Abstract
Objectives. We conducted 2 studies to determine the impact of text message immunization reminder–recalls in an urban, low-income population.
Methods. In 1 study, text message immunization reminders were sent to a random sample of parents (n = 195) whose children aged 11 to 18 years needed either or both meningococcal (MCV4) and tetanus–diphtheria–acellular pertussis (Tdap) immunizations. We compared receipt of MCV4 or Tdap at 4, 12, and 24 weeks with age- and gender-matched controls. In the other study, we compared attendance at a postshortage Haemophilus influenzae B (Hib) immunization recall session between parents who received text message and paper-mailed reminders (n = 87) and those who only received paper-mailed reminders (n = 87).
Results. Significantly more adolescents with intervention parents received either or both MCV4 and Tdap at weeks 4 (15.4% vs 4.2%; P < .001), 12 (26.7% vs 13.9%; P < .005), and 24 (36.4% vs 18.1%; P < .001). Significantly more parents who received both Hib reminders attended a recall session compared with parents who only received a mailed reminder (21.8% vs 9.2%; P < .05). After controlling for age, gender, race/ethnicity, insurance status, and language, text messaging was still significantly associated with both studies’ outcomes.
Conclusions. Text messaging for reminder–recalls improved immunization coverage in a low-income, urban population.
Immunization reminder–recalls are widely recommended as a way to increase immunization uptake.1–5 They may be particularly effective at providing cues to action when parents are unfamiliar with changes in vaccine recommendations. New additions to routine vaccine schedules (such as pertussis and meningococcal vaccines for adolescents6) and interruptions in vaccine supply (such as those that occur during shortages7) may leave parents unaware that their child is not fully immunized, making them ideal candidates for reminder–recalls. Low-income families, who are at high risk for limited health literacy,8 may be at particular risk for not having needed immunizations.9,10 Unfortunately, among low-income urban populations and adolescents, traditional mail or telephone reminder–recall interventions have had limited impact on immunization outcomes.11–13
Cellular telephone technology provides a novel method for implementing reminder–recalls. Wireless telephone networks have penetrated 96% of the total US population.14 In hard-to-reach low-income communities, wireless market penetration is especially high; thus, text messaging may be a particularly effective mechanism for delivering reminder–recalls in this population.15–19 Families and patients seem interested in vaccine-related text messages, but the efficacy of reminder–recalls for pediatric or adolescent immunizations remains underexamined.18,20–22
We conducted 2 independent, randomized studies to assess the feasibility and efficacy of text message reminder–recalls in an urban, low-income population. In the first study, Text4Health–Adolescents, we assessed the impact of text message reminder–recalls on young patients' return to their medical home for a needed routine vaccination. In the second study, Text4Health–Peds, we assessed the impact of using text messages to mobilize parents of children underimmunized for Haemophilus influenzae B (Hib) to attend special immunization sessions.
METHODS
We conducted the studies between January 2009 and June 2009 in a network of community-based clinics affiliated within an academic medical center in New York City, primarily serving a low-income, minority population. The studies were approved by the medical center's institutional review board with a waiver of consent.
We built a text-messaging platform and integrated it with the hospital's immunization information system, EzVac, which is linked to the hospital's registration and computerized provider order entry systems. EzVac synchronizes the hospital's primary care patients' immunization data with the New York Citywide Immunization Registry, allowing inclusion of vaccines provided outside practice sites. This registry captures more than 85% of immunizations administered in New York City in general and 93% of free Vaccines for Children–distributed immunizations.23,24 We identified parents’ cell phone numbers from the clinics’ registration system.
Text4Health–Adolescents
Conducted from January 2009 to April 2009, Text4Health–Adolescents was a randomized text messaging intervention with age- and gender-matched controls. The purpose was to assess the effect of text messaging on receipt of 1 or both of 2 routinely recommended adolescent vaccines: meningococcal (MCV4) and tetanus–diphtheria–acellular pertussis (Tdap).
Parents or guardians were eligible to participate in the study if (1) they had an 11- to 18-year-old child with any visit (including sick visits) at a study site within the previous 12 months, (2) the patient was in need of either or both MCV4 and Tdap, and (3) a cell phone number was recorded in the registration system. Parents of patients who had not received the Tdap vaccine but had received another tetanus-containing vaccine within the previous 2 years were excluded from study.25
We included all 6 sites affiliated with the ambulatory care network; 2 intervention sites and 4 control sites were assigned to provide comparable baseline populations and coverage rates for MCV4 and Tdap. Although the numbers of patients in the 2 groups (intervention and control) were similar, individual sites had varying numbers of patients. Sites were part of the same ambulatory care network, serving primarily minority, publicly insured patients. Providers in this network are typically advised to review immunizations at every visit, although adherence to this policy was unknown.
Weekly from January 2009 to April 2009, we used a computer algorithm to automatically select a random sample of patients from the intervention sites whose parents met the eligibility criteria. The group of eligible patients was updated during the intervention to reflect changing vaccination status. Intervention patients were then matched by gender and age (± 1 year) to randomly selected eligible patients from control sites, when such were available. During the 4 months when patients were randomly selected, there were 1656 eligible patients at the intervention sites and 1460 at control sites who needed Tdap or MCV. Of these, 625 (20%) had parents with a cell phone number in the registration system (cell phone numbers were not routinely collected during the registration process until 2008). The cell phone contact rates varied among individual sites.
Intervention parents received a series of automated text messages notifying them of their child's need for vaccination. Each parent received text messages at weeks 1, 2, 3, 6, and 7.26 Messages were stopped if receipt of MCV4 or Tdap was documented in EzVac. Text messages, developed with community input, were personalized to include the patient's first name, clinic name, and a listing of times when immunizations could be administered at the clinic.20 Messages were sent in English or Spanish, based on the parent's language preference recorded in the care network's electronic registration system. If sent in English, the first message included instructions on receiving future messages in Spanish. Families were also told how to decline further messages. Control parents received the standard of care at the practice sites, which did not include immunization reminders.
The primary outcome for this study was receipt of MCV4 or Tdap at 4, 12, and 24 weeks after randomization. A secondary outcome was receipt of any vaccine, which included MCV4 or Tdap along with all other vaccines. We did not include the human papillomavirus (HPV) and influenza vaccines in the primary outcome because their uptake may have reflected unique parental or provider-related attitudes and beliefs; in addition, the intervention extended past the influenza season.27–29 However, we included receipt of these vaccines in the secondary outcome. We collected immunization data from EzVac. We collected demographic data, including age, gender, race/ethnicity, insurance status, and language preference from the clinics’ registration system.
We analyzed differences between intervention and control groups with regard to receipt of an additional adolescent vaccine (MCV4 or Tdap) and receipt of any vaccine with the χ2 test at 4, 12, and 24 weeks. We conducted both intention-to-treat analyses and per-protocol analyses, adjusting for undeliverable messages and incorrect phone numbers. With a sample size of 150, a power of 80%, and an α of 5%, the study was (conservatively) powered to detect a 15-percentage-point difference between intervention and control participants. We performed nested analysis of variance (ANOVA) to assess the contribution of between-site variability to outcomes. This analysis is useful when sites are nested in intervention groups, to indicate whether observed differences between groups are likely to be attributable to the intervention rather than to baseline variability between sites.30 We used multivariable logistic regression to assess the impact of age, gender, race/ethnicity, insurance status, and language. We conducted all analyses with Stata version 9.1 (StataCorp LP, College Station, TX).
Text4Health–Peds
Conducted from May to June 2009, Text4Health–Peds was a quality initiative to mobilize parents to attend special Hib immunization recall sessions for children overdue for primary vaccination because of the national shortage that occurred in response to a voluntary recall.31,32 Eligible families had (1) a child aged 7 to 22 months lacking 1 Hib dose needed to complete his or her primary series, (2) a visit for that child in the past 12 months at 1 of 4 pediatric clinical sites, and (3) a cell phone number recorded in the clinic registration system. There were a total of 390 children in need of an Hib vaccine, which was 13.3% of those aged 7 to 22 months with a visit in the previous 12 months; 174 of the 390 (44.6%) had parents with a cell phone number recorded. Parents of children in need of an Hib vaccine who were ineligible for the study were contacted by their child's medical home.
Two weeks before the sessions, parents were randomized to receive a paper mailing alone or a paper mailing plus up to 3 text message notifications. The decision to provide a paper mailing to all participants was made by the medical director of the ambulatory care network. Text messages and letters notified parents that their child was in need of an Hib vaccine because of a shortage and included the location, times, and dates of the special immunization sessions. Sessions were held at 2 clinics. Parents were invited to an evening and Saturday session at the clinic nearest to them; 44% of control families and 43% of intervention families were directed to a site other than their medical home. All text messages were sent in English or Spanish, based on language preference in the registration system. All letters were in English and Spanish.
The outcome for this study was attendance at special immunization recall sessions. We chose this outcome to assess whether text messaging could be used to mobilize families to go someplace other than their medical home at a specific time to get vaccinated. We also assessed receipt of Hib dose within 2 weeks from the date the first set of text messages was sent. We collected children's age, gender, race/ethnicity, insurance status, and language from the registration system.
We conducted the χ2 or Fisher's exact test to assess differences in attendance between groups using intention-to-treat and per-protocol analyses. With 80% power and an α of 5%, the study required a sample size of 90 in each group to detect a difference of 15 percentage points, assuming a baseline attendance rate of 5%. In addition, we used multivariable logistic regression analyses to assess the impact of age, gender, race/ethnicity, insurance status, and language. We used the χ2 test to compare receipt of Hib dose.
RESULTS
We conducted these 2 complementary studies separately to assess the impact of text messaging for differing target populations and outcomes. Therefore, we report the results in 2 parts.
Text4Health–Adolescents
A total of 195 parents were randomized to the intervention group, and there were 166 controls available. There were no significant baseline differences between intervention and control participants (Table 1). Baseline adolescent and childhood immunization rates did not differ across study groups (Tdap: 66.9% vs 65.2%; MCV: 69.2% vs 69.8%; age-appropriate series aged 7 months to 35 months: 78.9% vs 77.4%). Each group of sites (intervention and control) contained a site with the highest and lowest immunization coverage in each study. At all study sites, there were no differences in age, race/ethnicity, gender, or insurance status between those with and without cell phones; those with a documented cell phone number were more likely to be primarily Spanish speakers compared with those without a documented cell phone number (57% vs 49%; P < .001).
TABLE 1—
Characteristics of Study Populations: Text4Health–Adolescents and Text4Health–Peds, New York City, 2009
| Intervention, No. (%) or Mean ±SD | Control, No. (%) or Mean ±SD | P | |
| Text4Health–Adolescentsa | |||
| Age, y | 16.3 ±1.7 | 16.1 ±1.7 | .172 |
| Sex | .275 | ||
| Male | 77 (39.5) | 75 (45.2) | |
| Female | 118 (60.5) | 91 (54.8) | |
| Race/ethnicityb | .572 | ||
| Black, non-Latino | 32 (16.7) | 21 (14.1) | |
| Latino | 100 (52.1) | 86 (57.7) | |
| White, non-Latino | 5 (2.6) | 6 (4.0) | |
| Other | 55 (28.6) | 36 (24.2) | |
| Insurance status | .438 | ||
| Uninsured | 23 (11.8) | 18 (10.8) | |
| Medicaid/SCHIP | 153 (78.5) | 137 (82.5) | |
| Private | 19 (9.7) | 11 (6.6) | |
| Primary language | .835 | ||
| English | 81 (41.5) | 67 (41.0) | |
| Spanish | 111 (56.9) | 94 (56.6) | |
| Other | 3 (1.5) | 4 (2.4) | |
| Text4Health–Pedsc | |||
| Age, mo | 14.3 ±3.1 | 14.6 ±3.3 | .562 |
| Sex | .879 | ||
| Male | 43 (49.4) | 44 (50.6) | |
| Female | 44 (50.6) | 43 (49.4) | |
| Race/ethnicity | .132 | ||
| Black, non-Latino | 9 (10.3) | 16 (18.4) | |
| Latino | 59 (67.8) | 44 (50.6) | |
| White, non-Latino | 1 (1.1) | 2 (2.3) | |
| Other | 18 (20.7) | 25 (28.7) | |
| Insurance | .812 | ||
| Uninsured | 13 (14.9) | 16 (18.4) | |
| Medicaid/SCHIP | 67 (77.0) | 65 (74.7) | |
| Private | 7 (8.0) | 6 (6.9) | |
| Primary language | .457 | ||
| English | 32 (36.8) | 27 (31.0) | |
| Spanish | 55 (63.2) | 59 (67.8) | |
| Other | 0 (0) | 1 (1.1) | |
Note. SCHIP = State Children's Health Insurance Program. Student t test used for continuous variables, and Pearson χ2 test used for categorical variables.
Intervention n = 195; control n = 166.
Does not equal total number because of missing data.
Intervention n = 87; control n = 87.
It took an estimated 470 hours to develop de novo the text messaging system, including connecting to our registry and developing protocols and messages. We used an additional 3 hours per week to monitor the text messaging platform. We sent a total of 821 text messages. Of the 195 parents, 12 numbers were incorrect or messages were undeliverable (6.2%); 5 families (2.6%) declined further messages. The median number of messages sent before a Text4Health–Adolescents participant received a needed vaccine was 3 (interquartile range = 3). When we assessed the source of the immunization data (i.e., EzVac vs the Citywide Immunization Registry), 94.6% of immunizations administered during the study period were given at practice sites.
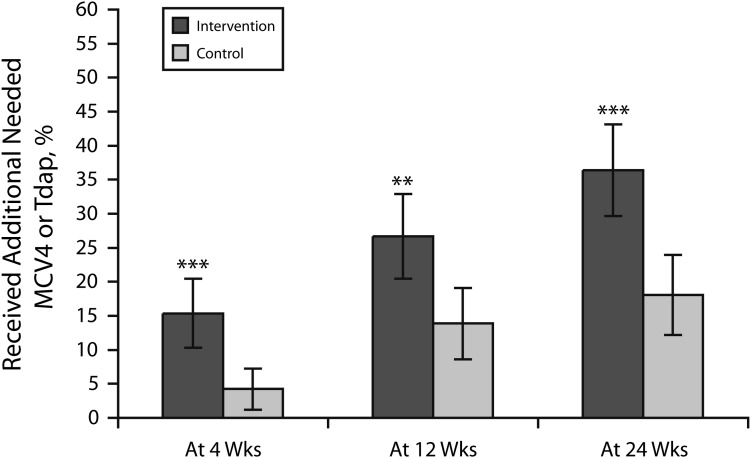
More (15.4%) Text4Health–Adolescents participants whose parents were sent a text message received 1 or both of MCV4 or Tdap at 4 weeks postrandomization than controls (4.2%; 11.2 percentage-point difference; 95% confidence interval [CI] = 5.3, 17.1; P = .001; Figure 1). At 12 weeks, 26.7% versus 13.9% received MCV4 or Tdap (12.8 percentage-point difference; 95% CI = 4.7, 20.9; P = .003), and at 24 weeks, 36.4% versus 18.1% received MCV4 or Tdap (18.3 percentage-point difference; 95% CI = 9.4, 27.3; P = .001). Per-protocol analyses were consistent. The nested ANOVA did not detect variability between study sites at 4 weeks (F = 0.38; P = .823), 12 weeks (F = 0.74; P = .568), or 24 weeks (F = 0.91; P = .458), but nested ANOVA did detect significant differences between intervention groups at all 3 time points.
FIGURE 1—
Percentages of patients aged 11–18 years who received an additional needed adolescent vaccine at 4, 12, and 24 weeks: Text4Health–Adolescents, New York City, 2009.
Note. MCV4 = meningococcal; Tdap = tetanus–diphtheria–acellular pertussis.**P < .01; ***P < .001.
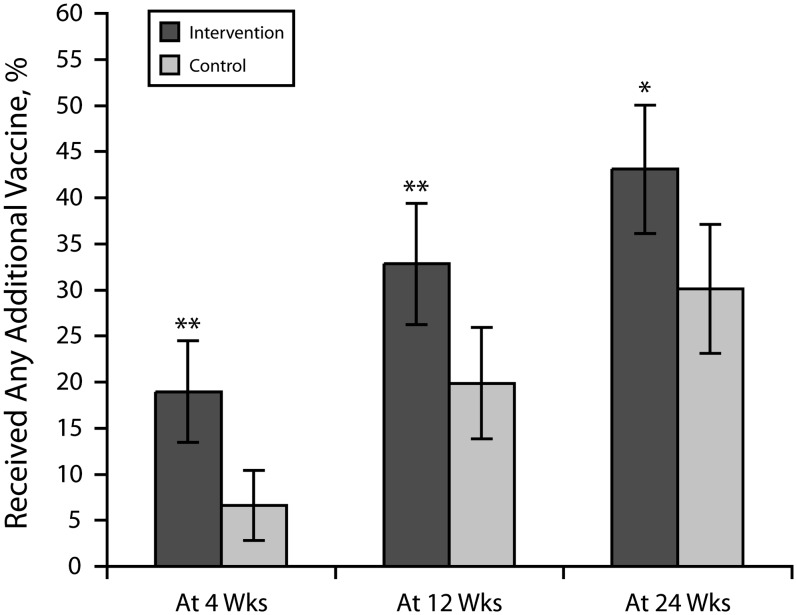
We also found significant differences in receipt of any vaccine at all 3 time points (Figure 2). Nested ANOVA again did not detect variability between study sites at all 3 time points for receipt of any vaccine, but it did detect significant differences between intervention groups at 4 and 12 weeks (P < .05). We detected no difference at 24 weeks for either intention-to-treat (P = .071) or per-protocol analyses (P = .053). Multivariable analyses at 4, 12, and 24 weeks found only intervention status to be significantly associated with receipt of MCV, Tdap, or any vaccine (Table 2).
FIGURE 2—
Percentages of patients aged 11–18 years who received any vaccination at 4, 12, and 24 weeks: Text4Health–Adolescents, New York City, 2009.
Note. Most common vaccines administered included meningococcal (MCV4), tetanus–diphtheria–acellular pertussis (Tdap), human papillomavirus (HPV), hepatitis A, influenza, varicella, and measles–mumps–rubella.*P < .05; **P < .01.
TABLE 2—
Impact of Text Message Interventions on Receipt of Needed Vaccines (Text4Health–Adolescents) and on Attendance at Special Immunization Session (Text4Health–Peds): New York City, 2009
| AOR (95% CI) | |
| Text4Health–Adolescents | |
| Weeks after randomization to MCV4 or Tdap | |
| 4 | 4.57 (1.83, 11.42) |
| 12 | 2.17 (1.23, 3.82) |
| 24 | 2.48 (1.49, 4.13) |
| Weeks after randomization to any vaccine | |
| 4 | 3.77 (1.74, 8.16) |
| 12 | 2.02 (1.21, 3.36) |
| 24 | 1.77 (1.12, 2.80) |
| Text4Health–Peds | |
| Attendance at special immunization sessions | 2.78 (1.10, 6.98) |
Note. AOR = adjusted odds ratio; CI = confidence interval; MCV4 = meningococcal; Tdap = tetanus–diphtheria–acellular pertussis. All analyses controlled for impact of age, race/ethnicity, gender, language preference, and insurance status. For both studies, the reference was the control group.
Text4Health–Peds
We sent 87 families reminders via letter and text messages, and we sent another 87 families only a letter reminder. No significant differences existed between the 2 groups (Table 1). It took an additional estimated 10 hours to design the messages, identify eligible children, and monitor the delivery of text messages. Of the 87 families, 4 had incorrect numbers listed (4.6%), 2 families called for more information, and no families asked to stop receiving text messages.
Significantly more children whose families received a letter and text messages attended a special immunization recall session compared with those who were only sent a letter. According to the intention-to-treat analysis, 21.8% of families who received a text message attended a session compared with 9.2% of those who received just a letter (P = .021). Only the intervention status had an effect on session attendance in multivariable analysis (adjusted odds ratio [AOR] = 2.78; 95% CI = 1.10, 6.98; Table 2). Per-protocol analyses were consistent. Overall, there was no significant difference in attendance among those directed to an immunization site that was their medical home versus one that was not (19.2% vs 10.7%; P = .12).
Among children of intervention families, 20.7% received an Hib dose at 2 weeks compared with 11.5% of those from control families (P = .15). For the subset directed to a session outside their medical home, 18.9% of children of intervention families received a dose at 2 weeks compared with 2.6% of children of control families (P = .028).
DISCUSSION
These data demonstrate the efficacy of text message reminder–recalls for low-income populations who were identified as having a cell phone. Text4Health–Adolescents participants whose parents received text messages were significantly more likely to receive a needed vaccination, and the increase in receipt of vaccination was higher than was the median reported by other researchers, despite our study population having limited resources.4,5 Among our pediatric population, text messaging with paper mailing was more successful than were paper mailings alone in affecting parents's attendance at immunization recall sessions. It also equipped them to attend an immunization recall session that was outside their medical home for nearly half of the sample and that was held during nonregular office hours for all participants. Although studies differed relative to age of patient, vaccine, and study design, both revealed significant and clinically relevant differences between the intervention and control populations. Text messaging has been used for other interventions,19,22,33–35 but together these findings address an important gap in knowledge of the efficacy of text message reminder–recalls for pediatric or adolescent immunizations. These studies also include the first published demonstration of text message implementation linked to an immunization registry.
The positive impact of text message reminders demonstrated in this study differs from previous phone or mail reminder–recall studies conducted in low-income urban populations, which have shown only limited efficacy.11–13,36,37 One commonly reported barrier in low-income populations has been difficulty in reaching families because of frequently changing contact information.11–13,38 In our intervention, only 6% of cell phone numbers were incorrect. It is possible that in urban, low-income communities cell phones may ultimately provide a more accurate means of contacting families than land lines or residential mailings; further longitudinal studies are needed to answer this question. To be most effective, practices would need to make a concerted effort to record cell phone numbers as part of the registration process. During this study, the practice of collecting cell phone information was relatively new, and only approximately 20% of study participants had a cell phone number available. As of December 2010, an estimated 70% of families of children and adolescents who had had a clinic visit in the past year had an active cell phone number in our registration system that was able to receive text messages. A study in another low-income population showed that 92% owned a cell phone, and 96% of those could receive text messages.18 Similar rates of cell phone ownership and text-messaging capability have been seen within this population in a current survey (M. S. S., unpublished data, 2011).
The efficacy of our intervention may reflect intrinsic benefits of text messages compared with other strategies for communicating with families. In a qualitative study, parents reported that text messages solicit attention better than do letters, e-mail, or voicemail.20 Additionally, text messages can provide clinic addresses, phone numbers, and hours of operation directly to parents’ cell phones. The convenience and accessibility of this information may help facilitate visits. Also, in contrast to mail, there is little lag time between when the text message is sent and when it is received. Another likely strength of our intervention was that messages were personalized, created based on parental feedback, and originated from the medical home.20 The potential cost of each message, which can range from no cost to up to 30 cents for those without a text message plan, may have been a barrier; but in these studies, only 1.8% of parents asked to stop receiving messages. Text messaging in health care settings is fairly new in the United States, but some electronic health record systems are already capable of linking with text messaging platforms, allowing providers to automatically identify and notify families of children due for immunizations.39 In addition, there are now commercial providers that offer health-related text messaging.
Text messaging could also have an important public health impact. Multiple, personalized text message reminders can be sent to hundreds or thousands of patients with minimal additional costs or personnel time, particularly compared with paper mailings. For example, text messaging could be linked to a local, city, or state immunization information system or registry. These studies together illustrate the potential use of registry-linked text messages not only as immunization reminders but also as a way to tell patients or families where to receive immunizations, especially if the location is outside their medical home. For example, during previous influenza vaccine shortages, high-risk populations needed to seek vaccination at places other than their medical home, but many did not know where to go.40 Moreover, for the 2009 H1N1 vaccine, departments of health needed to tell the public where vaccine was available; this need could recur in the event of a future pandemic. Finally, even during a regular influenza season, primary care providers may need to notify patients of alternative hours or locations for vaccination.41 Text messages may therefore be a useful way for local health departments or health care organizations to inform large populations where to be vaccinated.
Limitations
Some limitations warrant comment. Despite mandated reporting to the Citywide Immunization Registry in New York City, some participants may have received undocumented vaccines. However, this should have equally affected both intervention and control groups. Second, these studies focused on parents with a recorded cell phone number. Although our system has increasingly had more cell phone numbers recorded, it is difficult to determine how parents with documented cell phone numbers differed from those without, beyond demographics. It is possible that parents with cell phones may also be more or less likely to have their child vaccinated, although that bias should have affected both intervention and control groups equally. Future studies could assess the impact of text messaging in clinic populations with higher numbers of recorded cell phones. Finally, race/ethnicity was recorded by clinical staff, but we were unable to ensure that the correct racial/ethnic identity was reported for each participant. Regardless, our samples for both studies were located in a primarily Latino, low-income community, possibly limiting generalizability of findings. However, low-income minority populations have been traditionally at higher risk for underimmunization than is the general population; thus, they represent an important community for targeted interventions.
For the Text4Health–Adolescents study, the control group did not receive a different type of reminder; this is common in other reminder–recall interventions,11,42 and future studies could compare the efficacy of text messaging with other types of reminders. Although our intervention and control sites may have differed at baseline in ways that could have affected adherence to immunization recommendations, all sites for both studies had similar immunization rates and numbers of patients served, and all sites were part of the same care network serving similar populations. Moreover, the nested ANOVA did not detect an effect attributable to baseline variability between sites for all outcomes; we did note a change in significance for receipt of any vaccine at 24 weeks. We chose not to randomize at the individual level to avoid a carryover effect, in which parents in the intervention group could raise vaccine awareness among providers also vaccinating control participants.
In addition, we had anticipated having equal numbers of eligible patients in each group in the Text4Health–Adolescents study, but ultimately we had too few control participants to match by gender and age. Reanalysis including only patients with a matched control achieved similar results for all analyses, although receipt of any vaccine was only borderline significant at 24 weeks when the entire sample (intention to treat) was used. Receipt of any vaccine was significant among those who completed the protocol as dictated (per protocol). In addition, when we controlled for the effect of site with the matched sample, we found the intervention effect on receipt of any vaccine was not significant at 12 weeks only when we used the entire sample (intention to treat); the intervention effect on receipt of any vaccine at 12 weeks was significant in the per protocol sample. Finally, we did not conduct a practice-wide intervention, and we limited the sample to patients with any visit in the past year. We chose this approach based on the clinics’ capacity to immunize more patients in a short period of time. Future studies could build upon our reported findings with a population-based methodology.
In the Text4Health–Peds study, the number of reminders in the text-messaging group was higher than that in the paper-mailing–alone group; yet a strength of text messaging is the ability to send multiple reminders at minimal additional cost and effort. In addition, although attendance rates among those sent texts were significantly higher, the absolute percentages were not large for either group. This recall occurred during the 2009 H1N1 epidemic, and families may have chosen to defer medical visits, even for a needed immunization. In addition, although recall sessions occurred on evenings or Saturdays, selected times may not have been optimal for parents.
Conclusions
Within their scopes, these complementary studies suggest that text messaging can be successfully used to deliver parental immunization-related reminder–recalls in urban populations. These studies highlight how an emerging technology, text messaging, can be used to make a beneficial impact on an important public health issue.
Acknowledgments
The adolescent study was supported by the Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services (grant R40MC08961).
V. I. Rickert is on the Adolescent and Adult Vaccine National Advisory Board of Merck and Company Inc. and has received research funding from Merck unrelated to this study. V. I. Rickert also serves on the Adolescent Immunization Leadership Council supported by Sanofi Pasteur Inc. M. Lara has filed a patent application on health care alerts and is a founder of a mobile health venture that had no role in this study.
We acknowledge NewYork Presbyterian Hospital for its support of the EzVac system and the NewYork Presbyterian Hospital Ambulatory Care Network clinic practices for participating in the studies. Balendu DasGupta, MS, Oscar Peña, JD, and Harrison Fox, MPH, made substantial contributions to the technical and administrative aspects of the studies.
Note. The contents of the study are solely the responsibility of the authors and do not necessarily represent the official views of the funding agency.
Human Participant Participation
This study was approved by the Columbia University Medical Center institutional review board.
References
- 1.Centers for Disease Control and Prevention Recommendations of the Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the American Academy of Family Physicians: use of reminder and recall by vaccination providers to increase vaccination rates. MMWR Morb Mortal Wkly Rep. 1998;47(34):715–717 [PubMed] [Google Scholar]
- 2.National Vaccine Advisory Committee Standards for child and adolescent immunization practices. Pediatrics. 2003;112(4):958–963 [PubMed] [Google Scholar]
- 3.Middleman AB, Rosenthal SL, Rickert VI, Neinstein L, Fishbein DB, D'Angelo L.Adolescent immunizations: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 2006;38(3):321–327 [DOI] [PubMed] [Google Scholar]
- 4.Briss PA, Rodewald LE, Hinman ARet al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(1, suppl):97–140 [DOI] [PubMed] [Google Scholar]
- 5.Jacobson VJ, Szilagyi P.Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention 2011 child and adolescent immunization schedules. Available at: http://www.cdc.gov/vaccines/recs/schedules/child-schedule.htm#printable. Accessed March 23, 2011
- 7.Interim recommendations for the use of Haemophilus influenzae type b (Hib) conjugate vaccines related to the recall of certain lots of Hib-containing vaccines (PedvaxHIB® and Comvax®). MMWR Dispatch. 2007;56:1–2 [PubMed] [Google Scholar]
- 8.Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America's Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). Washington, DC: US Dept of Education, National Center for Education Statistics; 2006 [Google Scholar]
- 9.Lannon C, Brack V, Stuart Jet al. What mothers say about why poor children fall behind on immunizations. A summary of focus groups in North Carolina. Arch Pediatr Adolesc Med. 1995;149(10):1070–1075 [DOI] [PubMed] [Google Scholar]
- 10.Scott TL, Gazmararian JA, Williams MV, Baker DW.Health literacy and preventive health care use among Medicare enrollees in a managed care organization. Med Care. 2002;40(5):395–404 [DOI] [PubMed] [Google Scholar]
- 11.Szilagyi PG, Schaffer S, Barth Ret al. Effect of telephone reminder/recall on adolescent immunization and preventive visits: results from a randomized clinical trial. Arch Pediatr Adolesc Med. 2006;160(2):157–163 [DOI] [PubMed] [Google Scholar]
- 12.Daley MF, Steiner JF, Brayden RM, Xu S, Morrison S, Kempe A.Immunization registry-based recall for a new vaccine. Ambul Pediatr. 2002;2(6):438–443 [DOI] [PubMed] [Google Scholar]
- 13.Irigoyen MM, Findley S, Wang Det al. Challenges and successes of immunization registry reminders at inner-city practices. Ambul Pediatr. 2006;6(2):100–104 [DOI] [PubMed] [Google Scholar]
- 14.CTIA-The Wireless Association Wireless Quick Facts December 2010, CTIA, The Wireless Association. Available at: http://www.ctia.org/media/index.cfm/AID/10323. Accessed March 22, 2011
- 15.Blumberg SJ, Luke JV, Cynamon ML.Telephone coverage and health survey estimates: evaluating the need for concern about wireless substitution. Am J Public Health. 2006;96(5):926–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blumberg SJ, Luke JV.Reevaluating the need for concern regarding noncoverage bias in landline surveys. Am J Public Health. 2009;99(10):1806–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castano P, Andres R, Lara M, Westhoff C.Assessing feasibility of text messaging to improve medication adherence. Obstet Gynecol. 2006;107(4 suppl):40S [Google Scholar]
- 18.Ahlers-Schmidt CR, Chesser A, Hart T, Paschal A, Nguyen T, Wittler RR.Text messaging immunization reminders: feasibility of implementation with low-income parents. Prev Med. 2010;50(5–6):306–307 [DOI] [PubMed] [Google Scholar]
- 19.Vilella A, Bayas JM, Diaz MTet al. The role of mobile phones in improving vaccination rates in travelers. Prev Med. 2004;38(4):503–509 [DOI] [PubMed] [Google Scholar]
- 20.Kharbanda EO, Stockwell MS, Fox HW, Rickert VI.Text4Health: a qualitative evaluation of parental readiness for text message immunization reminders. Am J Public Health. 2009;99(12):2176–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharbanda EO, Vargas CY, Castano PM, Lara M, Martinez RA, Stockwell MS.Exploring pregnant women's views on influenza vaccination and educational text messages. Prev Med. 2011;52(1):75–77 [DOI] [PubMed] [Google Scholar]
- 22.Kharbanda EO, Stockwell MS, Fox HW, Andres R, Lara M, Rickert VI.Text message reminders to promote human papillomavirus vaccination. Vaccine. 2011;29(14):2537–2541 [DOI] [PubMed] [Google Scholar]
- 23.Papadouka V, Zucker J, Balter S, Reddy V, Moore K, Metroka A.Impact of childhood hepatitis A vaccination: New York City. Presented at: 41st National Immunization Conference; Kansas City, MO; March 2007. Available at: http://cdc.confex.com/cdc/nic2007/recordingredirect.cgi/id/2057. Accessed December 21, 2010 [Google Scholar]
- 24.Metroka AE, Hansen MA, Papadouka V, Zucker JR.Using an immunization information system to improve accountability for vaccines distributed through the Vaccines for Children program in New York City, 2005–2008. J Public Health Manag Pract. 2009;15(5):E13–E21 [DOI] [PubMed] [Google Scholar]
- 25.New York State Department of Health Sixth grade entry law for Tdap immunizations—provider letter. Available at: http://www.nyhealth.gov/prevention/immunization/providers/sixth_grade_entry_law_for_tdap.htm. Accessed December 21, 2010
- 26.Findley S, Guzman L, Lara M, Sanchez M. A pilot test of cell phone text messages to remind parents to take their children for immunizations. Presented at: 41st National Immunization Conference; Kansas City, MO; March 2007 [Google Scholar]
- 27.Kahn JA, Zimet GD, Bernstein DIet al. Pediatricians’ intention to administer human papillomavirus vaccine: the role of practice characteristics, knowledge, and attitudes. J Adolesc Health. 2005;37(6):502–510 [DOI] [PubMed] [Google Scholar]
- 28.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM.Parental vaccine safety concerns in 2009. Pediatrics. 2010;125(4):654–659 [DOI] [PubMed] [Google Scholar]
- 29.Daley MF, Crane LA, Chandramouli Vet al. Misperceptions about influenza vaccination among parents of healthy young children. Clin Pediatr (Phila). 2007;46(5):408–417 [DOI] [PubMed] [Google Scholar]
- 30.Zar JH. Biostatistical Analysis. 4th ed Upper Saddle River, NJ: Prentice Hall; 1998 [Google Scholar]
- 31.Centers for Disease Control and Prevention Continued shortage of Haemophilus influenzae type b (Hib) conjugate vaccines and potential implications for Hib surveillance—United States, 2008. MMWR Morb Mortal Wkly Rep. 2008;57(46):1252–1255 [PubMed] [Google Scholar]
- 32.White KE, Pabst LJ, Cullen KA.Up-to-date Haemophilus influenzae type b vaccination coverage during a vaccine shortage. Pediatrics. 2011;127(3):e707–e712 [DOI] [PubMed] [Google Scholar]
- 33.Fairhurst K, Sheikh A.Texting appointment reminders to repeated non-attenders in primary care: randomised controlled study. Qual Saf Health Care. 2008;17(5):373–376 [DOI] [PubMed] [Google Scholar]
- 34.Franklin VL, Greene A, Waller A, Greene SA, Pagliari C.Patients’ engagement with “Sweet Talk”—a text messaging support system for young people with diabetes. J Med Internet Res. 2008;10(2):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodgers A, Corbett T, Bramley Det al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tob Control. 2005;14(4):255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeBaron CW, Starnes DM, Rask KJ.The impact of reminder–recall interventions on low vaccination coverage in an inner-city population. Arch Pediatr Adolesc Med. 2004;158(3):255–261 [DOI] [PubMed] [Google Scholar]
- 37.Hambidge SJ, Davidson AJ, Phibbs SLet al. Strategies to improve immunization rates and well-child care in a disadvantaged population: a cluster randomized controlled trial. Arch Pediatr Adolesc Med. 2004;158(2):162–169 [DOI] [PubMed] [Google Scholar]
- 38.Kempe A, Lowery NE, Pearson KAet al. Immunization recall: effectiveness and barriers to success in an urban teaching clinic. J Pediatr. 2001;139(5):630–635 [DOI] [PubMed] [Google Scholar]
- 39.eClinicalWorks eClinicalWorks announces eClinicalWorks P2P. Business Wire. Available at: http://findarticles.com/p/articles/mi_m0EIN/is_20090913/ai_n35649438. Published September 13, 2009. Accessed November 10, 2010
- 40.Centers for Disease Control and Prevention Experiences with obtaining influenza vaccination among persons in priority groups during a vaccine shortage—United States, October–November, 2004. MMWR Morb Mortal Wkly Rep. 2004;53(49):1153–1155 [PubMed] [Google Scholar]
- 41.Rand CM, Szilagyi PG, Yoo BK, Auinger P, Albertin C, Coleman MS.Additional visit burden for universal influenza vaccination of US school-aged children and adolescents. Arch Pediatr Adolesc Med. 2008;162(11):1048–1055 [DOI] [PubMed] [Google Scholar]
- 42.Kempe A, Daley MF, Barrow Jet al. Implementation of universal influenza immunization recommendations for healthy young children: results of a randomized, controlled trial with registry-based recall. Pediatrics. 2005;115(1):146–154 [DOI] [PubMed] [Google Scholar]