Abstract
Background
Our previous studies have shown that complete Freund’s adjuvant (CFA)-induced masseter inflammation and microinjection of the pro-inflammatory cytokine interleukin-1β (IL-1β) into the subnucleus interpolaris/subnucleus caudalis transition zone of the spinal trigeminal nucleus (Vi/Vc) can induce contralateral orofacial hyperalgesia in rat models. We have also shown that contralateral hyperalgesia is attenuated with a lesion of the rostral ventromedial medulla (RVM), a critical site of descending pain modulation. Here we investigated the involvement of the RVM-Vi/Vc circuitry in mediating contralateral orofacial hyperalgesia after an injection of CFA into the masseter muscle.
Results
Microinjection of the IL-1 receptor antagonist (5 nmol, n=6) into the ipsilateral Vi/Vc attenuated the CFA-induced contralateral hyperalgesia but not the ipsilateral hyperalgesia. Intra-RVM post-treatment injection of the NK1 receptor antagonists, RP67580 (0.5-11.4 nmol) and L-733,060 (0.5-11.4 nmol), attenuated CFA-induced bilateral hyperalgesia and IL-1β induced bilateral hyperalgesia. Serotonin depletion in RVM neurons prior to intra-masseter CFA injection prevented the development of contralateral hyperalgesia 1–3 days after CFA injection. Inhibition of 5-HT3 receptors in the contralateral Vi/Vc with direct microinjection of the select 5-HT3 receptor antagonist, Y-25130 (2.6-12.9 nmol), attenuated CFA-induced contralateral hyperalgesia. Lesions to the ipsilateral Vc prevented the development of ipsilateral hyperalgesia but did not prevent the development of contralateral hyperalgesia.
Conclusions
These results suggest that the development of CFA-induced contralateral orofacial hyperalgesia is mediated through descending facilitatory mechanisms of the RVM-Vi/Vc circuitry.
Keywords: Pain, Descending modulation, Serotonin, Substance P, IL-1β, Vi/Vc transition zone, Rat
Background
Orofacial muscle pain is a debilitating disorder that diminishes the quality of life and seriously inhibits the health of the patient by impairing a person’s ability to eat and drink. Trigeminal pain also spreads to wide orofacial areas and is a serious clinical problem. Reports have shown that patients with unilateral myofascial temporomandibular muscle and joint disorders (TMD) experience bilateral hyperalgesia [1,2]. Very little research has focused on the possible circuitry and mechanisms involved in the development of contralateral orofacial hyperalgesia after deep tissue orofacial injury.
Traditionally, orofacial pain research has focused on the activation of the subnucleus caudalis (Vc) of the spinal trigeminal nucleus (STN) [3-7]. Despite the emphasis of orofacial pain research in the Vc, the subnucleus interpolaris/subnucleus caudalis transition zone (Vi/Vc) of the STN has been more recently shown to be involved in mechanisms of deep tissue trigeminal pain [8-11]. We have shown that complete Freund’s adjuvant (CFA)-induced masseter inflammation and intra-Vi/Vc microinjection of the pro-inflammatory cytokine, interleukin-1β (IL-1β), induce contralateral orofacial hyperalgesia in rat [12,13]. However, contralateral hyperalgesia is not seen with intra-Vc IL-1β microinjection. Similar to the behavioral findings, our previous studies showed that CFA-induced masseter inflammation results in bilateral expression of Fos protein in the Vi/Vc and ipsilateral expression of Fos protein in the Vc [14].
We have also shown that IL-1β-induced contralateral hyperalgesia is attenuated with ibotenic acid lesions in the rostral ventromedial medulla (RVM), a midline site that is critical in descending pain modulation [12]. Our previous studies have shown that bilateral reciprocal connections exist from the Vi/Vc to the RVM [14]. This suggests that activation of RVM neurons may be necessary for the development of contralateral hyperalgesia. Immunohistochemical localization shows moderate amounts of the substance P receptor, neurokinin-1 receptor (NK1-R), in the RVM [15-17]. Research has shown that Substance P activation of NK1-R in the RVM is involved in descending facilitation of pain [18-25]. We have also shown that the serotonin system is involved in descending facilitation in the spinal cord after tissue and nerve injury [26]. Furthermore, spinal 5-HT3 receptor activation has been shown to contribute to the development of hyperalgesia after spinal cord injury [27,28].
Based on these studies of descending facilitation and behavioral hyperalgesia at the spinal and trigeminal levels, we propose that the activation of the NK1-R in the RVM by input from the ipsilateral Vi/Vc results in enhanced release of 5-HT from descending RVM-Vi/Vc neurons. Activation of 5-HT3 receptors in the contralateral Vi/Vc ultimately leads to contralateral hyperalgesia.
Results
Contralateral orofacial hyperalgesia is mediated via IL-1R activation in the ipsilateral Vi/Vc
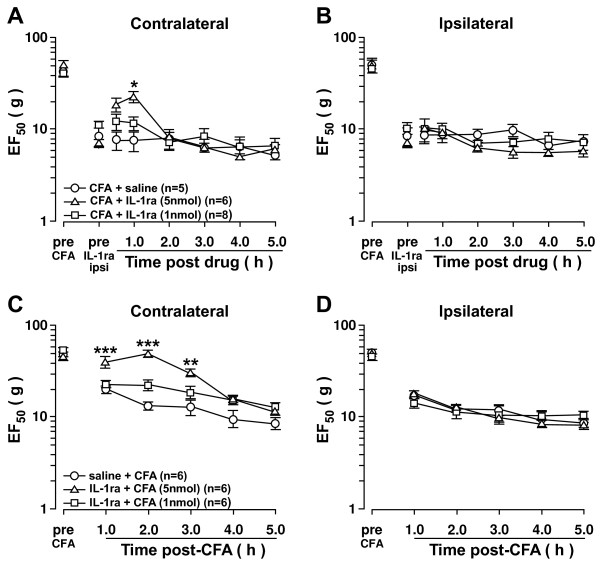
Our previous studies have shown that CFA injection into the masseter muscle and IL-1β injections into the Vi/Vc transition zone generate bilateral mechanical hyperalgesia as seen by the significant decreases in EF50 values [12,13]. To test whether activation of IL-1 receptors (IL-1R) in the Vi/Vc is involved in CFA-induced contralateral hyperalgesia, an IL-1R antagonist (IL-1ra) was injected. Microinjection of IL-1ra (5 nmol, n=6) into the ipsilateral Vi/Vc 24 h after CFA treatment attenuated contralateral hyperalgesia 1 h after microinjection as compared to saline-treated rats (Figure 1A). Intra-Vi/Vc pretreatment of IL-1ra (5 nmol, n=6) also attenuated CFA-induced contralateral hyperalgesia compared to saline-treated rats 1 h after CFA injection (Figure 1C). Pre-treatment and post-treatment of IL-1ra did not attenuate ipsilateral hyperalgesia (Figure 1B, D). These results suggest that IL-1R in the ipsilateral Vi/Vc is involved in CFA-induced contralateral orofacial hyperalgesia but not the ipsilateral orofacial hyperalgesia.
Figure 1.
Inhibition of IL-1R in the ipsilateral Vi/Vc attenuates contralateral hyperalgesia. Contralateral orofacial hyperalgesia is attenuated by IL-1R inhibition in the ipsilateral Vi/Vc. A, B. Post-treatment: CFA (0.05 ml; 1:1 oil/saline) was injected unilaterally into the masseter muscle 24 h prior to IL-1ra microinjection into the ipsilateral Vi/Vc (0.5μl). C, D. Pre-treatment: CFA (0.05 ml; 1:1 oil/saline) was injected unilaterally into the masseter muscle 15 min after IL-1ra microinjection into the ipsilateral Vi/Vc (0.5 μl). Pre-treatment and post-treatment of IL-1ra attenuated CFA-induced contralateral hyperalgesia as compared to saline treated rats. IL-1ra did not attenuate ipsilateral hyperalgesia. *: IL-1ra (5 nmol) vs saline. *: p<;0.05; **: p<;0.01; ***: p<;0.001 (ANOVA with repeated measures and Student Neuman-Keuls post-hoc test).
NK1-R activation in the RVM is critical to the development of bilateral orofacial hyperalgesia
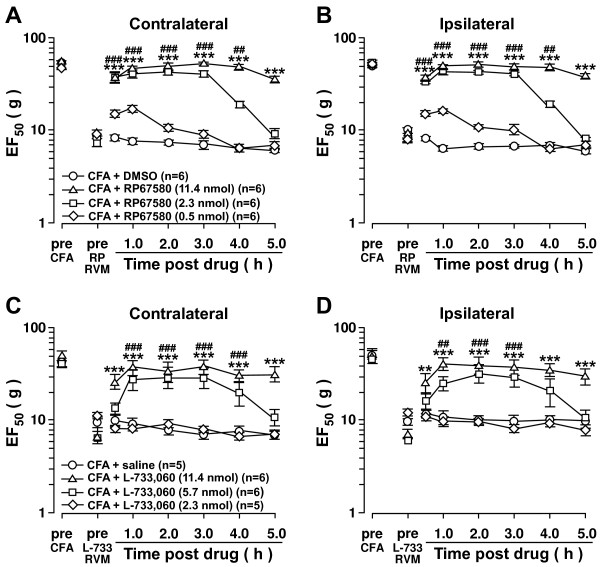
Previously we showed that intra-Vi/Vc IL-1β-induced contralateral orofacial hyperalgesia was mediated via RVM neurons [12]. The RVM is a midline structure that lacks laterality and projects neurons bilaterally to the ventral Vi/Vc [14]. NK1-R activation in the RVM has been shown to contribute to capsaicin-induced hyperalgesia [22]. Similarly, we showed that NK1-R activation in the RVM is involved in mediating behavioral hyperalgesia after hindpaw inflammation [24]. To test whether NK1-R activation in the RVM was involved in orofacial contralateral hyperalgesia, two NK1-R antagonists, RP67580 and L-733,060 were microinjected into the RVM. RP67580 attenuated CFA-induced bilateral hyperalgesia in a dose-dependent manner. Attenuation of hyperalgesia was seen 0.5-5 h post-injection with the highest dose of RP67580 (11.4 nmol; n=6) on the contralateral side (Figure 2A) and ipsilateral side (Figure 2B). Similar results were observed using the NK1-R antagonist L-733,060 (Figure 2C, D).
Figure 2.
Inhibition of NK1-R in the RVM attenuates inflammatory bilateral hyperalgesia. Intra-RVM microinjection of NK1-R antagonists attenuates CFA-induced bilateral hyperalgesia. CFA (0.05 ml; 1:1 oil/saline) was injected unilaterally into the masseter muscle 24 h prior to RP67580 (A, B) and L-733,060 (C, D) microinjection into the RVM (0.5 μl). +: lowest dose vs. saline. #: middle dose vs. saline. *: highest dose vs. saline. +,#,*: p<;0.05; ++,##,**: p<;0.01; +++,###,***: p<;0.001 (ANOVA with repeated measures and Student Neuman-Keuls post-hoc test).
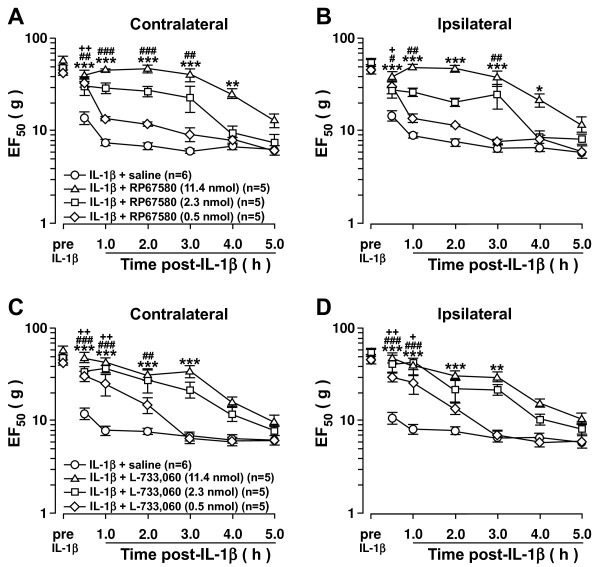
Inhibition of NK1-R activation in the RVM also attenuated IL-1β (160 fmol) – induced bilateral orofacial hyperalgesia. Microinjection of RP67580 and L-733,060 into the RVM 15 min after IL-1β injection into the Vi/Vc resulted in a dose-dependent attenuation of bilateral hyperalgesia. Application of RP67580 (11.4 nmol; n=5) resulted in attenuation of hyperalgesia 0.5-4 h post-injection on the contralateral side (Figure 3A) and ipsilateral side (Figure 3B). Similar results were observed using NK1-R antagonist L-733,060 (Figure 3C, D). These results suggest that contralateral and ipsilateral orofacial hyperalgesia are mediated by NK1-R activation in the RVM.
Figure 3.
Inhibition of NK1-R in the RVM attenuates IL-1β-induced bilateral hyperalgesia. Intra-RVM microinjection of NK1-R antagonists attenuates intra-Vi/Vc IL-1β-induced bilateral hyperalgesia. IL-1β (160 fmol/0.5 μl) was microinjected unilaterally into the Vi/Vc 15 min prior to RP67580 (A, B) and L-733,060 (C, D) microinjection into the RVM (0.5 μl). +: lowest dose vs. saline. #: middle dose vs. saline. *: highest dose vs. saline. +,#,*: p<;0.05; ++,##,**: p<;0.01; +++,###,***: p<;0.001 (ANOVA with repeated measures and Student Neuman-Keuls post-hoc test).
5-HT depletion in the RVM inhibits the development of CFA-induced contralateral orofacial hyperalgesia
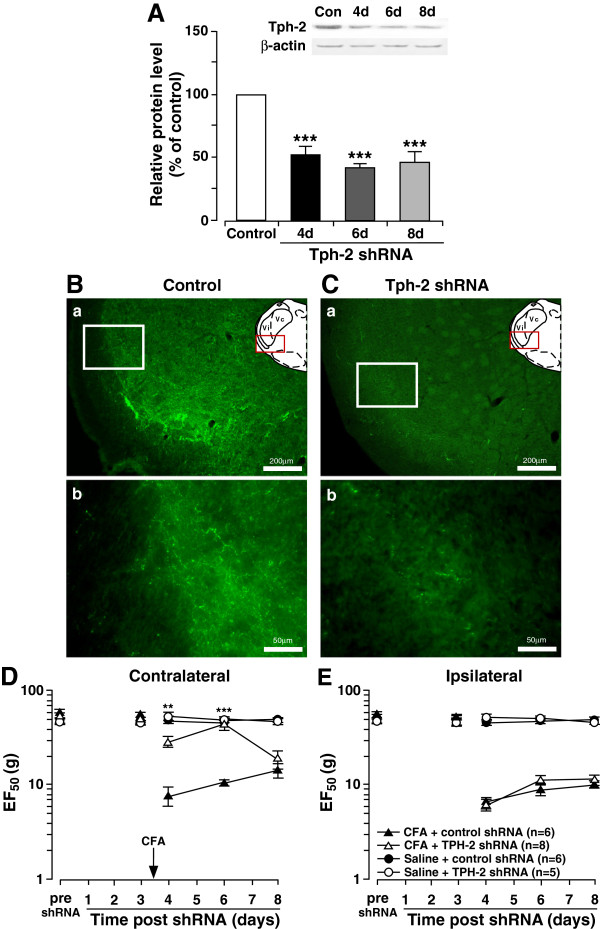
The results suggest that NK1-R activation of RVM neurons may lead to descending facilitation of pain on the contralateral side and ipsilateral side. We previously showed that depletion of 5-HT in the RVM neurons inhibits injury-induced hyperalgesia [26] and an intrathecal 5-HT3 receptor antagonist blocked intra-RVM SP-induced hyperalgesia [24]. Thus, we tested the hypothesis that NK1-R activation in the RVM could ultimately result in 5-HT-dependent descending facilitation to the contralateral Vi/Vc. To evaluate the role of 5-HT in the development of contralateral orofacial hyperalgesia, down regulation of tryptophan hydroxylase-2 (Tph-2), a rate limiting enzyme for 5-HT synthesis in RVM neurons was achieved by RNA interference. Since the RVM is a midline structure that projects bilaterally, RNA interference would result in bilateral 5-HT depletion at the Vi/Vc. In CFA-induced hyperalgesic rats, western blot showed that Tph-2 expression in the RVM was significantly down regulated at 4–8 days after Tph-2 shRNA administration when compared to control (n=4; p<;0.001) (Figure 4A). Immunostaining at 4 days after shRNA administration shows a reduction of 5-HT immunoreactivity in the ventral Vi/Vc axons of CFA-induced hyperalgesic rats when compared to control shRNA (Figure 4B, C). 5-HT depletion inhibits the development of CFA-induced contralateral orofacial hyperalgesia 4–6 days after CFA injection (Figure 4D). However, 5-HT depletion did not inhibit development of ipsilateral hyperalgesia after CFA masseter muscle injection (Figure 4E). These data suggests that 5-HT-dependent descending facilitation of pain is necessary for the development of contralateral hyperalgesia but not the ipsilateral hyperalgesia.
Figure 4.
5-HT depletion in the RVM prevents the development of contralateral hyperalgesia. RNAi of Tph-2 in the RVM prevents the development of CFA-induced contralateral hyperalgesia. A. Western blot analysis of Tph-2 in the RVM. CFA (0.05 ml; 1:1 oil/saline) injected unilaterally into the masseter muscle 3 days after shRNA administration. Tph-2 protein expression was measured in rats at 4 day (n=4), 6 days (n=4) and 8 days (n=4) after shRNA was administered. Tph-2 protein expression was measured in rats given control shRNA (n=4) 4 days after the shRNA was administered. B, C. Immunohistochemical fluorescent staining of 5-HT in the Vi/Vc of shRNA treated rats (n=3) 24 h after CFA administration shows decreased 5-HT expression in the Vi/Vc following Tph-2 depletion in rats as compared to control shRNA (n=3). CFA was administered 3 days after shRNA administration. (Ba: control shRNA, 10x; Bb: control shRNA,40x. Ca: Tph-2 shRNA,10x; Cb: Tph-2 shRNA,40x). D, E. Mechanical hyperalgesia was tested in 5-HT depleted rats with CFA-induced hyperalgesia. Control or Tph-2 shRNA was administered and CFA was injected 3 days after. Mechanical hyperalgesia was inhibited in 5-HT depleted rats as compared to control on the contralateral side (D), but not the ipsilateral side (E). *: CFA + Tph-2 shRNA vs. CFA + control shRNA, p<;0.05; **: p<;0.01; ***: p<;0.001 (ANOVA with repeated measures and Student Neuman-Keuls post-hoc test).
Vi/Vc 5-HT3 receptor activation is necessary for CFA-induced contralateral orofacial hyperalgesia
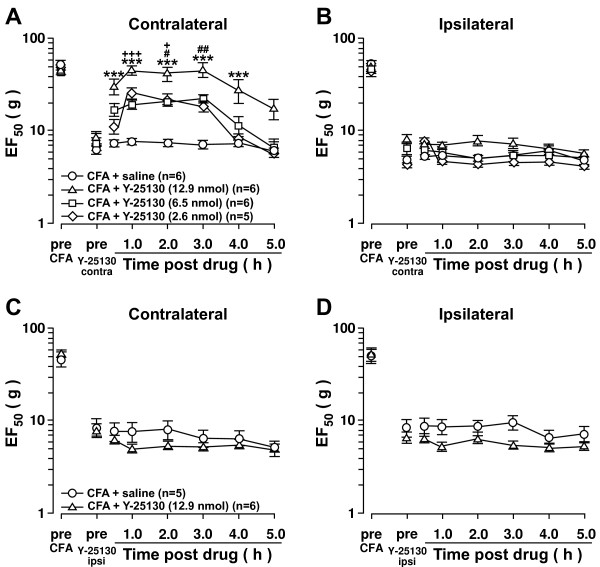
Previous studies have shown that 5-HT3 receptor expression is upregulated in the spinal cord and trigeminal nuclei in animal models of persistent pain [29,30]. In similar models of persistent pain, hyperalgesia is attenuated following 5-HT3 receptor inhibition [28,29,31]. To test whether the 5-HT3 receptor was involved in contralateral orofacial hyperalgesia, a 5-HT3 receptor antagonist, Y-25130 was microinjected into the contralateral Vi/Vc following CFA masseter muscle injection. Contralateral orofacial hyperalgesia was dose-dependently attenuated following Y-25130 (n=6) (Figure 5A). In contrast, Y-25130 microinjected into the contralateral Vi/Vc did not attenuate the ipsilateral hyperalgesia (Figure 5B). Microinjection of Y-25130 into the ipsilateral Vi/Vc did not attenuate either ipsilateral or contralateral hyperalgesia (Figure 5C, D). These results suggest that 5-HT3 receptor activation in the contralateral Vi/Vc facilitates the CFA-induced contralateral hyperalgesia but may not be necessary for the CFA induced ipsilateral hyperalgesia.
Figure 5.
Vi/Vc 5-HT3receptor inhibition attenuates contralateral hyperalgesia. Inhibition of the 5-HT3 receptor in the contralateral Vi/Vc attenuates CFA-induced contralateral hyperalgesia. CFA (0.05 ml; 1:1 oil/saline) was injected into the masseter muscle and 24 h later, Y-25130 was microinjected (0.5 μl) into the contralateral Vi/Vc (A, B). Y-25130 microinjection into the contralateral Vi/Vc attenuated contralateral hyperalgesia (A) but not ipsilateral hyperalgesia (B). Y-25130 microinjection into the ipsilateral Vi/Vc (C, D) did not attenuate ipsilateral or contralateral hyperalgesia. +: lowest dose vs. saline. #: middle dose vs. saline. *: highest dose vs. saline. +,#,*: p<;0.05; ++,##,**: p<;0.01; +++,###,***: p<;0.001 (ANOVA with repeated measures and Student Neuman-Keuls post-hoc test).
Ipsilateral Vc input is necessary for the development of CFA-induced ipsilateral orofacial hyperalgesia
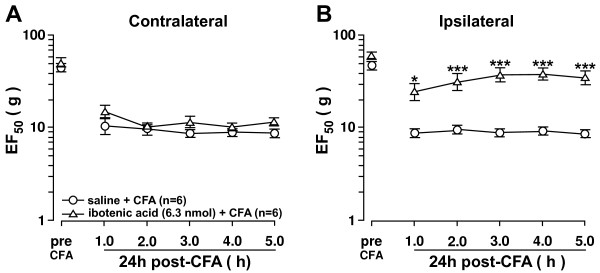
The present data provide evidence that Vi/Vc was necessary for contralateral orofacial hyperalgesia. Our present data also showed that injection of IL-1ra into the Vi/Vc did not affect the ipsilateral hyperalgesia. Our previous studies have shown that afferent nociceptive input into the Vc is critical for the development of ipsilateral hyperalgesia but does not affect contralateral hyperalgesia [13]. This suggests that the ipsilateral and contralateral hyperalgesia may be transmitted through different circuits in the trigeminal system. To test this, a lesion was made in the ipsilateral Vc using 6.3 nmol of the excitotoxin, ibotenic acid. Ibotenic acid induces glutamate-mediated toxicity to neuron cell bodies through activation of glutamate receptors. An ipsilateral Vc lesion prior to CFA injection inhibited the development of ipsilateral hyperalgesia (Figure 6B) but did not inhibit contralateral hyperalgesia development (Figure 6A). This suggests that ipsilateral Vc input is primarily involved in the development of ipsilateral hyperalgesia while Vi/Vc input is necessary for the development of hyperalgesia contralateral to the site of injury.
Figure 6.
Vc activation is necessary for the development of ipsilateral hyperalgesia. Lesion of the ipsilateral Vc prior to CFA injection into the master muscle prevents the development of ipsilateral hyperalgesia. Ibotenic Acid (6.3 nmol) was administered to unilaterally to the Vc between vertebrae C1-C2, 7 days prior to ipsilateral CFA injection into the masseter muscle. Lesion of the ipsilateral Vc did not prevent the development of contralateral hyperalgesia (A). However, lesion of the ipsilateral Vc did prevent the development of ipsilateral hyperalgesia (B). *: Ibotenic Acid (6.3 nmol) vs. saline, p<;0.05; **: p<;0.01; ***: p<;0.001 (ANOVA with repeated measures and Student Neuman-Keuls post-hoc test).
Histological confirmation of injection sites in the RVM and Vi/Vc transition zone
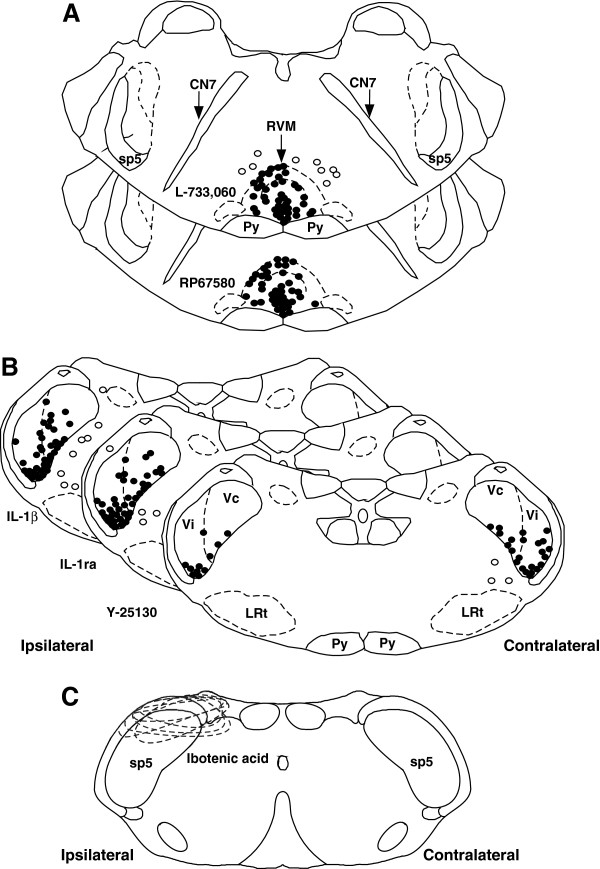
Animals were perfused with 4% paraformaldehyde at the conclusion of the experiment and the sections of brain stem tissues were stained with cresyl violet for histological verification of the sites of injection in the Vi/Vc, RVM, and Vc (Figure 7A, B, C). Animals with missed sites of injection showed no significant effect compared to control and were excluded from experimental groups.
Figure 7.
Histological representation of injection sites within the RVM and Vi/Vc. Histological representation of the cannula tip placement within the RVM (A), the Vi/Vc transition zone (B), and Vc (C). Diagrams are separated according to the drug administered. RVM: L-733,060 and RP67580; Vi/Vc: IL-1β. IL-1ra, and Y-25130; Vc: ibotenic acid. Closed circles represent cannula tips accurately placed within the site of interest. Open circles represent cannula tips placed outside the site of interest. Dashed circles represent affected area from ibotenic acid injection, which involved dorsal mandibular and middle maxillary divisions. Animals with missed sites of injection showed no significant effect compared to control and were excluded from experimental groups. CN 7: facial nerve; sp5: Spinal trigeminal nucleus; Py; pyramidal tract; LRt: lateral reticulate nucleus.
Discussion
Our previous studies have shown that CFA-induced masseter inflammation and intra-Vi/Vc microinjection of IL-1β result in contralateral orofacial hyperalgesia [12,13]. We have also shown that lesions in the RVM prevented the development of contralateral hyperalgesia [12]. The present study further shows that the development of contralateral orofacial hyperalgesia involves IL-1R activation in the ipsilateral Vi/Vc that ultimately leads to NK1-R activation in the RVM and 5-HT3 receptor activation in the contralateral Vi/Vc.
The RVM is a critical site in descending modulation of pain [32,33]. Much research has focused on the descending inhibitory affects of the opioid system within the RVM [34,35]. Our lab has shown that upregulation and phosphorylation of AMPA receptors in the RVM can lead to descending inhibition of inflammatory pain [36,37]. However, there has been mounting evidence of RVM-mediated descending facilitation [38-42]. The RVM has been shown to have reciprocal connections with trigeminal neurons in the Vi/Vc transition zone [14]. Our previous studies indicated that intra-masseter muscle CFA injection and intra-Vi/Vc IL-1β injection produces bilateral orofacial hyperalgesia. The RVM was previously shown to mediate the IL-1β-induced hyperalgesia on the contralateral side [12].
The contralateral effect was also produced by an IL-1β injection into the Vi/Vc but was not produced after IL-1β injection into the Vc [13]. Rats with masseter muscle inflammation exhibit increased IL-1β expression in Vi/Vc astroglia [43]. Furthermore, IL-1 receptor (IL-1R) activation induces a protein kinase-C dependent signaling cascade that leads to N-methyl-D-aspartate receptor phosphorylation and ultimately behavioral hyperalgesia [43]. In our present study, inhibition of IL-1R activation in the ipsilateral Vi/Vc before CFA treatment prevented the development of contralateral orofacial hyperalgesia. Similarly, inhibition of IL-1R activation in the ipsilateral Vi/Vc after CFA treatment resulted in an attenuation of the contralateral hyperalgesia. These results support the view that activation of IL-1R in the ipsilateral Vi/Vc is involved in the development and maintenance of deep tissue orofacial pain on the contralateral site.
Substance P has been shown to be involved in RVM descending modulation of pain [22,25]. NK1-R activation in the RVM enhances excitatory glutamatergic inputs to RVM neurons in rats with persistent pain [44]. NK1-R antagonism in the RVM attenuated CFA-induced thermal hyperalgesia in the hind paw [23]. Our previous studies have shown that NK1-R expression is increased in the RVM following hind paw inflammation and direct injection of substance P into the RVM can induce bilateral thermal hyperalgesia in the hind paw [24]. We also showed that Substance P-induced hyperalgesia can be mediated by 5-HT, NMDA, and GABAA circuits [24]. We have now shown that NK1-R inhibition in the RVM attenuated inflammatory orofacial hyperalgesia bilaterally. These results suggest that NK1-R activation in the RVM contributes to descending facilitation that mediates inflammatory orofacial hyperalgesia to the ipsilateral and contralateral side.
In the spinal cord, administration of a 5-HT3 receptor antagonist inhibits evoked responses of dorsal horn neurons after noxious stimulation [29] and intra-RVM cholecystokinin-induced mechanical and thermal hyperalgesia [45]. Similarly, intrathecal administration of a 5-HT3 receptor antagonist attenuates formalin-induced hind paw flinching and spinal ERK activation [46]. We have previously shown that after the depletion of endogenous 5-HT while maintaining the viability and function of serotonergic neurons, descending facilitation is dependent on the activation of serotonergic neurons in the RVM [26]. The present study shows that descending serotonergic facilitation is necessary for the development and maintenance of contralateral orofacial hyperalgesia after CFA-induced masseter inflammation. Furthermore, activation of Vi/Vc 5-HT3 receptors is necessary for induction of the CFA-induced contralateral orofacial hyperalgesia to develop.
Interestingly, our results show that IL-1R inhibition and 5-HT3 receptor blockade in the ipsilateral Vi/Vc does not affect ipsilateral hyperalgesia. This finding suggests that Vc activation from afferent input is important for the ipsilateral hyperalgesia while the development of contralateral hyperalgesia requires Vi/Vc-RVM circuitry. Supporting this hypothesis, lesions of the Vc inhibited the development of ipsilateral hyperalgesia without affecting contralateral hyperalgesia. It appears that the afferent drive into the Vc is the primary mechanism involved in ipsilateral hyperalgesia consistent with our previous findings [13].
A puzzling observation from this study is that shRNA treatment of Tph-2 in the RVM, which down regulates 5-HT bilaterally, did not affect ipsilateral hyperalgesia. One would expect that hyperalgesia would be attenuated bilaterally. One possible explanation is that ipsilateral hyperalgesia is dominated by hyperexcitability and central sensitization in the Vc driven by peripheral input which masks the loss of descending facilitation. An alternative explanation is that the absence of attenuation of ipsilateral hyperalgesia may be related to the finding of varying degrees of descending inhibitory and facilitatory input into the contralateral and ipsilateral medullary/spinal dorsal horn. It has been shown that descending pain modulation is in dynamic balance between inhibitory and facilitatory signals [47]. After hind paw inflammation, there is a time-dependent enhancement of descending inhibition on the ipsilateral side [48,49]. Descending 5-HT projections from the RVM also produce dual inhibitory and facilitatory effects due to activation of different 5-HT receptor subtypes [50]. The net effect of descending modulation may be facilitatory on the contralateral side and inhibitory on the ipsilateral side in this masseter inflammation model. Thus, depletion of descending 5-HT on the ipsilateral side would reduce predominant inhibitory signals and the hyperalgesia would be sustained.
Conclusions
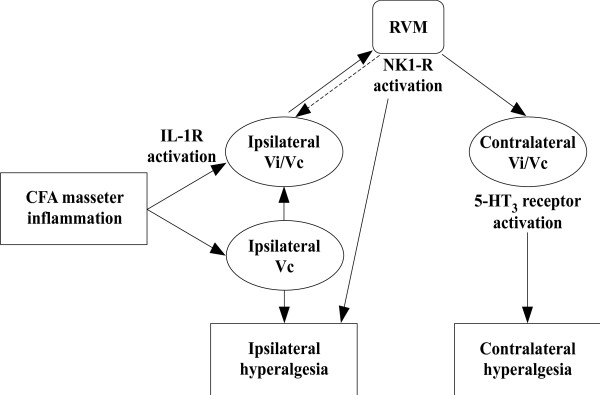
Overall, our results support the hypothesis that the Vi/Vc–RVM circuitry mediates the development of hyperalgesia on the contralateral side after ipsilateral deep tissue injury. CFA-induced inflammatory orofacial hyperalgesia produces an increase in IL-1β activation of IL-1R in the ipsilateral Vi/Vc. IL-1R activation in the Vi/Vc ultimately leads to NK1-R activation in the RVM. This leads to activation of descending serotonin-containing RVM neurons that project to the Vi/Vc bilaterally. Activation of 5-HT3 receptors in the contralateral Vi/Vc results in deep tissue orofacial pain in the contralateral masseter muscle (Figure 8). The findings further suggest that descending facilitatory mechanisms may also contribute to the development of referred pain at widespread sites via RVM descending circuitry.
Figure 8.
Vi/Vc-RVM circuitry is necessary for the development of contralateral hyperalgesia. The proposed pathway involved in the development of contralateral orofacial hyperalgesia following unilateral deep tissue inflammatory hyperalgesia. Unilateral CFA masseter inflammation leads to increased IL-1β activation of IL-1R in the ipsilateral Vi/Vc. Direct or indirect activation of the RVM through NK1-R activation leads to descending serotonergic facilitation of pain to the contralateral Vi/Vc. Activation of 5-HT3 receptors in the contralateral Vi/Vc leads to contralateral orofacial hyperalgesia.
Methods
Animals
Male Sprague–Dawley rats (n=222 total) weighing 200-300g (Harlan) were used for all experiments. Female rats were excluded to avoid complications of the estrous cycle. Rats were provided food and water ad libitum on a 12-h light/dark schedule. The experiments were approved by the Institutional Animal Care and Use Committee at the University of Maryland Dental School.
Surgical preparation/cannulation
Rats were anesthetized using 50 mg/kg of pentobarbital sodium (i.p) and 2-3% isoflurane inhalation in a 30/70% oxygen/nitrogen gas mixture. Rats were placed in a stereotaxic device (Kopf Instruments, Model 900). A midline incision was made in the scalp after debridement and sterilization of the surgical field with iodine wash. For administration of drugs via microinjection, guide cannulae (C315G, 26 gauge, Plastics One, Roanoke, VA) were implanted and cemented into the skull. A midline opening was made in the skull using a dental drill and a guide cannula was lowered into the ventral Vi/Vc transition zone or RVM by referring to the rat brain atlas [51]. The RVM is termed for the collective structures that consist of the midline nucleus raphe magnus (NRM) and the adjacent gigantocellular reticular nucleus pars α (NGC α). The guide cannula was then secured with cranioplastic cement. The wounds were cleaned with antiseptic solution and closed with 4–0 silk sutures. Animals were allowed to recover for 1 week before further experimentation. A group of rats received unilateral intra-masseter muscle injections of the inflammatory agent, complete Freund’s adjuvant (CFA, 0.05ml; 1:1 oil/saline). Twenty-four hours later, drugs were injected into the ventral Vi/Vc transition zone or RVM through a 33-gauge injection cannula (C315I, Plastic One) inserted through the tip of the guide cannula. The injection cannula was connected to a 1-μl Hamilton syringe by polyethylene-10 tubing. All injections (0.5 μl) were performed by delivering drug or vehicle solutions slowly over a 2-min period. Recombinant rat IL-1β, IL-1 receptor antagonist (PeproTech; Rocky Hill, NJ) and 5-HT3 receptor antagonist Y-25130 (Tocris Bioscience; Ellisville, MO) were microinjected into the Vi/Vc transition zone. The Neurokinin-1 receptor antagonists L-733,060 and RP67580 (Tocris Bioscience; Ellisville, MO) were microinjected into the RVM. IL-1β, IL-1 receptor antagonist, Y-25130, and L-733,060 were reconstituted in deionized water while RP67580 was reconstituted in DMSO.
shRNA
shRNA plasmids containing the 5-HT synthesizing enzyme Tryptophan Hydroxylase – 2 (Tph-2) or a scrambled sequence control was administered into the RVM with a 1-μL Hamilton syringe (0.5 μg/0.5 μl) over 5 min. Fifteen minutes after the injection, the syringe was slowly removed and a pair of Teflon-coated silver positive and negative electrodes spaced 3 mm apart were placed in a rostrocaudal direction at the injection site for electroporation (7 square-wave pulses; 50 ms, 40 V, 1 Hz). The rats were allowed to heal for 3 days before unilateral masseter injection of CFA. Behavioral tests were performed 24 h post-CFA.
Western blot
Naïve and treated rats were anesthetized with 50mg/kg pentobarbital sodium (i.p) and decapitated. The RVM tissue was removed as previously described [44]. The RVM tissue was homogenized and centrifuged at 20,200 x g for 10 min at 4°C, and the supernatant was removed. The protein concentration was determined. Each sample contained proteins from one animal. The proteins (50 μg) were separated on a 7.5% SDS-PAGE gel and blotted to a nitrocellulose membrane (GE Healthcare Biosciences, Pittsburgh, PA). The blot was incubated with rabbit anti-Tph-2 antibody overnight at 4°C. The membrane was washed with TBS and incubated for 1 h with anti-goat IgG horseradish peroxidase (HRP) (1:3000; Santa Cruz Biotechnology, Santa Cruz, CA) in 5% milk/TBS. The immunoreactivity was detected using enhanced chemiluminescence (ECL) (GE Healthcare, Pittsburgh, PA). The loading and blotting of equal amount of proteins were verified by reprobing the membrane with anti-β-actin antiserum (Sigma, St. Louis, MO). The ECL-exposed films were digitized, and densitometric quantification of immunoreactive bands was performed using UN-SCAN-IT gel (version 4.3, Silk Scientific). Photoshop software was utilized to construct the figure from the raw western blot data.
Immunohistochemistry
Rats were anesthetized at various time points after gene transfer with 50mg/kg of pentobarbital sodium (i.p) and perfused transcardially with 0.9% saline solution followed by 4% paraformaldehyde in 0.1M phosphate buffer solution. The brainstem and cervical spinal cord (C1-C2) was removed and immersed in the same fixative overnight at 4°C and transferred to 30% sucrose (w/v) in 0.1 M phosphate buffer solution for cryoprotection. After several days, 30 μm thick coronal sections of the tissue sample were sectioned with a cryostat at −20°C. Free floating tissue sections of the Vi/Vc were incubated with rabbit anti-5-HT (1:4000, Immunostar, Hudson,WI) antibody overnight at room temperature. After serial washes, the sections were incubated with Cy2-conjugated goat anti-rabbit IgG (1:500, Jackson ImmunoResearch, West Grove, PA). Control staining procedure was performed by omission of the primary antibody. Photoshop software was utilized to construct the figure from the original microscope photos.
Vc lesion
Vc lesions were made using 1 μg/0.5 μl of ibotenic acid injected into the Vc unilaterally [14]. Rats were anesthetized and a 1-μl Hamilton syringe was placed between C1-C2 vertebrae and ibotenic acid was injected into the ipsilateral Vc over a 2-min period. The rats were allowed to heal over 5–7 days. CFA was injected into the ipsilateral side and behavioral testing was performed after 24 h. Ibotenic acid was reconstituted using deionized water.
CFA and behavioral testing
Behavioral tests were conducted under blind conditions as described elsewhere [52]. A series of calibrated von Frey filaments were applied to the skin above the masseter muscle. Filaments were applied to the masseter muscle in increasing forces. Each von Frey filament was applied 5 times at intervals of a few seconds. A positive response was regarded as an active withdrawal of the head from the probing filament. The response frequencies [(number of responses/number of stimuli) X100%] to a range of von Frey filament forces were determined and a stimulus–response (S-R) curve plotted. After a non-linear regression analysis (GraphPad Prism), an EF50 value, defined as the von Frey filament force (g) that produces a 50% response frequency, was derived from the S-R curve. We used EF50 values as a measure of mechanical sensitivity. A leftward shift of the S-R curve, resulting in a reduction of EF50, occurred after inflammation [14]. This shift of the curve suggests the presence of mechanical hyperalgesia and allodynia since there was an increase in response to suprathreshold stimuli and a decreased response threshold for nocifensive behavior. Data are presented as mean ± S.E.M. Statistical comparisons were made by two-way ANOVA and ANOVA with repeated measures and post hoc comparisons (Neuman-Keuls). P<;0.05 was considered significant.
Abbreviations
TMD: Temporomandibular joint disorder; CFA: Complete Freund’s adjuvant; STN: Spinal trigeminal nucleus; Vc: Subnucleus caudalis of the STN; Vi/Vc: Subnucleus caudalis/subnucleus interpolaris transition zone of the spinal trigeminal nucleus; RVM: Rostral ventromedial medulla; EF50: The effective force that produces 50% response frequency; IL-1β: Interleukin-1β; IL-1R: Interleukin-1 receptor; IL-1ra: Interleukin-1 receptor antagonist; NK1-R: Neurokinin-1 receptor; Tph-2: Tryptophan hydroxylase-2; i.p: intraperitoneal.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BC was involved in carrying out the behavioral, pharmacological, RNAi, immunostaining, and histological studies. BC was also involved in the experimental design and drafted the manuscript. WG was involved in the experimental design and RNAi and Western blot studies. FW contributed to the experimental design and the RNAi and immunostaining experiments. KR and RD conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Bryan Chai, Email: bchai001@umaryland.edu.
Wei Guo, Email: wguo@umaryland.edu.
Feng Wei, Email: fwei@umaryland.edu.
Ronald Dubner, Email: rdubner@umaryland.edu.
Ke Ren, Email: kren@umaryland.edu.
Acknowledgements
This work was supported by National Institute of Health, DE021942, DE11964, and DE0212804.
References
- Fernández-de-Las-Peñas C, Galan-del-Rio F, Fernandez-Carnero J, Pesquera J, Arendt-Nielsen L, Svensson P. Bilateral widespread mechanical pain sensitivity in women with myofascial temporomandibular disorder: evidence of impairment in central nociceptive processing. J Pain. 2009;10:1170–1178. doi: 10.1016/j.jpain.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C, Galan-del-Rio F, Ortega-Santiago R, Jimenez-Garcia R, Arendt-Nielsen L, Svensson P. Bilateral thermal hyperalgesia in trigeminal and extra-trigeminal regions in patients with myofascial temporomandibular disorders. Exp Brain Res. 2010;202:171–179. doi: 10.1007/s00221-009-2121-x. [DOI] [PubMed] [Google Scholar]
- Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ren K. Brainstem mechanisms of persistent pain following injury. J Orofacial Pain. 2004;18:299–305. [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Bereiter DA. Chronic inflammation and estradiol interact through MAPK activation to affect TMJ nociceptive processing by trigeminal caudalis neurons. Neuroscience. 2009;164(4):1813. doi: 10.1016/j.neuroscience.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M, Tsuboi Y, Takamiya K, Huganir R, Kondo M, Shinoda M, Oi Y, Iwata K. Involvement of GluR2 and GluR3 subunit C-termini in the trigeminal spinal subnucleus caudalis and C1-C2 neurons in trigeminal neuropathic pain. Neurosci Lett. 2011;491(1):8–12. doi: 10.1016/j.neulet.2010.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbe H, Dubner R, Ren K. Masseteric inflammation-induced fos protein expression in the trigeminal interpolaris/caudalis transition zone: Contribution of somatosensory-vagal-adrenal integration. Brain Res. 1999;845:165–175. doi: 10.1016/S0006-8993(99)01913-7. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Imbe H, Dubner R, Ren K. Persistent fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: Implications for persistent orofacial pain. J Comp Neurol. 1999;412:276–291. doi: 10.1002/(SICI)1096-9861(19990920)412:2<276::AID-CNE7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ro JY, Harriott A, Crouse U, Capra NF. Innocuous jaw movements increase c-fos expression in trigeminal sensory nuclei produced by masseter muscle inflammation. Pain. 2003;104:539–548. doi: 10.1016/S0304-3959(03)00093-9. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain. Int Rev Neurobiol. 2011;97:207–225. doi: 10.1016/B978-0-12-385198-7.00008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Chai B, LaGraize SC, Wei F, Dubner R, Ren K. Microinjection of IL-1beta into the trigeminal transition zone produces bilateral NMDA receptor dependent orofacial hyperalgesia involving descending circuitry. Open Pain J. 2009;2:76–83. doi: 10.2174/1876386300902010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, Dubner R, Ren K. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol Pain. 2009;5:75. doi: 10.1186/1744-8069-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats. J Comp Neurol. 2005;493:510–523. doi: 10.1002/cne.20797. [DOI] [PubMed] [Google Scholar]
- Maeno H, Kiyama H, Tohyama M. Distribution of the substance P receptor (NK-1 receptor) in the central nervous system. Brain Res Mol Brain Res. 1993;18:43–58. doi: 10.1016/0169-328x(93)90172-l. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- Saffroy M, Torrens Y, Glowinski J, Beaujouan JC. Autoradiographic distribution of tachykinin NK2 binding sites in the rat brain: comparison with NK1 and NK3 binding sites. Neuroscience. 2000;116:761–773. doi: 10.1016/s0306-4522(02)00748-0. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Nohr D, Krause JE, Weihe E. Inflammation-induced upregulation of NK1 receptor mRNA in dorsal horn neurones. Neuroreport. 1993;4:1007–1010. doi: 10.1097/00001756-199308000-00003. [DOI] [PubMed] [Google Scholar]
- McCarson KE, Krause JE. NK-1 and NK-3 type tachykinin receptor mRNA expression in the rat spinal cord dorsal horn is increased during adjuvant or formalin-induced nociception. J Neurosci. 1994;14:712–720. doi: 10.1523/JNEUROSCI.14-02-00712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Budai D, Khasabov SG, Mantyh PW, Simone DA. NK-1 receptors modulate the excitability of ON cells in the rostral ventromedial medulla. J Neurophysiol. 2007;97:1388–1395. doi: 10.1152/jn.00450.2006. [DOI] [PubMed] [Google Scholar]
- Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. NK-1 receptors in the rostral ventromedial medulla contribute to hyperalgesia produced by intraplantar injection of capsaicin. Pain. 2008;139:34–46. doi: 10.1016/j.pain.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Hamity MV, White SR, Hammond DL. Effects of neurokinin-1 receptor agonism and antagonism in the rostral ventromedial medulla of rats with acute or persistent inflammatory nociception. Neuroscience. 2010;165:902–913. doi: 10.1016/j.neuroscience.2009.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGraize SC, Guo W, Yang K, Wei F, Ren K, Dubner R. NK-1 tachykinin receptors in the rostral ventromedial medulla mediate descending facilitation of inflammatory hyperalgesia linked to 5-HT3, NMDA and GABAA receptor dependent spinal circuitry. Neuroscience. 2010;71(4):1341–1356. doi: 10.1016/j.neuroscience.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink TS, Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. Differential modulation of neurons in the rostral ventromedial medulla by neurokin-1 receptors. J Neurophysiol. 2012;107(4):1210–1221. doi: 10.1152/jn.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Dubner R, Zou S, Ren K, Bai G, Wei D, Guo W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci. 2010;30:8624–8636. doi: 10.1523/JNEUROSCI.5389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Oatway MA, Weaver LC. Blockade of the 5-HT3 receptor for days causes sustained relief from mechanical allodynia following spinal cord injury. J Neuro Res. 2008;87(2):418–424. doi: 10.1002/jnr.21860. [DOI] [PubMed] [Google Scholar]
- Oatway MA, Chen Y, Weaver LC. The 5-HT3 receptor facilitates mechanical allodynia following spinal cord injury. Pain. 2004;110:259–268. doi: 10.1016/j.pain.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Rahman W, Suzuki R, Webber M, Hunt SP, Dickenson AH. Depletion of endogenous spinal 5-HT attenuates the behavioural hypersensitivity to mechanical and cooling stimuli induced by spinal nerve ligation. Pain. 2006;123:264–274. doi: 10.1016/j.pain.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Doucet E, Latrémolière A, Darmon M, Hamon M, Emerit MB. Immunolabelling of the 5-HT 3B receptor subunit in the central and peripheral nervous systems in rodents. Eur J Neurosci. 2007;26:355–366. doi: 10.1111/j.1460-9568.2007.05659.x. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174(4016):1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Fang FG, Haws CM, Drasner K, Williamson A, Fields HL. Opioid peptides (DAGO-enkephalin, dynorphin A(1–13), BAM 22P) microinjected into the rat brainstem: comparison of their antinociceptive effect and their effect on neuronal firing in the rostral ventromedial medulla. Brain Res. 1989;501(1):116–128. doi: 10.1016/0006-8993(89)91033-0. [DOI] [PubMed] [Google Scholar]
- Boyer JS, Morgan MM, Craft RM. Microinjection of morphine into the rostral ventromedial medulla produces greater antinociception in male compared to female rats. Brain Res. 1998;796(1–2):315–318. doi: 10.1016/s0006-8993(98)00353-9. [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo W, Zou SP, Dubner R, Ren K. Inflammation-induced upregulation of AMPA receptor subunit expression in brain stem pain modulatory circuitry. Pain. 2003;104(1–2):401–413. doi: 10.1016/s0304-3959(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo W, Robbins MT, Dubner R, Ren K. Changes in AMPA receptor phosphorylation in the rostral ventromedial medulla after inflammatory hyperalgesia in rats. Neurosci Lett. 2004;366(2):201–205. doi: 10.1016/j.neulet.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Wiertelak EP, Roemer B, Maier SF, Watkins LR. Comparison of the effects of nucleus tractus solitarius and ventral medial medulla lesions on illness-induced and subcutaneous formalin induced hyperalgesias. Brain Res. 1997;748:143–150. doi: 10.1016/S0006-8993(96)01289-9. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100(1–2):1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25(6):319–325. doi: 10.1016/S0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hammond DL. Substance P enhances excitatory transmission on spinally projecting neurons in the rostral ventromedial medulla after inflammatory injury. J Neurophysiol. 2009;102(2):1139–1151. doi: 10.1152/jn.91337.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogrul A, Ossipov MH, Porreca F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009;1280:52–59. doi: 10.1016/j.brainres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Tran TK, Fitzsimmons B, Yaksh TL, Hua XY. Descending serotonergic facilitation of spinal ERK activation and pain behavior. FEBS Lett. 2006;580:6629–6634. doi: 10.1016/j.febslet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas H, Schaible H-G. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. J Neurophysiol. 1996;76:3025–3037. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]
- Terayama R, Guan Y, Dubner R, Ren K. Activity-induced plasticity in brain stem pain modulatory circuitry after inflammation. Neuroreport. 2000;11:1915–1919. doi: 10.1097/00001756-200006260-00022. [DOI] [PubMed] [Google Scholar]
- Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol. 2011;22(5–6):390–404. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Amsterdam: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/S0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]