 (113.11) | 421.66 (122.26) |
| Hypertension | Systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg averaged over 2 assessments separated by about 5 min; high coded “1,” low or normal coded “0” | Systolic blood pressure | 128.50 (16.67) | 127.86 (20.28) |
| Diastolic blood pressure | 82.13 (11.54) | 82.18 (12.70) | ||
| Tuberculosis | Mantoux (purified protein derivative) test; reading of 5–9 mL inclusive with high risk, 10–14 mL with high or moderate risk, or ≥ 15 mL coded “1” (positive); others coded “0” (negative). | Mantoux | 0.48 (1.24) | 1.05 (4.28) |
| Hearing | Weber and Rinne tests | Hearing test | ||
| Oral health | Problems with tooth decay (any teeth with suspicious areas), evidence of gum problems or disease (plaque or bleeding), or a soft tissue condition (abnormalities such as cracking, discolorations, growths on tongue or inside of mouth) coded “1”; no significant findings coded “0” | Oral examination | ||
| Vision | 20/20 for both eyes (tested separately; with glasses or contacts, if needed) coded “0”; otherwise coded “1” | Acuity test |
Note. BMI = body mass index; BUN = blood urea nitrogen; CRP = C-reactive protein; EIA = enzyme immune assay; FTA = fluorescent treponemal antibody; HbA1C = hemoglobin A1C; RPR = rapid plasma reagin; WB = Western blot; WBC = white blood cell count.
Control variables. We included age in middle adulthood, gender, and race as control variables. To determine whether other factors might attenuate the relationship between child maltreatment and adult health outcomes, we also controlled for childhood socioeconomic status (SES; derived from a report of parents’ receiving welfare when the participant was a child); adult SES (earnings at interview 3: $0 coded “0,” < $25 000 “1,” $25 000– < $50 000 “2,” ≥ $50 000 “3” or if missing earnings but a high school graduate and holding a full-time job, case was coded “2”); unhealthy behaviors (alcohol or drug abuse diagnosis at interview 1 or body mass index [defined as weight in kilograms divided by the square of height in meters] at overweight level at interview 3, count 0–2); smoking (total pack-years, i.e., the number of cigarettes smoked per day divided by 20 cigarettes per pack, multiplied by the number of years of smoking)46; and mental health problems (diagnosis of major depression or posttraumatic stress disorder or neither at first interview, coded “0,” “1,” or “2”).
Analyses
We conducted cross-tabulations and logistic regressions with SPSS 17.0 (SPSS, Inc., Chicago, IL) to assess differences between the abuse and neglect and control groups for dichotomous outcomes. We have reported odds ratios (ORs) that indicate the magnitude of effect and 95% confidence intervals (CI). We conducted ordinary least square (OLS) regressions to assess the effect of group (child abuse and neglect vs control) on continuous outcomes and reported standardized parameter estimates and significance levels. Logistic and OLS regressions were repeated with adjustments for age, gender, and race and with each of the additional controls individually and simultaneously. Finally we examined gender by abuse and neglect and race by abuse and neglect interactions in separate analyses. The number of participants varied slightly because of missing information.
RESULTS
Table 2 shows physical health outcomes according to blood test results in middle adulthood. Child maltreatment (controlling for age, race, and gender) predicted above normal hemoglobin A1C (HbA1c; OR = 1.93; 95% CI = 1.12, 3.32)—indicating poor glycemic control and risk for diabetes—and lower levels of albumin (B = −0.11; P < .01), and continued to be predictive in the fully adjusted model.
TABLE 2—
Blood Test Results: Adult Health Outcomes for Abused and Neglected Children and Matched Controls, Midwestern United States, 2003–2005
| Control Group, No., %, or Mean ±SD | Abuse and Neglect Group |
Physical Abuse Group |
Sexual Abuse Group |
Neglect Group |
|||||
| Health Outcome | No., %, or Mean ±SD | OR (95% CI) or B | No., %, or Mean ±SD | OR (95% CI) or B | No., %, or Mean ±SD | OR (95% CI) or B | No., %, or Mean ±SD | OR (95% CI) or B | |
| CRP | |||||||||
| No. | 237 | 338 | 57 | 47 | 275 | ||||
| Unadjusted, % | 24.5 | 29.9 | 1.29 (0.88, 1.89) | 29.8 | 1.31 (0.69, 2.49) | 38.3 | 1.92** (0.99, 3.70) | 29.8 | 1.30 (0.88, 1.93) |
| Age, race, gender | 1.27 (0.86, 1.87) | 1.60 (0.81, 3.15) | 1.63 (0.81, 3.29) | 1.31 (0.88, 1.96) | |||||
| All control variables | 1.26 (0.82, 1.92) | 1.88* (0.88, 3.99) | 1.67 (0.80, 3.48) | 1.30 (0.83, 2.04) | |||||
| HbA1C | |||||||||
| No. | 238 | 338 | 56 | 47 | 276 | ||||
| Unadjusted, % | 9.2 | 16.0 | 1.91** (1.12, 3.26) | 16.1 | 1.88 (0.81, 4.34) | 14.9 | 1.72 (0.69, 4.29) | 15.9 | 1.83** (1.06, 3.16) |
| Age, race, gender | 1.93** (1.12, 3.32) | 2.35* (0.95, 5.79) | 2.05 (0.76, 5.59) | 1.91** (1.10, 3.33) | |||||
| All control variables | 1.81** (1.02, 3.23) | 3.26** (1.19, 8.96) | 1.93 (0.70, 5.36) | 2.89** (1.02, 3.47) | |||||
| Anemia | |||||||||
| No. | 236 | 332 | 55 | 47 | 271 | ||||
| Unadjusted, % | 28.4 | 17.8 | 0.54*** (0.36, 0.80) | 14.5 | 0.43** (0.19, 0.96) | 17.0 | 0.52 (0.23, 1.17) | 19.2 | 0.60** (0.40, 0.92) |
| Age, race, gender | 0.53*** (0.35, 0.80) | 0.52 (0.23, 1.18) | 0.51 (0.22, 1.21) | 0.60** (0.40, 0.92) | |||||
| All control variables | 0.54*** (0.35, 0.85) | 0.56 (0.23, 1.34) | 0.48 (0.20, 1.17) | 0.59** (0.37, 0.95) | |||||
| Malnutrition | |||||||||
| No. | 232 | 334 | 56 | 47 | 272 | ||||
| Unadjusted, % | 23.3 | 27.5 | 1.30 (0.87, 1.93) | 41.1 | 2.30*** (1.24, 4.24) | 34.0 | 1.70 (0.87, 3.34) | 25.7 | 1.13 (0.75, 1.70) |
| Age, race, gender | 1.29 (0.87, 1.93) | 2.39*** (1.27, 4.49) | 1.65 (0.81, 3.36) | 1.12 (0.74, 1.69) | |||||
| All control variables | 1.51* (0.98, 2.33) | 3.16† (1.53, 6.50) | 2.16** (1.02, 4.61) | 1.39 (0.87, 2.21) | |||||
| Hepatitis C | |||||||||
| No. | 231 | 334 | 57 | 46 | 272 | ||||
| Unadjusted, % | 7.4 | 10.5 | 1.43 (0.78, 2.62) | 8.8 | 1.21 (0.43, 3.43) | 15.2 | 2.26 (0.88 5.81) | 9.9 | 1.40 (0.74, 2.64) |
| Age, race, gender | 1.44 (0.78, 2.67) | 0.98 (0.33, 2.92) | 2.76* (0.97, 7.88) | 1.42 (0.75, 2.70) | |||||
| All control variables | 1.26 (0.65, 2.44) | 0.99 (0.30, 3.26) | 2.16 (0.71, 6.59) | 1.18 (0.59, 2.38) | |||||
| HIV | |||||||||
| No. | 235 | 335 | 56 | 46 | 272 | ||||
| Unadjusted, % | 0.4 | 2.1 | 4.88 (0.60, 39.96) | 1.8 | 4.26 (0.26, 69.08) | 2.2 | 5.20 (0.32, 84.67) | 1.8 | 3.52 (0.39, 31.70) |
| Age, race, gender | 5.14 (0.62, 42.52) | 6.18 (0.29, 132.15) | 5.23 (0.26, 106.58) | 3.87 (0.42, 35.66) | |||||
| All control variables | 5.45 (0.57, 52.66) | NE | 7.09 (0.27, 184.56) | 4.29 (0.40, 45.57) | |||||
| Syphilis | |||||||||
| No. | 234 | 338 | 57 | 47 | 275 | ||||
| Unadjusted, % | 0.9 | 0.6 | 0.68 (0.09, 4.83) | 1.8 | 2.07 (0.19, 23.25) | 0.0 | 0.7 | 0.86 (0.12, 6.13) | |
| Age, race, gender | 0.71 (0.10, 5.15) | 3.14 (0.24, 41.46) | 0.90 (0.12, 6.55) | ||||||
| All control variables | 0.55 (0.07, 4.58) | 6.28 (0.25, 155.98) | 0.67 (0.08, 5.68) | ||||||
| Albumin/dL | |||||||||
| No. | 236 | 338 | 57 | 47 | 275 | ||||
| Unadjusted, % | 4.34 ±0.34 | 4.27 ±0.30 | −0.120*** | 4.25 ±0.32 | −0.110* | 4.24 ±0.28 | −0.120** | 4.27 ±0.29 | −0.110*** |
| Age, race, gender | −0.110*** | −0.130** | −0.050 | −0.110*** | |||||
| All control variables | −0.090** | −0.140** | −0.040 | −0.100** | |||||
| BUN mg/dL | |||||||||
| No. | 238 | 338 | 57 | 47 | 275 | ||||
| Unadjusted, % | 13.62 ±4.14 | 13.82 ±4.50 | 0.020 | 15.60 ±4.45 | 0.180† | 13.09 ±4.07 | −0.050 | 13.66 ±4.56 | 0.010 |
| Age, race, gender | 0.030 | 0.180† | 0.001 | 0.010 | |||||
| All control variables | 0.060 | 0.200† | −0.010 | 0.050 | |||||
Note. BUN = blood urea nitrogen; CI = confidence interval; CRP = C-reactive protein; HbA1c = hemoglobin A1C; OR = odds ratio. Percentages refer to the presence of an out of acceptable range blood test score. Line 1 = unadjusted; line 2 = adjusted with controls for age, gender, and race; and line 3 = adjusted with all control variables, including age, race, and gender; childhood socioeconomic status (according to report of parents’ receiving welfare when the person was a child); adult socioeconomic status (earnings at interview 3, $0 coded as “0”; < $25 000 coded as “1”; $25 000– < $50 000 coded as “2”; ≥ $50 000 coded as “3”; if missing earnings but a high school graduate and holding a full-time job, case was coded “2”); unhealthy behaviors (count 0–3: alcohol or drug abuse diagnosis at interview 1 or body mass index [defined as weight in kg divided by the square of height in m] at overweight level at interview 3); smoking (pack-years—the usual current number or when the person last smoked regularly); and number of mental health problems (diagnosis of major depression or posttraumatic stress disorder or none at first interview, 0, 1, or 2).
*P < .1; **P < .05; ***P < .01; †P < .001.
Specific types of abuse and neglect were associated with different health consequences. Childhood physical abuse predicted increased risk for malnutrition (OR = 2.39; 95% CI = 1.27, 4.49), lower albumin levels (B = −0.13; P < .05), higher blood urea nitrogen levels (B = 0.18; P < .001), and above normal HbA1c (OR = 2.35; 95% CI = 0.95, 5.79; P < .10), which became significant in the fully adjusted model. One additional trend is worth noting: physical abuse predicted increased risk for above normal C-reactive protein (CRP; OR = 1.88; 95% CI = 0.88, 3.99), significant in the fully adjusted model, suggesting risk for heart disease. For sexual abuse, there were nonsignificant trends for hepatitis C (OR = 2.76; 95% CI = 0.97, 7.88) and HIV (OR = 5.23; 95% CI = 0.26, 106.58), and a significant increase in risk for malnutrition (OR = 2.16; 95% CI = 1.02, 4.61) in the fully adjusted model. Neglect predicted above normal HbA1c (OR = 1.91; 95% CI = 1.10, 3.33), intensified in the fully adjusted model (OR = 2.89; 95% CI = 1.02, 3.47), and lower levels of albumin (B = −0.11; P < .01). In contrast to expectations, child maltreatment overall (OR = 0.53; 95% CI = 0.35, 0.80) and neglect in particular (OR = 0.60; 95% CI = 0.40, 0.92) decreased risk for anemia, and these results persisted despite the inclusion of all other variables.
Table 3 presents the results of measured health outcomes. Overall, with controls for age, gender, and race, child maltreatment predicted poor peak airflow (OR = 3.45; 95% CI = 0.98, 12.13) and vision problems (OR = 1.51; 95% CI = 1.05, 2.18). However, both relationships were attenuated in the fully adjusted model to nonsignificant trends. Neglect increased risk for poor peak airflow (OR = 4.36; 95% CI = 1.22, 15.52) and persisted with all additional variables (OR = 4.04; 95% CI = 1.07, 15.26). Neglect also increased risk for oral health (OR = 1.55; 95% CI = 1.10, 2.18) and vision problems (OR = 1.58; 95% CI = 1.08, 2.30), although risk was attenuated in the fully adjusted model. There was a trend for sexual abuse to predict decreased risk for oral health problems (OR = 0.52; 95% CI = 0.27, 1.02; P < .1) that became significant with the introduction of the additional factors.
TABLE 3—
Measured Test Results: Adult Health Outcomes for Abused and Neglected Children and Matched Controls, Midwestern United States, 2003–2005
| Control Group, No. or % | Abuse and Neglect Group |
Physical Abuse Group |
Sexual Abuse Group |
Neglect Group |
|||||
| Health Outcome | No. or % | OR (95% CI) | No. or % | OR (95% CI) | No. or % | OR (95% CI) | No. or % | OR (95% CI) | |
| Peak airflow | |||||||||
| No. | 261 | 360 | 59 | 49 | 295 | ||||
| Unadjusted, % | 1.1 | 4.2 | 3.65** (1.05, 12.75) | 1.7 | 1.48 (0.15, 14.51) | 2.0 | 1.79 (0.18, 17.59) | 4.7 | 4.32** (1.23, 15.19) |
| Age, race, gender | 3.45** (0.98, 12.13) | 1.41 (0.14, 14.52) | 1.54 (0.15, 16.21) | 4.36** (1.22, 15.52) | |||||
| All control variables | 3.22* (0.87, 11.95) | 0.55 (0.04, 8.80) | 2.53 (0.17, 37.41) | 4.04** (1.07, 15.26) | |||||
| Blood pressure | |||||||||
| No. | 261 | 360 | 59 | 49 | 295 | ||||
| Unadjusted, % | 29.9 | 30.8 | 1.04 (0.73, 1.47) | 37.3 | 1.40 (0.77, 2.52) | 36.7 | 1.36 (0.72, 2.58) | 28.8 | 0.96 (0.67, 1.38) |
| Age, race, gender | 1.07 (0.74, 1.54) | 1.46 (0.78, 2.73) | 1.63 (0.82, 3.26) | 1.00 (0.68, 1.45) | |||||
| All control variables | 1.16 (0.79, 1.71) | 1.43 (0.73, 2.79) | 1.48 (0.73, 3.01) | 1.12 (0.74, 1.70) | |||||
| Mantoux (PPD) | |||||||||
| No. | 218 | 318 | 51 | 45 | 260 | ||||
| Unadjusted, % | 1.8 | 2.8 | 1.51 (0.46, 4.96) | 2.0 | 1.07 (0.12, 9.78) | 0 | NE | 3.1 | 1.71 (0.51, 5.76) |
| Age, race, gender | 1.46 (0.44, 4.82) | 1.02 (0.11, 9.61) | 1.72 (0.51, 5.56) | ||||||
| All control variables | 1.19 (0.33, 4.28) | 0.75 (0.07, 8.58) | 1.18 (0.32, 4.39) | ||||||
| Hearing | |||||||||
| No. | 260 | 358 | 58 | 48 | 294 | ||||
| Unadjusted, % | 4.2 | 7.5 | 1.99* (0.95, 4.19) | 8.6 | 2.14 (0.71, 6.40) | 10.4 | 2.63* (0.87, 7.95) | 7.1 | 1.75 (0.83, 3.71) |
| Age, race, gender | 1.96 (0.93, 4.16) | 2.21 (0.71, 6.86) | 2.12 (0.67, 6.73) | 1.74 (0.82, 3.71) | |||||
| All control variables | 1.74* (0.79, 3.85) | 2.37* (0.68, 8.26) | 2.23 (0.66, 7.57) | 1.72 (0.74, 4.01) | |||||
| Oral health | |||||||||
| No. | 258 | 360 | 59 | 49 | 295 | ||||
| Unadjusted, % | 49.6 | 55.8 | 1.29 (0.93, 1.79) | 44.1 | 0.80 (0.45, 1.41) | 32.7 | 0.49** (0.26, 0.94) | 60.0 | 1.51** (1.08, 2.11) |
| Age, race, gender | 1.34* (0.97, 1.87) | 0.82 (0.40, 0.99) | 0.52* (0.27, 1.02) | 1.55*** (1.10, 2.18) | |||||
| All control variables | 0.98 (0.67, 1.42) | 0.70 (0.37, 1.35) | 0.43** (0.21, 0.87) | 1.07 (0.72, 1.59) | |||||
| Vision | |||||||||
| No. | 262 | 361 | 59 | 49 | 296 | ||||
| Unadjusted, % | 65.3 | 74.2 | 1.51** (1.06, 2.15) | 64.4 | 0.96 (0.53, 1.74) | 79.6 | 2.08** (0.99, 4.35) | 74.0 | 1.50** (1.04, 2.16) |
| Age, race, gender | 1.51** (1.05, 2.18) | 0.81 (0.43, 1.53) | 1.39 (0.63, 3.07) | 1.58** (1.08, 2.30) | |||||
| All control variables | 1.12 (0.75, 1.66) | 0.58 (0.29, 1.17) | 1.22 (0.53, 2.78) | 1.17 (0.76, 1.78) | |||||
Note: CI = confidence interval; NE = not estimable; OR = odds ratio; PPD = purified protein derivative. Line 1 = unadjusted; line 2 = adjusted with controls for age, gender, and race; and line 3 = adjusted with all control variables, including age, race, gender; childhood socioeconomic status (according to report of parents’ receiving welfare when the person was a child); adult socioeconomic status (earnings at interview 3, $0 coded as “0”; < $25 000 coded as “1”; $25 000– < $50 000 coded as “2”; ≥ $50 000 coded as “3”; if missing earnings but a high school graduate and holding a full-time job, case was coded “2”); unhealthy behaviors (count 0–3: alcohol or drug abuse diagnosis at interview 1 or body mass index [defined as weight in kg divided by the square of height in m] at overweight level at interview 3); smoking (pack-years—the usual current number or when the person last smoked regularly); and number of mental health problems (diagnosis of major depression or posttraumatic stress disorder or none at first interview, 0, 1, or 2). Percentages refer to the presence of test scores in the problem range.
*P < .1; **P < .05; ***P < .01; †P < .001.
Gender and Race Differences
The presence or absence of main effects for child abuse and neglect does not necessarily indicate that health outcomes are the same for subgroups, because a strong positive effect for 1 group (e.g., men) and negative for another group (e.g., women) produces a nonsignificant main effect. We tested for interactions of child abuse and neglect by gender and child abuse and neglect by race across the health outcomes and found few significant interactions.
There was a significant child abuse and neglect by gender interaction for above normal HbA1c, for abuse and neglect overall (OR = 5.83; 95% CI = 1.88, 18.08), and for neglect (OR = 7.09; 95% CI = 2.17, 23.17). Maltreated females overall and neglected females in particular were at increased risk for above normal HbA1c (OR = 4.13; 95% CI = 1.85, 9.24 and OR = 4.61; 95% CI = 2.01, 10.56, respectively), whereas the results for males were in the opposite direction (OR = 0.71; 95% CI = 0.32, 1.58 and OR = 0.65; 95% CI = 0.28, 1.53, respectively). There was also a significant sexual abuse by gender interaction for anemia (OR = 0.12; 95% CI = 0.02, 0.75). Sexually abused females were at decreased risk of anemia, compared with control females (OR = 0.29; 95% CI = 0.10, 0.83), whereas the effect for sexually abused males was in the opposite direction (OR = 2.48; 95% CI = 0.52, 11.96).
We found child abuse and neglect by race interactions for above normal CRP: abuse and neglect overall (OR = 2.62; 95% CI = 1.18, 5.80) and neglect (OR = 3.27; 95% CI = 1.42, 7.51). Among Whites, child abuse and neglect in general and neglect in particular predicted above normal CRP (OR = 1.88; 95% CI = 1.13, 3.14 and OR = 2.11; 95% CI = 1.24, 3.57, respectively). Among Blacks, child abuse and neglect in general and neglect in particular did not predict CRP.
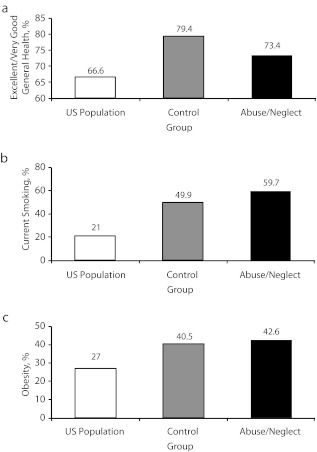
Comparison With the US General Population
Finally, we compared self-reports from our sample to results from a national sample of the US population on 3 prominent health indicators. Figure 1 has 3 components and presents 3 patterns of results. Abused and neglected individuals were less likely to report that their general health is excellent or very good compared with controls but more likely compared with Americans in general.30 Individuals with histories of abuse and neglect were more likely to report currently smoking, compared with controls and Americans in general. Both groups (individuals with documented histories of abuse and neglect and controls) were more likely to be obese than were Americans surveyed in 2009, but they did not differ from each other in terms of obesity.44 However, it should be noted that physically abused individuals had significantly higher body mass index scores than did controls.44
FIGURE 1—
Comparison of US population health reports of (a) excellent/very good health, (b) current smoking, and (c) obesity: Adult Health Outcomes for Abused and Neglected Children and Matched Controls, Midwestern United States, 2003–2005.
Note. Assessed in middle adulthood (aged approximately 41 years). Adults with documented histories of abuse and neglect were less likely than were controls to report excellent or very good health (P < .05), as opposed to fair or poor health, and both groups were more likely than was the general population (P < .001). Abused and neglected individuals were more likely than were controls (P < .001) and the US population (P < .001) to report current smoking. Individuals with documented histories of abuse and neglect and controls were both significantly more likely to be obese than was the general US population (P < .001) but did not differ from each other.35
DISCUSSION
This prospective long-term investigation of physical health outcomes in abused and neglected children followed into middle adulthood shows evidence of the impact of these childhood experiences 30 years later on important health indicators. According to blood test results and direct physical measurements, these new findings provide clear evidence that child abuse and neglect affect adult health status in terms of increased risk for diabetes, lung disease, malnutrition, and vision problems. Because these findings are derived from assessed health status, they reflect untreated risks and do not reflect the extent to which these participants may have disorders controlled by medication, diet, or other treatments.
These results also reveal differences in long-term health outcomes associated with the specific types of maltreatment. Neglected children were at increased risk for diabetes, poorer lung functioning, and vision and oral health problems. Physical abuse increased risk for diabetes and malnutrition and showed a trend for CRP. Sexual abuse showed nonsignificant trends for hepatitis C, HIV, and oral health problems but a significant increase in risk for malnutrition when all other factors were controlled. For physical and sexual abuse, however, small sample sizes limited our power, possibly explaining why these were trends rather than significant findings.
These new findings raise questions about the mechanisms underlying these increases in risk for diabetes, poorer lung functioning, and malnutrition, and the unexpected decreased risk for anemia. A possible explanation for the increased risk for maltreated children to have poor glycemic control (diabetes) or poor lung functioning may rest in higher body mass index for diabetes and smoking for lung functioning. However, we controlled for these factors, ruling them out as possible explanations.
Although our findings reveal important adult health outcomes that abused and neglected children are at increased risk for, our findings are not nearly as expansive as those of other researchers.6,7 What might explain the discrepancy between our findings and those of other researchers? One important difference is the study design. We followed a group of individuals with documented histories of child abuse and neglect into adulthood, whereas most studies are cross-sectional and rely on retrospective reports of childhood maltreatment. In addition, our findings are derived from measured health outcomes (physical examination and blood test results), whereas previous research typically relies on self-reports of health status.11 Thus, earlier cross-sectional studies involve recall problems in both sides of the equation (child maltreatment and health outcomes). Finally, a critical component of this study involves the matched control group. Because the SES of both groups in this sample is skewed toward the lower end of the socioeconomic spectrum, it is possible that there is more similarity in the health problems of the maltreated and control children because of the overlay of poverty.47,48 These analyses reveal the effect of both childhood and adult SES on health outcomes. For example, childhood social class in part explained increases in the risk for malnutrition, peak airflow, and oral health and vision problems in adulthood. Further work is clearly needed to understand why individuals with histories of abuse and neglect are at increased risk for these health outcomes and the gender and race differences in these relationships. Nonetheless, it is likely that behaviors, lifestyle factors, access to health care, and neighborhood characteristics (e.g., environmental toxins) may act as mediators between childhood abuse and neglect and long-term physical health consequences.
Despite numerous strengths, limitations need to be noted. Our findings are derived from cases of childhood abuse and neglect drawn from official court records and are not generalizable to unreported cases of child abuse and neglect. If there is unreported abuse or neglect in the control group, this may underestimate the association between child maltreatment and adult health. Officially reported cases of child abuse and neglect are generally skewed toward the lower end of the socioeconomic spectrum. Thus, these findings cannot be generalized to child abuse and neglect cases that occur in middle- or upper-class children and their families. These findings represent the experiences of children aged 0 to 11 years and may not generalize to children abused or neglected at an older age (i.e., in adolescence).
Nonetheless, these new findings show that child abuse and neglect have long-lasting physical health consequences that are manifested 30 years after these childhood experiences. Given the magnitude of the problem of child maltreatment in the United States and around the world,49 these findings provide direct support for early health care prevention efforts targeted at abused and neglected children. Understanding the mechanisms that place abused and neglected children at higher risk for these adult physical health outcomes will help focus these efforts.
Acknowledgments
This research was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Development (grant HD40774), the National Institute of Mental Health (grants MH49467 and MH58386), the National Institute of Justice (grants 86-IJ-CX-0033 and 89-IJ-CX-0007), the National Institute on Drug Abuse (grants DA17842 and DA10060), the National Institute on Alcohol Abuse and Alcoholism (grants AA09238 and AA11108), and the Doris Duke Charitable Foundation.
The authors express appreciation to Melissa Frederikse, MD, for her role in supervising the feedback provided to participants in the study; Harold Cohen, DDS, and Michael Glick, DDS, for assistance with the dental assessment; and Annabella Bernard and Melville Malone Francis for assistance with the preparation of the article.
Note. Points of view are those of the authors and do not necessarily represent the position of the US Department of Justice.
Human Participant Protection
City University of New York’s institutional review board approved this phase of this study, and participants provided written informed consent. For individuals with limited reading ability, we explained the consent form verbally.
References
- 1.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities. JAMA. 2009;301(21):2252–2259 [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services Child Maltreatment 2009. Administration for Children and Families; 2010. Available at: http://www.acf.hhs.gov/programs/cb/pubs/cm09. Accessed November 10, 2011 [Google Scholar]
- 3.Dong M, Giles WH, Felitti VJet al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110(13):1761–1766 [DOI] [PubMed] [Google Scholar]
- 4.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104(4):1319–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubowitz H. The impact of child maltreatment on health. : Starr RH, Jr, Wolfe DA, The Effects of Child Abuse and Neglect: Issues and Research. New York: Guilford Press; 1991: 278–294 [Google Scholar]
- 6.Lissau I, Sorensen TIA. Parental neglect during childhood and increased risk of obesity in young adulthood. Lancet. 1994;343(8893):324–327 [DOI] [PubMed] [Google Scholar]
- 7.Felitti VJ, Anda RF, Nordenberg Det al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) study. Am J Prev Med. 1998;14(4):245–258 [DOI] [PubMed] [Google Scholar]
- 8.Walker EA, Gelfand A, Katon WJ, Von Korff M, Bernstein D, Russo J. Adult health status of women with histories of childhood abuse and neglect. Am J Med. 1999;107(4):332–339 [DOI] [PubMed] [Google Scholar]
- 9.Flaherty EG, Thompson R, Litrownik AJet al. Effect of early childhood adversity on child health. Arch Pediatr Adolesc Med. 2006;160(12):1232–1238 [DOI] [PubMed] [Google Scholar]
- 10.Chartier MJ, Walker JR, Naimark B. Child abuse, adult health, and health care utilization: results from a representative community sample. Am J Epidemiol. 2007;165(9):1031–1038 [DOI] [PubMed] [Google Scholar]
- 11.Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med. 2009;71(8):805–812 [DOI] [PubMed] [Google Scholar]
- 12.Arnow BA, Hart S, Scott C, Dea R, O’Connell L, Taylor CB. Childhood sexual abuse, psychological distress and medical use. Psychosom Med. 1999;61(6):762–770 [DOI] [PubMed] [Google Scholar]
- 13.Hulme PA. Symptomatology and health care utilization of women primary care patients who experienced childhood sexual abuse. Child Abuse Negl. 2000;24(11):1471–1484 [DOI] [PubMed] [Google Scholar]
- 14.Tang B, Jamieson E, Boyle M, Libby A, Gafni A, MacMillan H. The influence of child abuse on the pattern of expenditures in women’s adult health service utilization in Ontario, Canada. Soc Sci Med. 2006;63(7):1711–1719 [DOI] [PubMed] [Google Scholar]
- 15.Walker EA, Unutzer J, Rutter Cet al. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch Gen Psychiatry. 1999;56(7):609–613 [DOI] [PubMed] [Google Scholar]
- 16.Widom CS, Raphael KG, DuMont K. The case for prospective longitudinal studies in child maltreatment research: commentary on Dube, Williamson, Thompson, Felitti, and Anda (2004). Child Abuse Negl. 2004;28(7):715–722 [DOI] [PubMed] [Google Scholar]
- 17.Brewin CR, Andrews B, Gotlib IH. Psychopathology and early experience: a reappraisal of retrospective reports. Psychol Bull. 1993;113(1):82–98 [DOI] [PubMed] [Google Scholar]
- 18.Fergusson DM, Horwood JL, Woodward LJ. The stability of child abuse reports: a longitudinal study of the reporting behaviour of young adults. Psychol Med. 2000;30(3):529–544 [DOI] [PubMed] [Google Scholar]
- 19.Fivush R. Developmental perspectives on autobiographical recall. : Goodman GS, Bottoms BL, Child Victims, Child Witnesses: Understanding and Improving Testimony. New York: Guilford Press; 1993:1–24 [Google Scholar]
- 20.Henry B, Moffitt TE, Caspi A, Langley J, Silva PA. On the “remembrance of things past”: a longitudinal evaluation of the retrospective method. Psychol Assess. 1994;6(2):92–101 [Google Scholar]
- 21.Offer D, Kaiz M, Howard KI, Bennett ES. The altering of reported experiences. J Am Acad Child Adolesc Psychiatry. 2000;39(6):735–742 [DOI] [PubMed] [Google Scholar]
- 22.Raphael KG, Cloitre M. Does mood-congruence or causal search govern recall bias? A test of life event recall. J Clin Epidemiol. 1994;47(5):555–564 [DOI] [PubMed] [Google Scholar]
- 23.Schraedley PK, Turner RJ, Gotlib IH. Stability of retrospective reports in depression: traumatic events, past depressive episodes, and parental psychopathology. J Health Soc Behav. 2002;43(3):307–316 [PubMed] [Google Scholar]
- 24.Williams LM. Recall of childhood trauma: a prospective study of women’s memories of child sexual abuse. J Consult Clin Psychol. 1994;62(6):1167–1176 [DOI] [PubMed] [Google Scholar]
- 25.Goodwin RD, Stein M. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med. 2004;34(3):509–520 [DOI] [PubMed] [Google Scholar]
- 26.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64(1):49–56 [DOI] [PubMed] [Google Scholar]
- 27.Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156(8):1223–1229 [DOI] [PubMed] [Google Scholar]
- 28.Widom CS, Kuhns JB. Childhood victimization and subsequent risk for promiscuity, prostitution, and teenage pregnancy: a prospective study. Am J Public Health. 1996;86(11):1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez CM, Widom CS. Childhood victimization and long-term intellectual and academic outcomes. Child Abuse Negl. 1994;18(8):617–633 [DOI] [PubMed] [Google Scholar]
- 30.National Center for Health Statistics Health, United States, 2009 With a Special Feature on Medical Technology. Hyattsville, MD: US Department of Health and Human Services; 2009 [PubMed] [Google Scholar]
- 31.Leventhal JM. Research strategies and methodologic standards in studies of risk factors for child abuse. Child Abuse Negl. 1982;6(2):113–123 [DOI] [PubMed] [Google Scholar]
- 32.Schulsinger F, Mednick SA, Knop J. Longitudinal Research: Methods and Uses in Behavioral Sciences. Boston: Martinus Nijhoff Publishers; 1981 [Google Scholar]
- 33.Widom CS. Child abuse, neglect and adult behavior: research design and findings on criminality, violence, and child abuse. Am J Orthopsychiatry. 1989;59(3):355–367 [DOI] [PubMed] [Google Scholar]
- 34.Widom CS. The cycle of violence. Science. 1989;244(4901):160–166 [DOI] [PubMed] [Google Scholar]
- 35.MacMillan HL, Fleming JE, Streiner DLet al. Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry. 2001;158(11):1878–1883 [DOI] [PubMed] [Google Scholar]
- 36.Case A, Fertig A, Paxson C. The lasting impact of childhood health and circumstance. J Health Econ. 2005;24(2):365–389 [DOI] [PubMed] [Google Scholar]
- 37.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399 [DOI] [PubMed] [Google Scholar]
- 38.Conroy K, Sandel M, Zuckerman B. Poverty grown up: how childhood socioeconomic status impacts adult health. J Dev Behav Pediatr. 2010;31(2):154–160 [DOI] [PubMed] [Google Scholar]
- 39.Adler NE, Boyce T, Chesney MAet al. Socioeconomic status and health: the challenge of the gradient. Am Psychol. 1994;49(1):15–24 [DOI] [PubMed] [Google Scholar]
- 40.Watt NF. Longitudinal changes in the social behavior of children hospitalized for schizophrenia as adults. J Nerv Ment Dis. 1972;155(1):42–54 [DOI] [PubMed] [Google Scholar]
- 41.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the pattern tells us. Am J Public Health. 2010;100(suppl 1):S186–S196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orsi JM, Margellos-Anast H, Whitman SN. Black–White health disparities in the United States and Chicago: a 15-year progress analysis. Am J Public Health. 2010;100(2):349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollingshead AB. Four-Factor Index of Social Status. New Haven: Yale University Press; 1975 [Google Scholar]
- 44.Bentley T, Widom CS. A 30-year follow-up of the effects of child abuse and neglect on obesity in adulthood. Obesity (Silver Spring). 2009;17(10):1900–1905 [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). Hyattsville, MD: Department of Health and Human Services; 1999–2000.
- 46.Cruickshanks KJ, Klein R, Klein BE, Wiley TL, Nondahl DM, Tweed TS. Cigarette smoking and hearing loss: the epidemiology of hearing loss study. JAMA. 1998;279(21):1715–1719 [DOI] [PubMed] [Google Scholar]
- 47.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252 [DOI] [PubMed] [Google Scholar]
- 48.Nikulina V, Widom CS, Czaja S. The role of childhood neglect and childhood poverty in predicting mental health, academic achievement and crime in adulthood. Am J Community Psychol. 2011;48(3–4):309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373(9657):68–81 [DOI] [PubMed] [Google Scholar]