Abstract
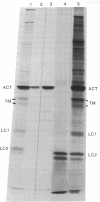
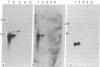
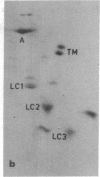
The construction and partial characterization of recombinant bacterial plasmids carrying DNA sequences that hybridize with rat skeletal muscle actin and a myosin light chain mRNA is described. DNA of one clone hybridizes specifically with the muscle-specific alpha-actin mRNA. Three plasmid clones contain DNA inserts that hybridize with muscle as well as with nonmuscle actin mRNA. A fifth plasmid contains sequences complementary to mRNA coding for myosin light chain 2. DNA of this plasmid hybridizes specifically with RNA extracted from muscle and differentiated muscle cultures but not with RNA extracted from proliferating mononucleated myogenic cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Carmon Y., Neuman S., Yaffe D. Synthesis of tropomyosin in myogenic cultures and in RNA-directed cell-free systems: qualitative changes in the polypeptides. Cell. 1978 Jun;14(2):393–401. doi: 10.1016/0092-8674(78)90124-1. [DOI] [PubMed] [Google Scholar]
- Chi J. C., Rubinstein N., Strahs K., Holtzer H. Synthesis of myosin heavy and light chains in muscle cultures. J Cell Biol. 1975 Dec;67(3):523–537. doi: 10.1083/jcb.67.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym H., Turner D. C., Eppenberger H. M., Yaffe D. Creatine kinase isoenzyme transition in actinomycin D-treated differentiating muscle cultures. Exp Cell Res. 1978 Apr;113(1):15–21. doi: 10.1016/0014-4827(78)90082-4. [DOI] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Crkvenjakov R., Boedtker H., Doty P. Construction and characterization of a 2.5-kilobase procollagen clone. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5417–5421. doi: 10.1073/pnas.75.11.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough J., Bischoff R. Differentiation of creatine phosphokinase during myogenesis: quantitative fractionation of isozymes. Dev Biol. 1977 Jun;57(2):330–344. doi: 10.1016/0012-1606(77)90219-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin synthesis in cultures of differentiating chicken embryo skeletal muscle. Dev Biol. 1972 Oct;29(2):113–138. doi: 10.1016/0012-1606(72)90050-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- REPORTER M. C., KONIGSBERG I. R., STREHLER B. L. Kinetics of accumulation of creatine phosphokinase activity in developing embryonic skeletal muscle in vivo and in monolayer culture. Exp Cell Res. 1963 Apr;30:410–417. doi: 10.1016/0014-4827(63)90313-6. [DOI] [PubMed] [Google Scholar]
- Roy R. K., Sreter F. A., Sarkar S. Changes in tropomyosin subunits and myosin light chains during development of chicken and rabbit striated muscles. Dev Biol. 1979 Mar;69(1):15–30. doi: 10.1016/0012-1606(79)90271-9. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Sreter F., Holtzer S., Gergely J., Holtzer H. Some properties of embryonic myosin. J Cell Biol. 1972 Dec;55(3):586–594. doi: 10.1083/jcb.55.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sréter F. A., Bálint M., Gergely J. Structural and functional changes of myosin during development: comparison with adult fast, slow and cardiac myosin. Dev Biol. 1975 Oct;46(2):317–325. doi: 10.1016/0012-1606(75)90108-6. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Rich A. Chick cytoplasmic actin and muscle actin have different structural genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2346–2350. doi: 10.1073/pnas.73.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Maier V., Eppenberger H. M. Creatine kinase and aldolase isoenzyme transitions in cultures of chick skeletal muscle cells. Dev Biol. 1974 Mar;37(1):63–89. doi: 10.1016/0012-1606(74)90170-5. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Mammalian cytoplasmic actins are the products of at least two genes and differ in primary structure in at least 25 identified positions from skeletal muscle actins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1106–1110. doi: 10.1073/pnas.75.3.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2018–2022. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka Z., Yaffe D. Synthesis of myosin light chains and accumulation of translatable mRNA coding for light chain-like polypeptides in differentiating muscle cultures. Differentiation. 1977 Oct 13;8(3):133–143. doi: 10.1111/j.1432-0436.1977.tb00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7(3):159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]