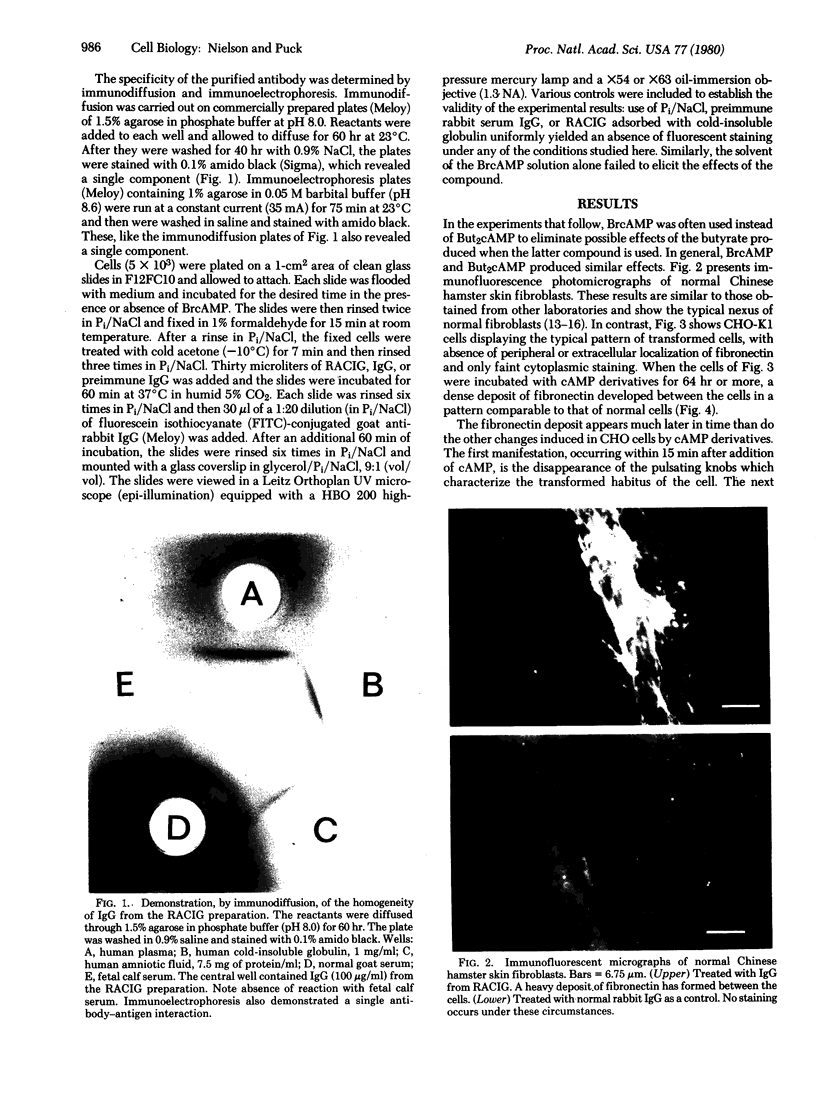
Abstract
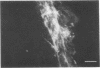
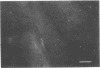
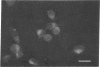
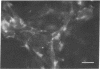
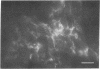
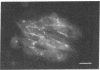
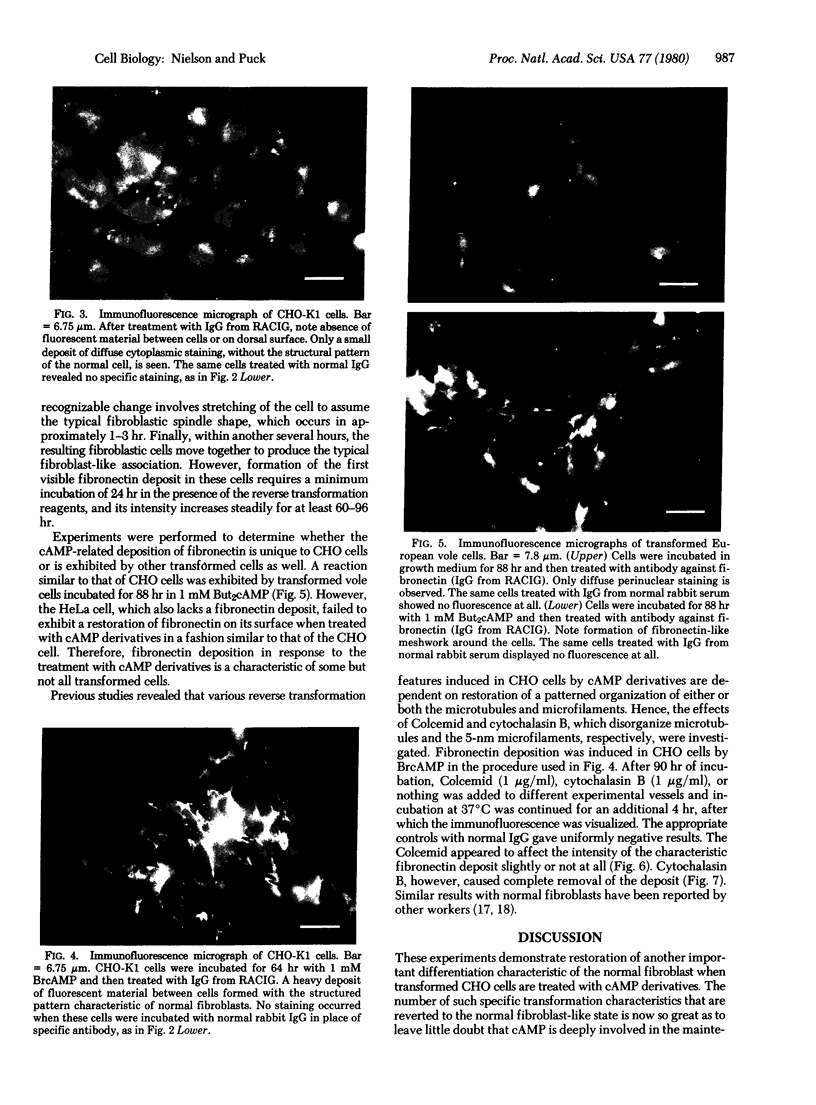
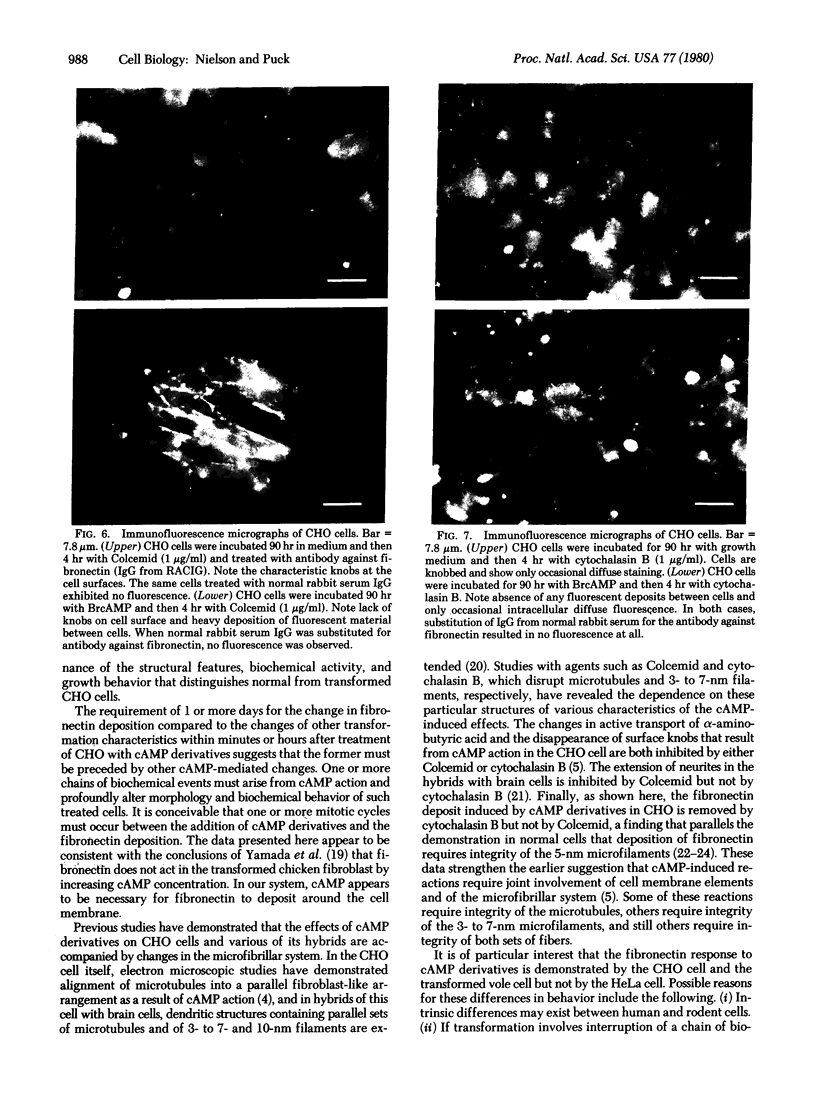
The Chinese hamster ovary (CHO) cell, like other transformed cells, has lost the fibronectin deposit around its membrane. Treatment with cyclic AMP derivatives restores the typical fibroblastic deposit of fibronectin. Thus, the reverse transformation process induced by cyclic AMP (cAMP) in the CHO cell restores this important property as well as other morphological, biochemical, and growth behavioral characteristics of the normal fibroblastic state. The fibronectin deposit occurs significantly later in time than do other characteristics of the reverse transformation reaction and may therefore reflect a metabolic action that requires other cAMP effect to precede it. The restoration of fibronectin deposition in response to cAMP derivatives is also exhibited by vole cells transformed by avian sarcoma virus, but it is not by HeLa cell. Addition of Colcemid, which disrupts microtubules, to CHO cells containing a fibronectin deposit induced by cAMP derivatives causes little or no erosion of the deposit, but cytochalasin B, which disrupts 5-nm microfilaments, eliminates it completely. Thus, various features of the action of cAMP derivatives on CHO and related cells require integrity of the cellular microfibrils--in some cases microtubules only, in some cases 5-nm microfilaments only, and in some cases both classes of fibrils.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Hynes R. O. Effects of cytochalasin B and colchicine on attachment of a major surface protein of fibroblasts. Biochim Biophys Acta. 1977 Nov 15;471(1):16–24. doi: 10.1016/0005-2736(77)90388-1. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Burridge K., Murray A., Walsh M. L., Copple C. D., Bushnell A., McDougall J. K., Gallimore P. H. Modulation of cell surface glycocalyx: studies on large, external, transformation-sensitive protein. Ann N Y Acad Sci. 1978 Jun 20;312:366–381. doi: 10.1111/j.1749-6632.1978.tb16814.x. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Murray A., Segal R. A., Bushnell A., Walsh M. L. Studies on intercellular LETS glycoprotein matrices. Cell. 1978 Jun;14(2):377–391. doi: 10.1016/0092-8674(78)90123-x. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Hayman M. J., Vogt P. K. Properties of mammalian cells transformed by temperature-sensitive mutants of avian sarcoma virus. Cell. 1977 Jul;11(3):513–521. doi: 10.1016/0092-8674(77)90069-1. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Furcht L. T., Mosher D. F., Wendelschafer-Crabb G. Effects of cell density and transformation on the formation of a fibronectin extracellular filamentous matrix on human fibroblasts. Cancer Res. 1978 Dec;38(12):4618–4623. [PubMed] [Google Scholar]
- Furcht L. T., Mosher D. F., Wendelschafer-Crabb G., Foidart J. M. Reversal by glucocorticoid hormones of the loss of a fibronectin and probollagen matrix around transformed human cells. Cancer Res. 1979 Jun;39(6 Pt 1):2077–2083. [PubMed] [Google Scholar]
- Hsie A. W., Jones C., Puck T. T. Further changes in differentiation state accompanying the conversion of Chinese hamster cells of fibroblastic form by dibutyryl adenosine cyclic 3':5'-monophosphate and hormones. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1648–1652. doi: 10.1073/pnas.68.7.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Ali I. U., Destree A. T., Mautner V., Perkins M. E., Senger D. R., Wagner D. D., Smith K. K. A large glycoprotein lost from the surfaces of transformed cells. Ann N Y Acad Sci. 1978 Jun 20;312:317–342. doi: 10.1111/j.1749-6632.1978.tb16811.x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T., Mautner V. M., Ali I. U. Synthesis, secretion, and attachment of LETS glycoprotein in normal and transformed cells. J Supramol Struct. 1977;7(3-4):397–408. doi: 10.1002/jss.400070311. [DOI] [PubMed] [Google Scholar]
- Jockusch B. M., Kelley K. H., Meyer R. K., Burger M. M. An efficient method to produce specific anti-actin. Histochemistry. 1978 Apr 4;55(3):177–184. doi: 10.1007/BF00495757. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Faik P., Puck T. T. Extension of branching processes from hybrids of brain and Chinese hamster ovary cells. Exp Cell Res. 1979 Aug;122(1):83–91. doi: 10.1016/0014-4827(79)90563-9. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Wartiovaara J., Vaheri A. Cytochalasin B releases a major surface-associated glycoprotein, fibronectin, from cultured fibroblasts. Exp Cell Res. 1978 Jan;111(1):127–137. doi: 10.1016/0014-4827(78)90243-4. [DOI] [PubMed] [Google Scholar]
- Mautner V., Hynes R. O. Surface distribution of LETS protein in relation to the cytoskeleton of normal and transformed cells. J Cell Biol. 1977 Dec;75(3):743–768. doi: 10.1083/jcb.75.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Saksela O., Keski-Oja J., Vaheri A. Distribution of a major surface-associated glycoprotein, fibronectin, in cultures of adherent cells. J Supramol Struct. 1977;6(4):551–557. doi: 10.1002/jss.400060408. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Puck T. T., Hsie A. W., Kelley D. An electron microscopy study of the effects on dibutyryl cyclic AMP on Chinese hamster ovary cells. Cell. 1974 Jul;2(3):145–162. doi: 10.1016/0092-8674(74)90089-0. [DOI] [PubMed] [Google Scholar]
- Puck T. T. Cyclic AMP, the microtubule-microfilament system, and cancer. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4491–4495. doi: 10.1073/pnas.74.10.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck T. T. Studies on cell transformation. Somatic Cell Genet. 1979 Nov;5(6):973–990. doi: 10.1007/BF01542655. [DOI] [PubMed] [Google Scholar]
- Puck T. T., Waldren C. A., Hsie A. W. Membrane dynamics in the action of dibutyryl adenosine 3':5'-cyclic monophosphate and testosterone on mammalian cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1943–1947. doi: 10.1073/pnas.69.7.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I. The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell. 1979 Mar;16(3):675–685. doi: 10.1016/0092-8674(79)90040-0. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]