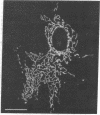
Abstract
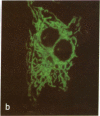
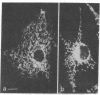
The laser dye rhodamine 123 is shown to be a specific probe for the localization of mitochondria in living cells. By virtue of its selectivity for mitochondria and its fluorescent properties, the detectability of mitochondria stained with rhodamine 123 is significantly improved over that provided by conventional light microscopic techniques. With the use of rhodamine 123, it is possible to detect alterations in mitochondrial distribution following transformation by Rous sarcoma virus and changes in the shape and organization of mitochondria induced by colchicine treatment.
Full text
PDF
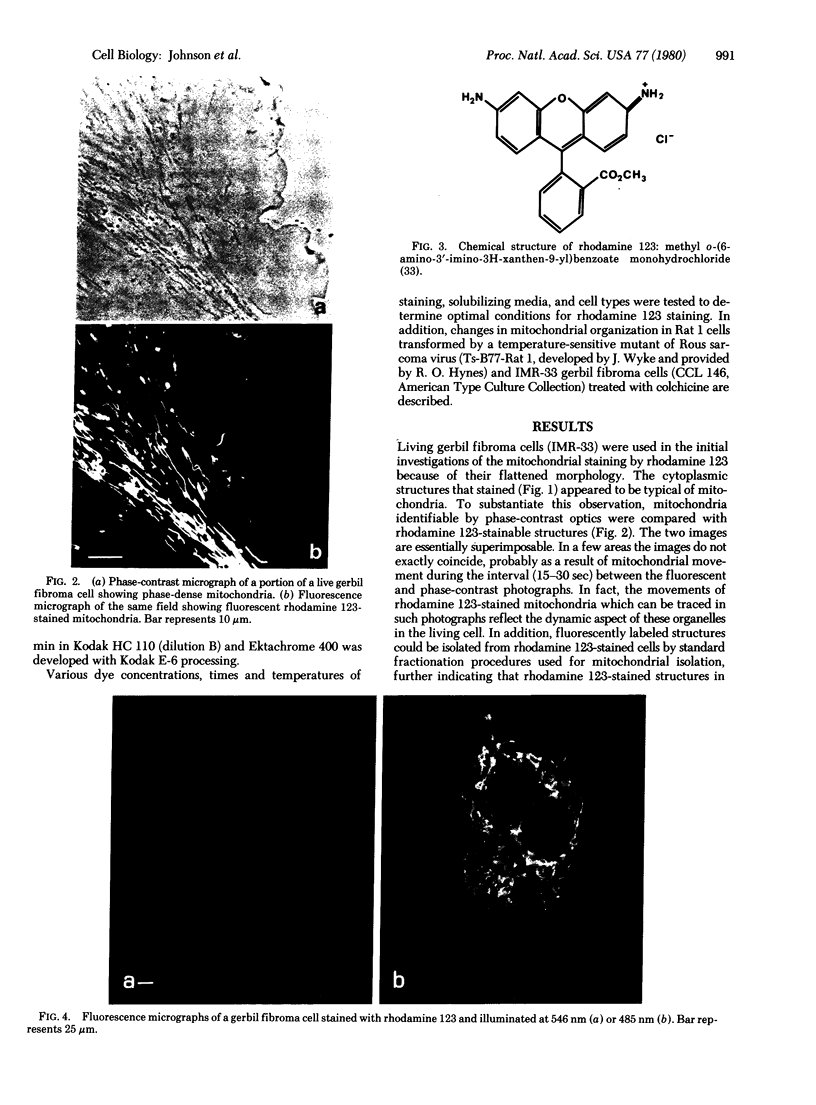
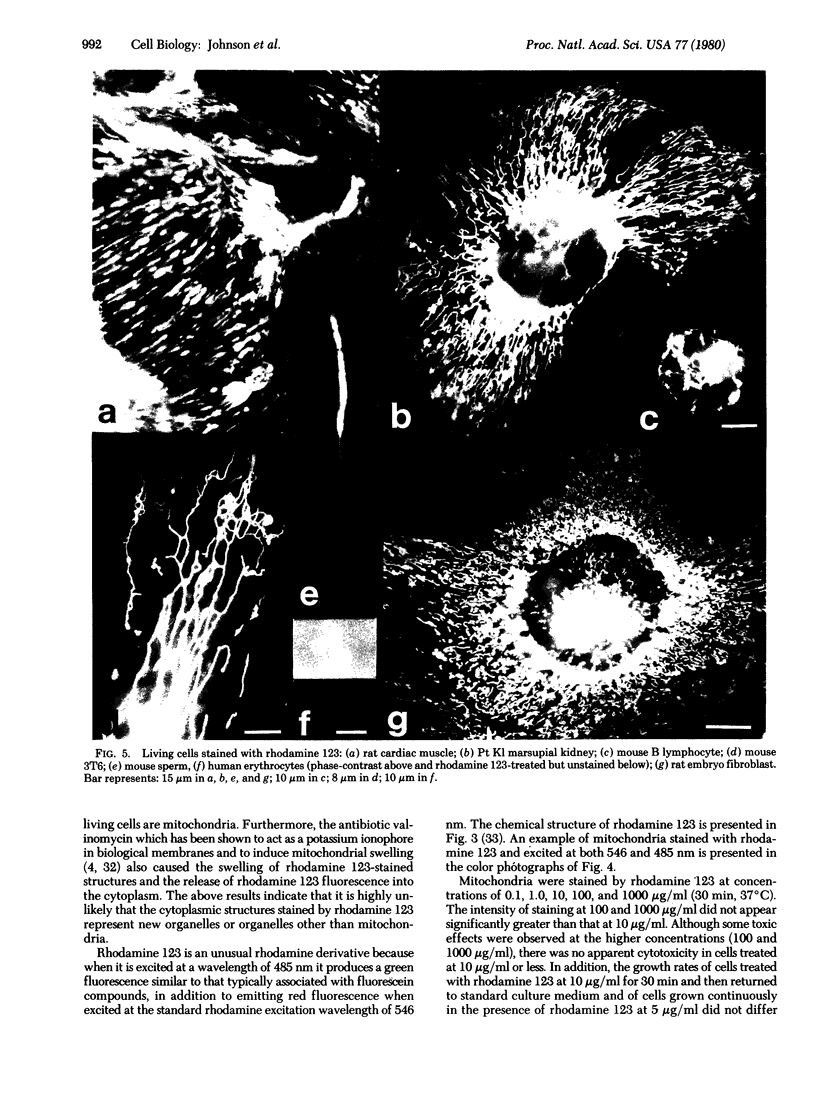
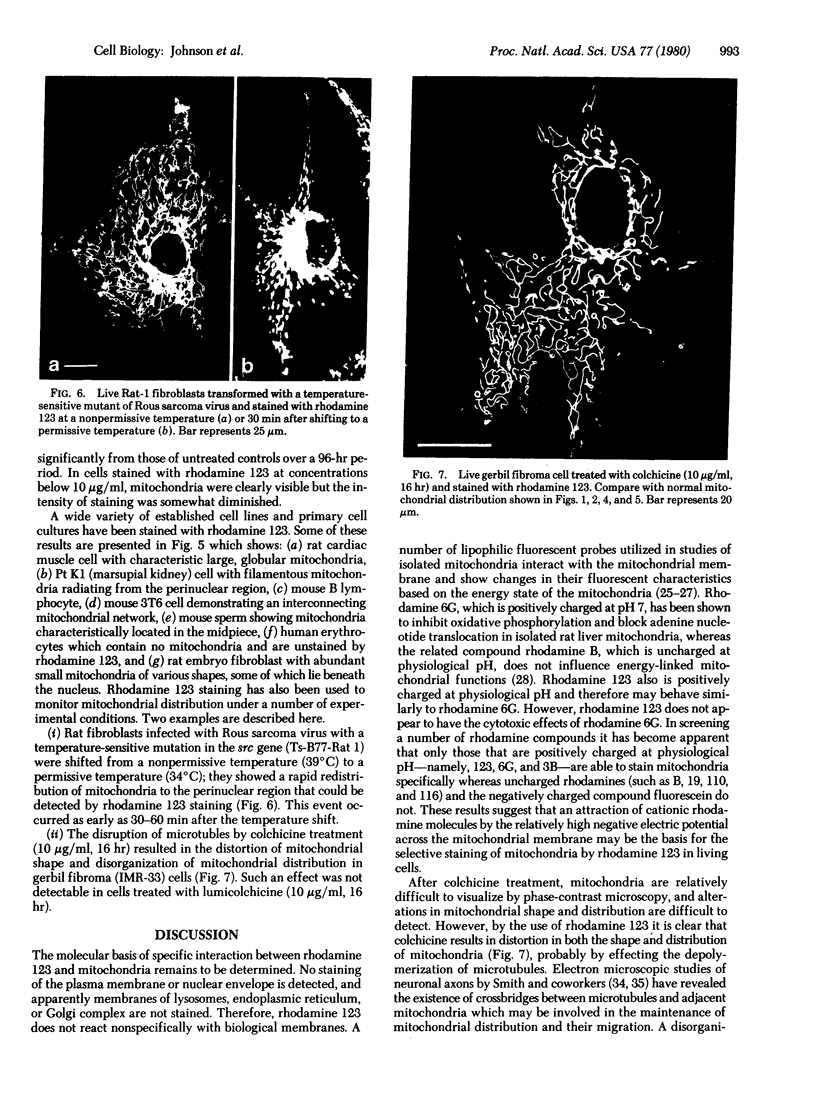

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A. The application of fluorescent probes in membrane studies. Q Rev Biophys. 1975 May;8(2):237–316. doi: 10.1017/s0033583500001803. [DOI] [PubMed] [Google Scholar]
- BIERLING R. Beobachtungen zum Verhalten der Mitochondrien von Zellen in vitro. Z Krebsforsch. 1954;60(1):31–46. [PubMed] [Google Scholar]
- BIESELE J. J., TOBIOKA M. Mitochondria in living cells: an analysis of movements. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):319–324. doi: 10.1083/jcb.2.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. T., Martin A. P., Lucas F. V., Vorbeck M. L. The structure of rat liver mitochondria: a reevaluation. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1097–1104. doi: 10.1016/s0006-291x(74)80091-4. [DOI] [PubMed] [Google Scholar]
- Carignani G., Lancashire W. E., Griffiths D. E. Extra-chromosomal inheritance of rhodamine 6G resistance in Saccharomyces cerevisiae. Mol Gen Genet. 1977 Feb 28;151(1):49–56. doi: 10.1007/BF00446912. [DOI] [PubMed] [Google Scholar]
- Cohen J. D., Eaton N. R. Genetic analysis of multiple drug cross resistance in Saccharomyces cerevisiae: a nuclear-mitochondrial gene interaction. Genetics. 1979 Jan;91(1):19–33. doi: 10.1093/genetics/91.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEY G. O., SHAPRAS P., BORYSKO E. Activities and responses of living cells and their components as recorded by cinephase microscopy and electron microscopy. Ann N Y Acad Sci. 1954 Nov 17;58(7):1089–1109. doi: 10.1111/j.1749-6632.1954.tb45895.x. [DOI] [PubMed] [Google Scholar]
- Gear A. R. Rhodamine 6G. A potent inhibitor of mitochondrial oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3628–3637. [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol. 1966 Aug;30(2):269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol. 1968 May;37(2):345–369. doi: 10.1083/jcb.37.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Simon M., Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle P. C., McCarty R. E. How cells make ATP. Sci Am. 1978 Mar;238(3):104-17, 121-3. doi: 10.1038/scientificamerican0378-104. [DOI] [PubMed] [Google Scholar]
- Kára J., Mach O., Cerná H. Replication of Rous sarcoma virus and the biosynthesis of the oncogenic subviral ribonucleoprotein particles ("virosomes") in the mitochondria isolated from Rous sarcoma tissue. Biochem Biophys Res Commun. 1971 Jul 2;44(1):162–169. doi: 10.1016/s0006-291x(71)80173-0. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L. Water uptake and extrusion by mitochondria in relation to oxidative phosphorylation. Physiol Rev. 1962 Jul;42:467–517. doi: 10.1152/physrev.1962.42.3.467. [DOI] [PubMed] [Google Scholar]
- PALADE G. E. An electron microscope study of the mitochondrial structure. J Histochem Cytochem. 1953 Jul;1(4):188–211. doi: 10.1177/1.4.188. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Posakony J. W., England J. M., Attardi G. Morphological heterogeneity of HeLa cell mitochondria visualized by a modified diaminobenzidine staining technique. J Cell Sci. 1975 Nov;19(2):315–329. doi: 10.1242/jcs.19.2.315. [DOI] [PubMed] [Google Scholar]
- ROUILLER C. Physiological and pathological changes in mitochondrial morphology. Int Rev Cytol. 1960;9:227–292. doi: 10.1016/s0074-7696(08)62748-5. [DOI] [PubMed] [Google Scholar]
- Rosenbaum R. M., Wittner M., Lenger M. Mitochondrial and other ultrastructural changes in great alveolar cells of oxygen-adapted and poisoned rats. Lab Invest. 1969 Jun;20(6):516–528. [PubMed] [Google Scholar]
- SJOSTRAND F. S. Electron microscopy of mitochondria and cytoplasmic double membranes. Nature. 1953 Jan 3;171(4340):30–32. doi: 10.1038/171030a0. [DOI] [PubMed] [Google Scholar]
- Sato T., Tauchi H. The formation of enlarged and giant mitochondria in the aging process of human hepatic cells. Acta Pathol Jpn. 1975 Jul;25(4):403–412. doi: 10.1111/j.1440-1827.1975.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Smith D. S., Järlfors U., Cameron B. F. Morphological evidence for the participation of microtubules in axonal transport. Ann N Y Acad Sci. 1975 Jun 30;253:472–506. doi: 10.1111/j.1749-6632.1975.tb19223.x. [DOI] [PubMed] [Google Scholar]
- Smith D. S., Järlfors U., Cayer M. L. Structural cross-bridges between microtubules and mitochondria in central axons of an insect (Periplaneta americana). J Cell Sci. 1977;27:255–272. doi: 10.1242/jcs.27.1.255. [DOI] [PubMed] [Google Scholar]
- Tandler B., Erlandson R. A., Wynder E. L. Riboflavin and mouse hepatic cell structure and function. I. Ultrastructural alterations in simple deficiency. Am J Pathol. 1968 Jan;52(1):69–96. [PMC free article] [PubMed] [Google Scholar]
- Wang E., Goldman R. D. Functions of cytoplasmic fibers in intracellular movements in BHK-21 cells. J Cell Biol. 1978 Dec;79(3):708–726. doi: 10.1083/jcb.79.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weakley B. S. Variations in mitochondrial size and ultrastructure during germ cell development. Cell Tissue Res. 1976 Jul 6;169(4):531–550. doi: 10.1007/BF00218151. [DOI] [PubMed] [Google Scholar]