Abstract
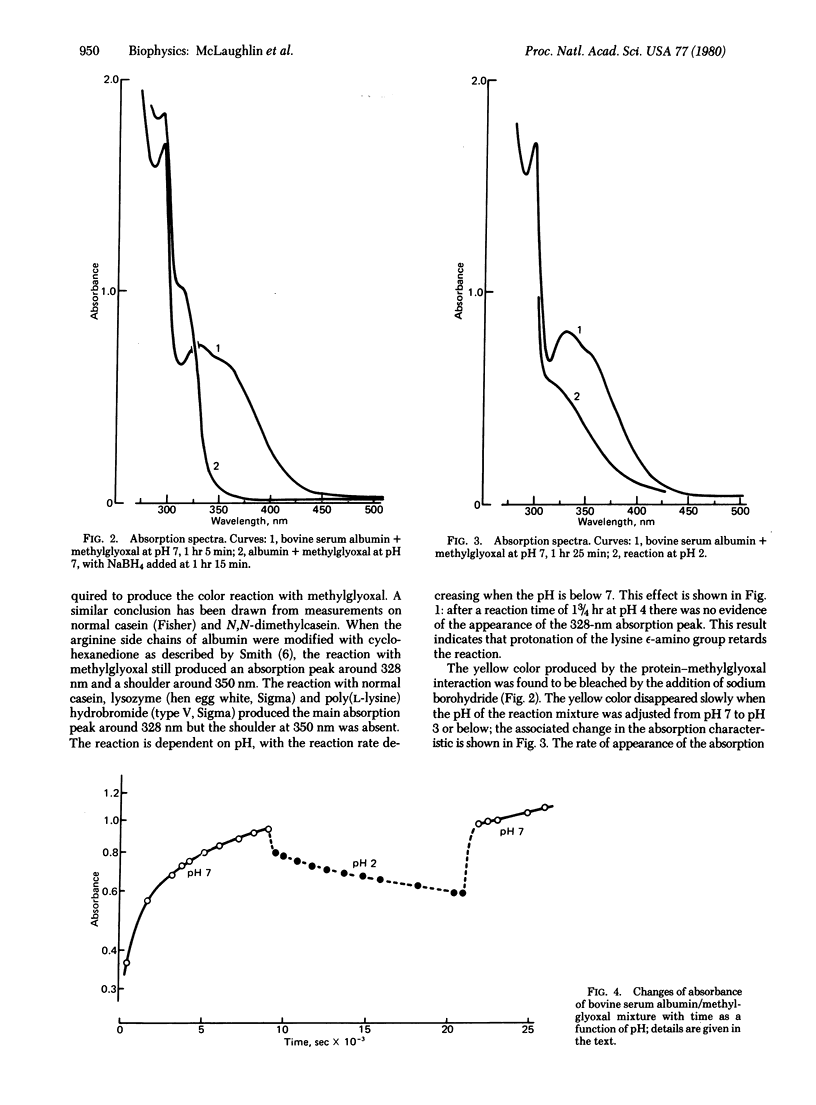
Spectroscopic measurements are reported for the effects of pH, time, solvent, and chemical modification of arginine and lysine side chains on the reaction of proteins with methylglyoxal. The reaction responsible for the appearance of a brown coloration and increased submolecular electronic activity in the proteins involves the epsilon-amino groups of the lysine residues. It is concluded that the primary step in the reaction involves the formation of a Schiff base linkage between the lysine side chain and methylglyoxal. These findings reaffirm the concept that, by the formation of Schiff bases, aldehydes can act as electron acceptors in charge transfer interactions with proteins.
Full text
PDF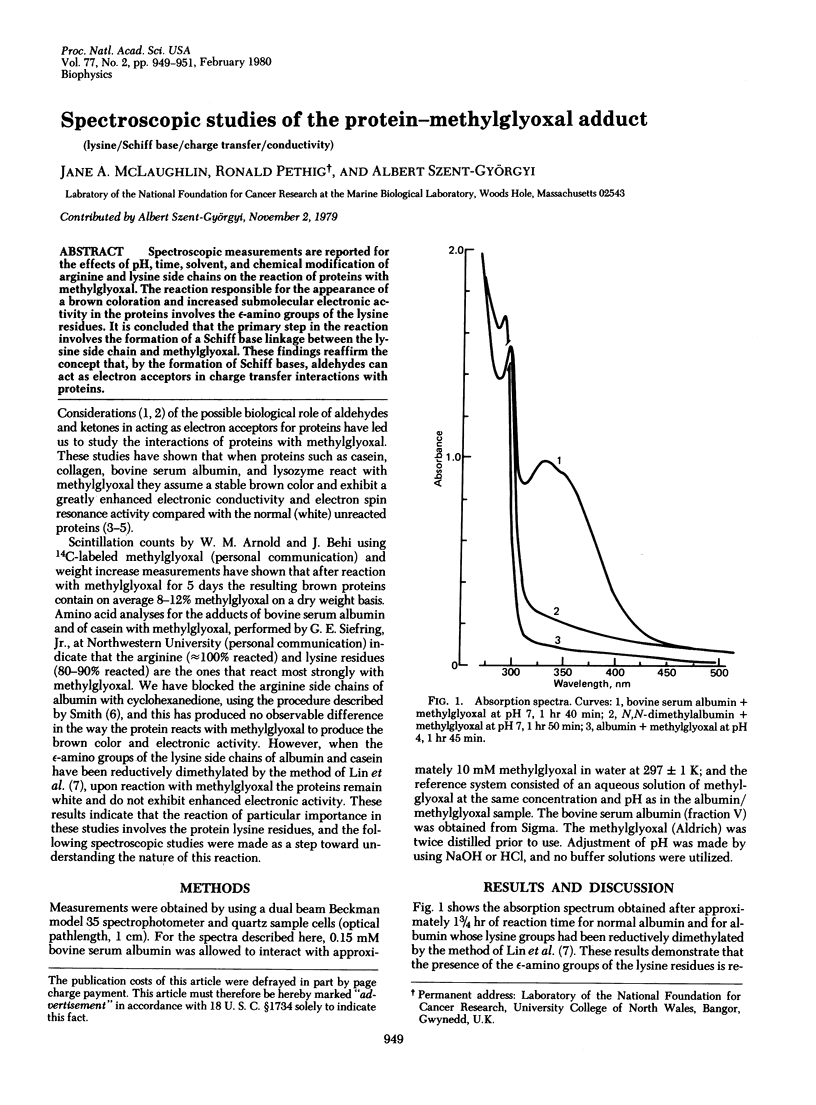

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonsignore A., Leoncini G., Audisio G., Zetta L., Ferruti P. Characterization of the polymer formed from methylglyoxal in the presence of L(+)-lysine. Ital J Biochem. 1977 Mar-Apr;26(2):162–168. [PubMed] [Google Scholar]
- Lin Y., Means G. E., Feeney R. E. The action of proteolytic enzymes on N,N-dimethyl proteins. Basis for a microassay for proteolytic enzymes. J Biol Chem. 1969 Feb 10;244(3):789–793. [PubMed] [Google Scholar]
- Otto P., Ladik J., Laki K., Szent-Györgyi A. Internal charge transfer in proteins to the Schiff bases of their lysine side chains. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3548–3550. doi: 10.1073/pnas.75.8.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethig R., Szent-Györgyi A. Electronic properties of the casein-methylglyoxal complex. Proc Natl Acad Sci U S A. 1977 Jan;74(1):226–228. doi: 10.1073/pnas.74.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. L. Reversible blocking at arginine by cyclohexanedione. Methods Enzymol. 1977;47:156–161. doi: 10.1016/0076-6879(77)47019-8. [DOI] [PubMed] [Google Scholar]


