Abstract
High-affinity K+ uptake in plant roots is rapidly up-regulated when K+ is withheld and down-regulated when K+ is resupplied. These processes make important contributions to plant K+ homeostasis. A cDNA coding for a high-affinity K+ transporter, HKT1, was earlier cloned from wheat (Triticum aestivum L.) roots and functionally characterized. We demonstrate here that in both barley (Hordeum vulgare L.) and wheat roots, a rapid and large up-regulation of HKT1 mRNA levels resulted when K+ was withdrawn from growth media. This effect was specific for K+; withholding N caused a modest reduction of HKT1 mRNA levels. Up-regulation of HKT1 transcript levels in barley roots occurred within 4 h of removing K+, which corresponds to the documented increase of high-affinity K+ uptake in roots following removal of K+. Increased expression of HKT1 mRNA was evident before a decline in total root K+ concentration could be detected. Resupply of 1 mm K+ was sufficient to strongly reduce HKT1 transcript levels. In wheat root cortical cells, both membrane depolarizations in response to 100 μm K+, Cs+, and Rb+, and high-affinity K+ uptake were enhanced by K+ deprivation. Thus, in both plant systems the observed physiological changes associated with manipulating external K+ supply were correlated with levels of HKT1 mRNA expression. Implications of these findings for K+ sensing and regulation of the HKT1 mRNA levels in plant roots are discussed.
The absorption of K+ by plant roots is mediated by at least two general transport systems. At low external [K+], high-affinity transport systems, referred to as mechanism I by Epstein et al. (1963) or HATS by Guy et al. (1988), are saturable and have apparent Km values for K+ uptake in the micromolar range (Epstein et al., 1963; Glass, 1976; Kochian and Lucas, 1982). Rates of high-affinity K+ uptake are extremely sensitive to plant K+ status; Vmax values for K+ (86Rb+) influx decline and Km values increase as the K+ content of roots increases (Glass, 1976). By contrast, there is a rapid up-regulation of high-affinity K+ uptake when the exogenous K+ supply is interrupted (Glass, 1975; Kochian and Lucas, 1982; Drew et al., 1984; Fernando et al., 1990). Under these conditions, root K+ concentrations are rapidly depleted by K+ translocation to the shoot and by root growth (Hooymans, 1974; Glass, 1978; Drew and Saker, 1984; Walker et al., 1996a). Likewise, when K+ uptake is limited by pruning the root system (Claassen and Barber, 1977) or by restricting the K+ supply to certain regions of the root (Drew et al., 1984), high-affinity K+ uptake increases to compensate for the reduced absorptive surface.
The elevated rates of high-affinity K+ influx observed in K+-deprived plants are rapidly down-regulated when K+ is resupplied (Glass, 1976, 1978; Fernando et al., 1990). Recently, it was shown that in addition to these changes of K+ fluxes across the plasma membrane, adjustments in K+ fluxes across the tonoplast and K+ transport to the xylem contribute to K+ homeostasis (Fernando et al., 1992; Walker et al., 1996a).
At higher external [K+] a second class of K+ transport systems that is responsible for low-affinity K+ transport becomes evident (Epstein et al., 1963; Epstein and Rains, 1965; Glass and Dunlop, 1978; Kochian and Lucas, 1982). Unlike the high-affinity transporters, low-affinity transport systems appear to be insensitive to plant K+ status in barley (Hordeum vulgare L.) and ryegrass (Glass and Dunlop, 1978) and in corn (Kochian and Lucas, 1982), whereas in sunflower (Benlloch et al., 1989) and Arabidopsis (Maathuis and Sanders, 1995) low-affinity K+ uptake and K+ channel activity were slightly increased by K+ starvation.
Genes that encode K+-uptake function (AKT1 and KAT1) were identified from a higher plant by complementing a yeast strain defective in K+ uptake with a cDNA from Arabidopsis (Anderson et al., 1992; Sentenac et al., 1992). Translation of the KAT1 cRNA in Xenopus laevis oocytes conferred functional expression of the classical properties of plant inward-rectifying K+ channels, including the lack of inactivation, time and voltage dependences, external K+ dependence, K+ selectivity, and pharmacological block (Schachtman et al., 1992). In Brassica napus high levels of the AKT1 gene expression were evident in plants supplied with 5 mm, 0.25 mm, and <5 μm K+ (Lagarde et al., 1996), and it was concluded that withholding K+ from B. napus plants did not alter the expression levels of AKT1. This observation indicates that AKT1 may thus be expressed constitutively, in agreement with the proposed constitutive expression of low-affinity K+ uptake activity in barley, ryegrass, and maize (Glass and Dunlop, 1978; Kochian and Lucas, 1982).
By complementation of yeast mutants with cDNAs from K+-starved roots, a cDNA encoding a high-affinity transporter for K+ (HKT1) was identified in wheat (Triticum aestivum L.; Schachtman and Schroeder, 1994). HKT1 shows homology to the high-affinity K+ transporters (encoded by TRK1 and TRK2 genes) of the yeast plasma membrane (Ko and Gaber, 1991). HKT1 expression in yeast yields saturable kinetics with apparent Km values in the range of 3 μm for K+ uptake and 30 μm for Rb+ uptake. Patterns of in situ RNA hybridization of wheat seedlings showed that HKT1 is expressed in root cortical cells and in cells adjacent to the vascular tissue in leaves, indicative of a role in K+ transport (Schachtman and Schroeder, 1994). Further studies of HKT1 expression in Saccharomyces cerevisiae and X. laevis oocytes showed that this transporter behaves as a Na+-K+ symporter that is strongly selective for the uptake of K+ and Na+ relative to the other alkali cations, Rb+, Li+, and Cs+ (Rubio et al., 1995; Gassmann et al., 1996). The finding that HKT1 functions as a Na+-K+ symporter has led to the suggestion that HKT1 may represent one of the multiple molecular pathways contributing to high-affinity K+ uptake in vivo (Rubio et al., 1996). Recent research supports this hypothesis, showing that in Arabidopsis members of a large new gene family may contribute to high- and/or low-affinity K+ uptake (Quintero and Blatt, 1997; Santa-Maria et al., 1997; Fu and Luan, 1998; Kim et al., 1998).
High-affinity 86Rb+ (K+) uptake in barley and wheat has been demonstrated to be active in the absence of Na+ (Epstein and Hagen, 1952; Maathuis et al., 1996; Walker et al., 1996b). As a consequence, a role for HKT1 in mediating high-affinity K+ transport in higher plant roots has been questioned (Maathuis et al., 1996; Walker et al., 1996b) and remains an open question. In the present study we investigated the effects of manipulating external K+ supply to roots of barley and wheat on HKT1 mRNA transcript levels. We observed a rapid up-regulation of HKT1 expression during the first 4 h following withdrawal of K+ from external media, which corresponds with the documented increases of high-affinity K+ uptake systems in barley and maize roots under identical conditions (Glass, 1975, 1976; Kochian and Lucas, 1982; Fernando et al., 1990). The increased expression of HKT1 in roots preceded any detectable changes of root or shoot [K+], providing novel insights concerning the sensing of root or plant K+ status.
MATERIALS AND METHODS
Growth of Plants
Seeds of barley (Hordeum vulgare L. cvs Jackson and Klondike) and wheat (Triticum aestivum L. cv Atlas 66) were germinated for 4 d in a growth room maintained at 20°C on a day/night cycle of 16-h light/8-h dark. Light was provided by fluorescent lamps (Vita-Lite, Durotest, Fairfield, NJ), which produced approximately 300 μmol m−2 s−1 at plant level. Seeds were surface sterilized for 15 min using a 1% solution of commercial bleach and then subjected to at least six washes with distilled water to remove all traces. Barley seeds were then aerated for 24 h in distilled water before planting on plastic mesh on Plexiglas discs in sterilized, washed sand. By d 4 seedling roots had grown through the mesh to a length of approximately 50 mm. Seedlings were then treated according to the described experimental conditions. This typically involved transfer to hydroponic growth tanks in the same growth room. The solutions used for culture were based on Johnson's modified inorganic nutrient recipe (Epstein, 1972), diluted to one-fifth strength. The [K+] in this solution was 1.2 mm. At the times indicated, plants were transferred to otherwise identical solutions lacking K+. Wheat seedlings were germinated on filter paper wetted with distilled water for 4 d, and then transferred to hydroponic solutions containing 1 mm CaCl2 (K+ free) or 1 mm KCl and 1 mm NaCl for another 2 d. When plants were 6 d old, they were used for physiological and molecular investigations, as described below.
Isolation of RNA and Expression Analysis
All root samples for northern hybridization were cut from shoots and immediately frozen in liquid N2. Total barley root RNA was isolated using guanidium hydrochloride (Logemann et al., 1987). To isolate mRNA from wheat roots, 4-d-old wheat seedlings were transferred to hydroponic solutions containing 1 mm CaCl2 or 1 mm KCl. After 2 d, approximately 100 mg of root tissue per sample was homogenized in liquid N2, and total mRNA was isolated using the QuickPrep Micro mRNA purification kit (Pharmacia). The total amount of mRNA isolated was quantified spectroscopically.
Electrophoresis of the RNA was carried out in 1.2% agarose containing 17% formaldehyde, and RNA was blotted onto nylon membranes (Hybond N+, Amersham). Blots were probed by a 32P-labeled HindIII-XbaI fragment of the HKT1 clone (Schachtman and Schroeder, 1994) at 40°C for at least 16 h in 50% formamide, 5× SSPE (0.75 m NaCl, 50 mm NaH2PO4, and 5 mm EDTA, pH 7.4), 1% SDS, 5× Denhardt's solution (0.1% PVP and 0.1% BSA), and 125 mg mL−1 herring-sperm DNA. Membranes were washed twice at 40°C in 2× SSC (0.3 m NaCl and 0.03 m sodium citrate, pH 7), 0.1% SDS for 10 min, twice in 0.5× SCC, 0.1 SDS for 15 min, and twice in 0.25× SCC, 0.1 SDS for 20 min. Autoradiographs were exposed for 10 d. To generate an internal control for equivalent loading of lanes, the membranes were reprobed using a 32P-labeled 0.97-kb BstEII fragment of rice 28S rRNA excised from pRRbm2, which was constructed by subcloning the 3.7-kb BamHI fragment of pRR217 into pUC19 (Takaiwa et al., 1984). The autoradiograph was exposed for 10 to 20 min. Quantitative analysis of HKT1 mRNA levels in the time-course study was achieved by scanning x-ray films and measuring the relative densities of film exposure using NIH Image 1.54 software (National Institutes of Health, Bethesda, MD).
To determine the expression level of HKT1 mRNA in wheat roots, the method of quantitative competitive reverse-transcription PCR was used (Gilliland et al., 1990). For construction of the competitor molecule, the HKT1 cDNA subcloned into the SmaI site of the vector pGEM-HE (Liman et al., 1992) was cut with KpnI and StuI, blunted, and religated. This removed 783 bp from the N terminus of HKT1, including one EcoRI site at position 777. The religated vector with the C-terminal portion of the HKT1 cDNA contained two EcoRI sites at positions 1026 and 1113 of the full-length HKT1-coding region and one in the polylinker of pGEM-HE at the C-terminal end. To remove the EcoRI-EcoRI fragment from the HKT1-coding region, the DNA was partially digested with EcoRI. Isolation of the correct construct missing the 87-bp EcoRI-EcoRI fragment from the C-terminal part of the HKT1-coding region was verified by PCR. From this construct, mRNA was transcribed from the linearized plasmid using the mMESSAGE mMACHINE In Vitro Transcription Kit (Ambion, Inc., Austin, TX).
Oligo(dT)-primed reverse transcription was carried out with 20 μm of total mRNA and varying amounts of competitor RNA with the cDNA Cycle Kit (Invitrogen, San Diego, CA). The resulting single-stranded DNA was directly amplified in PCR reactions with the primers 5′-CATGATCAATAACCCCGAGG-3′ (forward, positions 900–919 of full-length HKT1 cDNA) and 5′-ATTAGCACAAACTTTCCTCC-3′ (reverse, positions 1516–1535) with the following cycle parameters: once at 94°C for 3 min; 40 times at 94°C for 1 min, 55°C for 2 min, 72°C for 3 min; and once at 72°C for 5 min. Amplified bands were analyzed on 2% agarose gels.
K+ Content and Uptake in Roots
At intervals after transfer to K+-free solutions, barley root and shoot samples were rinsed briefly in fresh K+-free solution and spun for 15 s in a basket centrifuge to remove superficial water. Samples were then weighed and placed in glass scintillation vials, which were ashed overnight in a furnace at 400°C. Ashes were dissolved in distilled water and analyzed for K+ with a flame photometer (Instrumentation Laboratory, Lexington, MA).
Uptake by wheat roots was measured by atomic-absorption spectrophotometry, as described by Benlloch et al. (1989). After 6 d of growth in hydroponic solutions, seedlings were rinsed in double-distilled water and for 5 min in a rinse buffer containing 10 mm Mes and 0.1 mm MgCl2, Ca(OH)2, pH 6.0. At time 0, seedlings were transferred to the uptake solution, which consisted of the rinse buffer plus 10 μm RbCl. Seedlings were removed from the uptake solution after incubations lasting up to 10 min and placed in an ice-cold buffer containing 5 mm CaCl2 and 1 mm KCl for 5 min. Subsequently, the plant tissue was carefully blotted on filter paper, and the roots were cut off to determine their fresh weights and then frozen at −80°C. For extraction of Rb+, the tissue was thawed and soaked in 1.5 mL of 10% acetic acid for 12 h and then washed with 10 mL of boiling water. The [Rb+] of the combined liquids was determined by atomic-absorption spectrophotometry.
Cation-Induced Depolarizations in Roots
The wheat plants from which HKT1 was isolated were used for cortical root-cell membrane depolarization measurements. Plants were grown hydroponically in the presence of either 1 mm CaCl2 or 1 mm KCl and 1 mm NaCl for 2 d after the 4-d germination period. Plant roots were pierced with quartz microelectrodes filled with 3 m KCl, and the membrane potentials of the cortical cells were measured using an electrometer (model DUO 773, World Precision Instruments, Sarasota, FL). Roots were perfused with 1.8 mm CaCl2, 6 mm MgCl2, and 10 mm Mes, adjusted to pH 6.0. K+, Cs+, and Rb+ were added as chloride salts to compare cation-induced depolarizations in K+-starved versus 1 mm K+-fed plants.
RESULTS
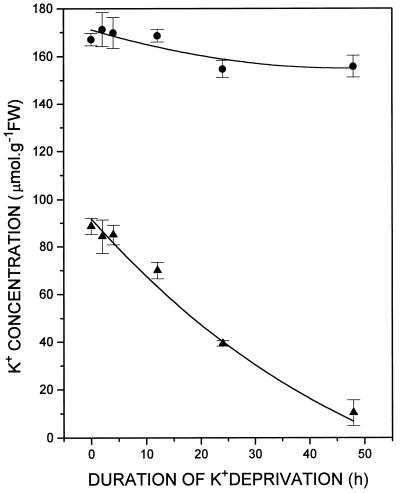
Barley plants previously grown in complete nutrient solution containing 1.2 mm K+ and then transferred to K+-free medium, had no significant reduction of either root or shoot K+ at 4 h (Fig. 1). By 12 h root [K+] had declined from 88.6 ± 3.5 μm mol g−1 at the time of transfer to 69.9 ± 3.5 μm mol g−1, a reduction of 21% (Fig. 1). During the same time there was no change in shoot [K+], which was 167 ± 2.6 μm mol g−1 at the time of transfer to minus K+ medium and remained at 168.6 ± 3 μm mol g−1 12 h later (Fig. 1).
Figure 1.
Time course of whole-root (▴) and whole-shoot (•) K+ concentration changes in the barley cv Klondike following transfer of intact plants from a complete inorganic nutrient medium containing 1.2 mm K+ to medium lacking K+. Means and se were determined for four replicates of approximately 10 plants each. FW, Fresh weight.
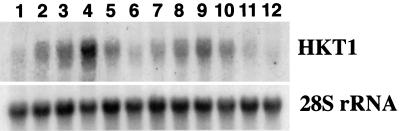
To investigate whether HKT1 mRNA levels are up-regulated during K+ deprivation, expression of HKT1 was investigated at intervals following removal of exogenous K+. Quantitative analysis of the northern blots presented in Figure 2 demonstrated that by 4 h after K+ withdrawal, the level of HKT1 expression had increased to >2-fold in the barley cv Jackson and approximately 1.5-fold in cv Klondike (Fig. 2, compare lanes 1 and 2 and 6 and 7). HKT1 RNA levels increased in spite of the lack of detectable changes in whole root or shoot [K+] by 4 h after withdrawing K+ (Fig. 1). Expression of HKT1 continued to increase until 12 h of K+ deprivation, both in cvs Jackson and Klondike (Fig. 2, lanes 4 and 9, respectively). Removing all sources of N from the growth medium for 4 and 16 h caused no increase of HKT1 expression; this treatment caused a 30% reduction of HKT1 expression (Fig. 2, lanes 11 and 12). By 24 h of K+ deprivation, HKT1 mRNA expression had declined slightly in cvs Jackson and Klondike from the 12-h peak levels (Fig. 2, lanes 5 and 10).
Figure 2.
Time course of HKT1 expression in roots of cvs Jackson (lanes 1–5) and Klondike (lanes 6–12). Plants were transferred to complete nutrient inorganic nutrient medium containing 1.25 mm K+ on d 4. On d 7 plants were transferred to medium lacking K+ for 0 h (lanes 1 and 6), 4 h (lane 2), 6 h (lanes 3 and 7), 8 h (lane 8), 12 h (lanes 4 and 9), and 24 h (lanes 5 and 10), or to medium lacking N for 16 h (lanes 11 and 12). Fifteen micrograms of total RNA was loaded into each lane. Lanes were probed with a 32P fragment of an HKT1 cDNA clone from wheat and reprobed with a 32P fragment of rice 28S rDNA as an internal control for gel loading.
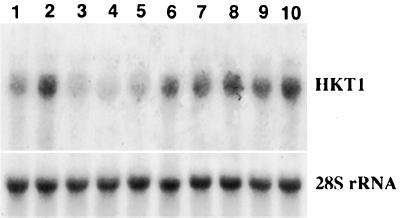
Studies of K+ (86Rb+) uptake in barley plants have commonly made use of plants grown in CaSO4 for 4 to 6 d to generate “low-salt” roots. These typically showed high rates of high-affinity K+ uptake (Epstein et al., 1963). The expression of the HKT1 gene was therefore examined in roots of barley plants grown for 4 or 5 d in CaSO4 without exogenous K+. As shown in Figure 3, HKT1 mRNA levels remained high after 4 d of K+ deprivation (Fig. 3, lane 1). HKT1 mRNA levels increased even further after 5 d of growth in K+-free medium (Fig. 3, lane 2), whereas HKT1 levels were low in 1.2 mm K+-grown controls (Figs. 2, lane 1, and 3, lane 3). Transfer of plants after d 4 to a complete medium containing 1.2 mm K+ for 1 d reduced the high level of HKT1 expression, but within 4 h of removing K+ (Fig. 3, lane 6) a high level of HKT1 expression was again restored to levels typical of 5 d of growth without K+ (Fig. 3, lane 2). Further growth without K+ (lanes 7–10) to a maximum of 24 h caused only a small increase of HKT1 expression beyond that at 4 h.
Figure 3.
Effect of growth on CaSO4 solution and subsequent provision and withdrawal of K+ on HKT1 mRNA expression in roots of the barley cv Jackson. Plants were grown on K+-free CaSO4 solution for 4 d (lane 1) or 5 d (lane 2). Plants were then exposed to 1.25 mm K+ for 1 d, which suppressed HKT1 mRNA levels. K+ was then withdrawn for 0 h (lane 3), 1 h (lane 4), 2 h (lane 5), 4 h (lane 6), 6 h (lane 7), 8 h (lane 8), 12 h (lane 9), and 24 h (lane 10). Fifteen micrograms of total RNA was loaded into each lane. Probing was as in Figure 2.
To distinguish the effects of the duration of K+ deprivation from any age dependency of the response, plants grown for 7, 8, or 9 d in a complete nutrient solution containing 1.25 mm K+ were subjected to 4 h of K+ deprivation, and the levels of HKT1 expression were determined by northern analysis. The rapid up-regulation of HKT1 was analyzed by measuring RNA levels after 4 h of K+ deprivation. Figure 4 reveals that the strongest response to K+ deprivation was at d 7. Beyond d 7, the relative increase in the level of HKT1 expression declined, although withholding K+ increased HKT1 mRNA expression in all cases.
Figure 4.
Effect of age and K+ deprivation on very rapid induction of HKT1 mRNA in roots of cv Jackson. RNA was extracted from 7-d (lanes 1 and 2), 8-d (lanes 3 and 4), or 9-d (lanes 5 and 6) seedlings that were either grown continuously in a complete inorganic nutrient medium containing 1.2 mm K+ (lanes 1, 3, and 5) or transferred from complete medium to a medium lacking K+ for 4 h before RNA extraction (lanes 2, 4, and 6).
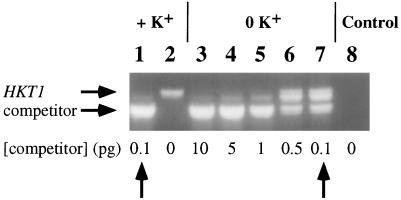
Because the HKT1 cDNA was initially isolated from wheat roots, the level of HKT1 mRNA was further analyzed in wheat plants. Competitive reverse-transcription PCR experiments (Gilliland et al., 1990) were performed on 6-d-old wheat seedlings to allow quantitative analysis of HKT1 mRNA levels. Roots grown in the presence of 1 mm K+ showed only very low HKT1 levels corresponding to less than 0.1 pg RNA (Fig. 5, lanes 1 and 2). Wheat roots grown in the absence of K+ (in 1 mm CaCl2) showed a strong enhancement in HKT1 mRNA levels 10- to 50-fold higher than roots grown in 1 mm KCl, based on quantitative PCR analysis (Fig. 5, lanes 3–7). These data show that in barley and wheat K+ deprivation leads to increased levels of HKT1 mRNA. Furthermore, in both species exposure of roots to only 1 to 1.2 mm extracellular K+ was sufficient to substantially reduce HKT1 mRNA levels.
Figure 5.
HKT1 mRNA levels in response to K+ deprivation in intact roots of the wheat cv Atlas 66. Agarose-gel analysis of a reverse-transcription competitive PCR experiment with mRNA isolated from roots grown in 1 mm K+ (lanes 1 and 2) and from roots grown without K+ in 1 mm CaCl2 (lanes 3–7). The amount of competitor RNA is given in picograms on the bottom. Expected band sizes for HKT1 mRNA and competitor RNA are indicated by horizontal arrows. The intermediate band most likely represents HKT1-competitor hybrids, as suggested by PCR experiments with purified DNA templates. Lane 8, Control experiment without added RNA.
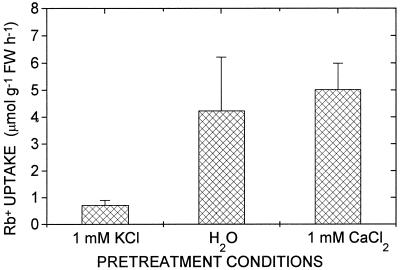
The question of whether the increased expression levels of HKT1 mRNA can be correlated with an increased high-affinity K+ uptake in roots, as is known for barley and maize (Glass, 1976; Kochian and Lucas, 1982; Fernando et al., 1992), was further analyzed by examining the response of wheat seedlings to altered K+ provision during growth. First, the effect of growing wheat seedlings in the presence of 1 mm extracellular K+ on high-affinity uptake was determined. When plants were pretreated with 1 mm K+, high-affinity Rb+ uptake in roots bathed in 10 μm Rb+ was low (Fig. 6). By contrast, plants grown in a K+-free medium (1 mm CaCl2 or water) showed high rates of high-affinity K+ (Rb+) uptake (Fig. 6). These data show that high-affinity uptake was strongly reduced in wheat roots grown in only 1 mm K+. The elevated rates of high-affinity Rb+ uptake in wheat roots evoked by K+ deprivation (Fig. 6) are similar to those reported for barley and other species (Williams, 1961; Epstein et al., 1963; Young and Sims, 1972; Glass and Dunlop, 1978; Kochian and Lucas, 1982; Fernando et al., 1990) and correlate with the strong level of expression of HKT1 mRNA in response to K+ deprivation (Figs. 2 and 5).
Figure 6.
High-affinity K+ (Rb+) uptake in wheat roots in response to K+ deprivation. High-affinity uptake was suppressed in roots grown in 1 mm KCl. In K+-depleted roots grown hydroponically in H2O or in 1 mm CaCl2, high-affinity Rb+ uptake was induced. Average uptake rates from 10 (CaCl2) or 3 (H2O) replicate experiments are illustrated. The [Rb+] was 10 μm. FW, Fresh weight.
Another method that may be used to analyze the up-regulation of high-affinity K+ uptake in K+-deprived roots is to measure depolarizations in roots in response to micromolar concentrations of extracellular K+ (Newman et al., 1987). Furthermore, analysis of depolarizations in wheat roots enables a comparison to be made with published biophysical transport properties of HKT1. Electrophysiological studies of HKT1 expressed in X. laevis oocytes have shown that the alkali cations K+, Rb+, and Cs+ can each cause depolarization (Rubio et al., 1996). This relatively low specificity among cations in triggering depolarizations via HKT1 was shown to be due to the characteristic reduction of HKT1-mediated outward currents by low concentrations of alkali cations and/or to cation uptake (Schachtman and Schroeder, 1994; Gassmann et al., 1996). Therefore, experiments were pursued to determine whether depolarizations in planta were also less specific among cations and whether these depolarizations were enhanced by K+ deprivation.
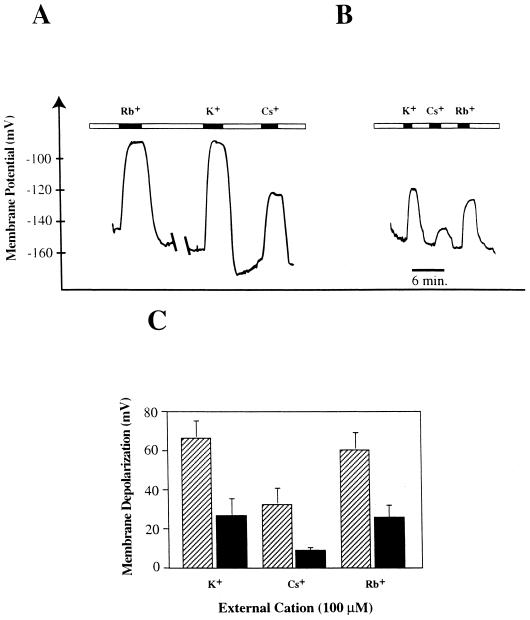
Depolarizations triggered by extracellular perfusion of roots with 100 μm K+, Cs+, or Rb+ were measured in wheat root cortical cells from plants grown in the absence and in the presence of 1 mm K+. In K+-depleted plants, exposure to 100 μm K+, Cs+, or Rb+ in the external solution caused large membrane depolarizations (Fig. 7A) that were substantially greater in magnitude than the depolarizations observed in 1 mm K+-grown plants under the same conditions (Fig. 7, B and C). These data show that alkali cation-induced depolarizations are enhanced by K+ starvation.
Figure 7.
Membrane potentials and depolarizations of wheat root cortical cells in response to alkali cations for plants grown in the presence or absence of K+. A, Membrane potential of root cortical cells of plants grown without K+ when the root was externally perfused with 100 μm Tris+ (open bars) and 100 μm K+, Cs+, or Rb+ (solid bars). B, Membrane potential of root cortical cells of plants grown in 1 mm K+ when the root was externally perfused with 100 μm Tris+ (open bars) and 100 μm K+, Cs+, or Rb+ (solid bars). C, Membrane depolarizations induced by 100 μm K+, Cs+, or Rb+ in K+-deprived plants (striped bars) and K+-supplied (1 mm) plants (solid bars). In K+-deprived plants 100 μm K+, Cs+, and Rb+ depolarized the membrane by 66.4 ± 9 mV (n = 13), 32 ± 8.8 mV (n = 4), and 60.5 ± 9 mV (n = 4), respectively. Corresponding values for 1 mm K+-grown plants were 26.4 ± 9 mV (n = 7), 8.3 ± 1.9 mV (n = 6), and 25 ± 6.8 mV (n = 7), respectively.
DISCUSSION
Our data show that HKT1 expression is rapidly up-regulated at the transcript level in roots of barley and wheat following withdrawal of extracellular K+. Down-regulation of HKT1 expression, by contrast, results from exposure of the roots to only 1 to 1.2 mm K+. Extensive earlier studies demonstrated the relative rapidity of increased or decreased plasma membrane K+ influx, respectively, in response to withholding or resupplying K+ to barley roots (Glass 1978; Fernando et al., 1990). In barley increased or decreased K+ fluxes were detected by 1 h after external [K+] was altered (Glass, 1978; Fernando et al., 1990). Furthermore, increases in both K+ uptake in roots and depolarizations by low alkali cation concentrations are elicited by withholding K+ and reduced by growth in 1 mm K+, which shows a correlation with previously described biophysical depolarization properties of HKT1. The correlations between patterns of high-affinity K+ influx in roots of barley and wheat and HKT1 expression are consistent with a contribution of HKT1 to the high-affinity K+-uptake systems described in classical uptake studies (Epstein et al., 1963; Glass, 1978; Kochian and Lucas, 1982).
The data presented in this report do not exclude contributions to (cation-induced) depolarizations and to measured high-affinity K+ uptake in plants from additional transporters, as pointed out previously (Schachtman and Schroeder, 1994; Maathuis et al., 1996; Rubio et al., 1996). Recently, a new gene family of plant K+-uptake transporters, named ATKT, HAK1, or AtKUP, was identified in Arabidopsis and barley (Quintero and Blatt, 1997; Santa-Maria et al., 1997; Fu and Luan, 1998; Kim et al., 1998). The barley HAK1 cDNA expressed in yeast and the AtKUP1 gene expressed in Arabidopsis were shown to mediate high-affinity Rb+ uptake (Santa-Maria, 1997; Kim et al., 1998). Additionally, AtKUP1 mediates a low-affinity K+ or Rb+ uptake at a high external K+ or Rb+ concentrations (Fu and Luan, 1998; Kim et al., 1998). The finding that HAK1 and AtKUP3 mRNA levels are also increased by withholding K+ supply (Santa-Maria et al., 1997; Kim et al., 1998) indicates that these specific members of this gene family may contribute to increased K+ influx associated with K+ deprivation. Time courses of expression and the transport mechanisms have not yet been determined for these HAK/KUP genes. Furthermore, the apparent complexity of high-affinity K+ uptake at the molecular level highlights the necessity for characterizing the time course of induction of individual components of transport to determine the relative importance of particular genes at different times and stages of development.
Regulation of HKT1 Expression by Root [K+]
The present findings provide experimental evidence for the hypothesis that the up-regulation of high-affinity K+ uptake in roots can occur rapidly via events affecting mRNA levels of K+ transporters. This need not preclude additional posttranslational regulation of K+-transport proteins. The age-dependence study of HKT1 expression in CaSO4-grown barley plants revealed that by d 4 the transcript level was already highly expressed in these K+-depleted plants compared with 1 mm K+-grown plants (Fig. 3). By d 5, HKT1 mRNA levels had increased further. Mechanistically, this gradual increase in HKT1 mRNA levels in CaSO4-grown plants over time may not be different from the rapid increase of HKT1 mRNA levels associated with the transfer of rapidly growing K+-replete plants to solutions lacking K+ (Fig. 2). Even without an exogenous source of K+, seeds are initially well supplied with K+ from seed reserves. Because these are diluted by growth without exogenous K+, influx increases over a period of approximately 8 d, after which time influx begins to decline (Fernando et al., 1990). Thus, the pattern of HKT1 expression and high-affinity K+ influx in barley were also correlated in this age-dependent manner. With increasing age the response to K+ deprivation declined (Fig. 4) so that by d 8 and 9 removal of K+ in K+-grown plants evoked a smaller enhancement in HKT1 mRNA expression levels. This result may have been due to the fact that by 8 and 9 d plants are larger and have accumulated sufficient K+ such that a short period of K+ deprivation (4 h) fails to perturb the K+ status as much as in younger plants.
Figures 1 and 2 indicate that following the removal of exogenous K+, increased levels of HKT1 expression were observed before a significant reduction in either whole-root or whole-shoot [K+] was apparent. Even after 24 h had elapsed, shoot [K+] was still at a level that was 93% of that before the K+ supply was interrupted (Fig. 1). However, by this time root [K+] had declined to 44% of its original value. In the analyses of gross tissue [K+] as presented here, it is largely vacuolar K+ that is being determined. Clearly, if the initiation of increased HKT1 transcription was evident after 4 h of K+ removal, before it was possible to detect changes in root [K+], it is likely that the signal responsible for regulating transcription is determined not by information from the vacuole, which is little changed by this stage, but by another pool, such as the extracellular space or cytosolic [K+].
It is now well established that cytoplasmic [K+] remains essentially constant when the K+ supply is perturbed (Memon et al., 1985; Walker et al., 1996a). If this is the case, then it is difficult to invoke cytoplasmic K+ as the K+ pool responsible for regulating HKT1 expression. Nevertheless, Hooymans (1974) observed that when exogenous K+ was withdrawn from barley roots, translocation of K+ to the shoot continued until root [K+] was reduced to the level of CaSO4-grown roots. This involved mobilization of vacuolar K+ for transport to the xylem via the cytoplasm. Therefore, the possibility exists that, in switching to vacuolar K+ as the source of K+ for xylem translocation, there may be a temporary perturbation of cytoplasmic K+ responsible for initiating the transcriptional events leading to elevated levels of HKT1 expression. Alternatively, the net flux of K+ from the vacuole to the cytoplasm may represent the responsible signal. During K+ translocation to the xylem, K+ is thought to be loaded from the cytoplasmic phase of xylem parenchyma cells (Lauchli, 1972; Drew et al., 1990). Using electron probe x-ray microanalysis, Drew et al. (1990) were able to demonstrate that the cytoplasmic [K+] of outer cortical cells was statistically lower than that of inner cortical cells in split-root experiments. Thus, although it is generally true that K+ homeostasis operates to maintain the constancy of cytoplasmic K+ as a first priority, perturbations of plant K+ resulting from modifying K+ supply to the roots may nevertheless cause some temporary perturbation of cytoplasmic [K+].
An alternative hypothesis is that induction of HKT1 is controlled by a K+ sensor located on the external surface of the plasma membrane of root cells that is capable of rapidly responding to altered concentrations of K+ in the extracellular space. However, this hypothesis appears insufficient to explain all of the observations, because in split-root experiments (Claassen and Barber, 1977; Drew et al., 1984), withdrawal of external K+ from one region of the root caused increased K+ influx into the K+-supplied roots as well as into the K+-deprived roots. Thus, up-regulation of K+ influx occurred in the K+-supplied roots despite a constant external [K+]. These results appear to signify a response to internal rather than external signals.
High-Affinity K+ Uptake and Depolarization in Roots
In addition to the above-discussed molecular and physiological events, exposure of maize roots to micromolar extracellular K+ concentrations results in immediate depolarizations (Newman et al., 1987). It is interesting that in wheat roots micromolar concentrations of the alkali cations K+, Rb+, and Cs+ all caused enhanced depolarizations in plants grown in K+-free medium (Fig. 7, A and C). Biophysical studies of HKT1 expressed in X. laevis oocytes have shown a low specificity for cation-induced depolarizations, which was attributed to inhibition of HKT1-mediated outward currents by low-permeability cations and/or by inward currents, depending on the cation and the conditions (Gassmann et al., 1996; Rubio et al., 1996). These analyses emphasize the fact that cation-induced depolarizations can occur through two simple mechanisms: inhibition of cation efflux or stimulation of cation influx. The assumption that membrane depolarization originates from an increased inward current through K+ transporters (Maathuis et al., 1996) is therefore not universally valid, and depolarizations cannot be used to unequivocally determine transport mechanisms of individual components. Nevertheless, the present observation that cation-induced depolarizations are strongly enhanced by K+ deprivation (Fig. 7) is consistent with the hypothesis that these depolarizations are directly linked to high-affinity K+-transport mechanisms, as originally revealed by Newman et al. (1987).
Multiple High-Affinity K+-Uptake Transporters
Despite the observed correlations between HKT1 expression levels and high-affinity K+-influx and membrane depolarizations in the present study, the finding that high-affinity K+ uptake via HKT1 is mediated by a Na+-K+ symport (Rubio et al., 1995; Gassman et al., 1996) has led to the suggestion that additional Na+-independent K+ transporters should exist in plants (Epstein et al.,1963; Rubio et al., 1995, 1996; Maathuis et al., 1996; Walker et al., 1996b). Na+-coupled high-affinity K+ uptake has been shown in charophyte algae (Smith and Walker, 1989), in root cells of Egeria and Elodea, and in leaves of Vallisneria (Maathuis et al., 1996). Whereas these studies demonstrate the presence of a Na+-coupled high-affinity K+-uptake mechanism in plants analogous to that of HKT1, the apparent absence of Na+-dependent uptake when using Rb+ as a tracer in barley, wheat, and Arabidopsis (Epstein and Hagen, 1952; Maathuis et al., 1996; Walker et al., 1996b) suggests that additional types of high-affinity K+ transporters exist in plants, as discussed previously (Rubio et al., 1996). Thus, the relative role of HKT1 expression, although correlated with high-affinity K+ influx, remains to be determined under different growth conditions and at different developmental stages.
The existence of multiple high-affinity transport systems for the acquisition of a single ion, e.g. the multiple high-affinity NO3− transporters of the crnA family (Trueman et al., 1996; Quesada et al., 1997), indicates that this pattern may be the norm. Recent studies have led to the identification of a novel class of K+ transporters in plants, with some members showing high-affinity 86Rb+ uptake in yeast (Santa-Maria et al., 1997) and in transgenic Arabidopsis cells (Kim et al., 1998). The demonstration that gene families with multiple members may also contribute to high-affinity K+ uptake indicates that we cannot at present determine the relative contributions of individual genes, including HKT1 or HAK/KUP, to high-affinity K+ uptake without the use of molecular genetic disruption (“knock out”) of individual genes. Nevertheless, although the precise role of the HKT1 system remains to be determined in vivo, it is interesting that its expression is so well correlated with patterns of high-affinity K+ influx and membrane depolarization in barley and wheat. Therefore, it is premature to presume that this system is of no significance (Walker et al., 1996b). The rapid up-regulation of HKT1 mRNA levels in response to K+ withdrawal provides a potent tool to further investigate the functional and molecular basis of K+ sensing in plants.
Footnotes
This research was supported by a Natural Sciences and Engineering Research Council of Canada grant to A.D.M.G. and by a U.S. Department of Agriculture grant to J.I.S.
LITERATURE CITED
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlloch M, Moreno I, Rodriguez-Navarro A. Two modes of rubidium uptake in sunflower plants. Plant Physiol. 1989;90:939–942. doi: 10.1104/pp.90.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen N, Barber SA. Potassium influx characteristics of corn roots and interaction with N, P, Ca and Mg influx. Agron J. 1977;69:860–864. [Google Scholar]
- Drew MC, Saker LR. Uptake and long-distance transport of phosphate, potassium and chloride in relation to internal ion concentration in barley: evidence of non-allosteric regulation. Planta. 1984;160:500–507. doi: 10.1007/BF00411137. [DOI] [PubMed] [Google Scholar]
- Drew MC, Saker LR, Barber SA, Jenkins W. Changes in the kinetics of phosphate and potassium absorption in nutrient-deficient barley roots measured by a solution-depletion technique. Planta. 1984;160:490–499. doi: 10.1007/BF00411136. [DOI] [PubMed] [Google Scholar]
- Drew MC, Webb J, Saker LR. Regulation of K+ uptake and transport to the xylem in barley roots; K+ distribution determined by electron probe x-ray microanalysis of frozen hydrated cells. J Exp Bot. 1990;41:815–826. [Google Scholar]
- Epstein E (1972) Mineral Nutrition of Plants: Principles and Perspectives. John Wiley, New York
- Epstein E, Hagen CE. A kinetic study of the absorption of alkali cations by barley roots. Plant Physiol. 1952;27:457–474. doi: 10.1104/pp.27.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Rains DW. Carrier-mediated cation transport in barley roots: kinetic evidence for a spectrum of active sites. Proc Natl Acad Sci USA. 1965;53:1320–1324. doi: 10.1073/pnas.53.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Rains DW, Elzam OE. Resolution of dual mechanisms of potassium absorption by barley roots. Proc Natl Acad Sci USA. 1963;49:684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando M, Kulpa J, Siddiqi MY, Glass ADM. Potassium-dependent changes in the expression of membrane-associated proteins in barley roots. 1. Correlations with K+ (86Rb+) influx and root K+ concentration. Plant Physiol. 1990;92:1128–1132. doi: 10.1104/pp.92.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando M, Mehroke J, Glass ADM. De novo synthesis of plasma membrane and tonoplast polypeptides of barley roots during short-term K+ deprivation. In search of the high-affinity K+ transport system. Plant Physiol. 1992;100:1269–1276. doi: 10.1104/pp.100.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H-H, Luan S. AtKUP1: A dual-affinity K+ transporter from Arabidopsis. Plant Cell. 1998;10:63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann W, Rubio F, Schroeder JI. Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J. 1996;10:869–882. doi: 10.1046/j.1365-313x.1996.10050869.x. [DOI] [PubMed] [Google Scholar]
- Gilliland G, Perrin S, Bunn HF. Competitive PCR for quantitation of mRNA. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. San Diego, CA: Academic Press; 1990. pp. 60–69. [Google Scholar]
- Glass ADM. The regulation of potassium absorption in barley roots. Plant Physiol. 1975;56:377–380. doi: 10.1104/pp.56.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass ADM. The regulation of potassium absorption in barley roots: an allosteric model. Plant Physiol. 1976;58:33–37. doi: 10.1104/pp.58.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass ADM. The regulation of K+ influx into intact roots of barley (Hordeum vulgare (L) cv. Conquest) by internal K+ Can J Bot. 1978;56:1759–1764. [Google Scholar]
- Glass ADM, Dunlop J. The influence of potassium content on the kinetics of potassium influx into excised ryegrass and barley roots. Planta. 1978;141:117–119. doi: 10.1007/BF00387753. [DOI] [PubMed] [Google Scholar]
- Guy M, Zabala G, Filner P. The kinetics of chlorate uptake by XD tobacco cells. Plant Physiol. 1988;86:817–821. doi: 10.1104/pp.86.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooymans JJM. Role of cell compartments in the redistribution of K and Na ions absorbed by the roots of intact barley plants. Z Pflanzenphysiol. 1974;73:234–242. [Google Scholar]
- Kim EJ, Kwak JM, Uozumi N, Schroeder JI. AtKUP1: An Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell. 1998;10:51–62. doi: 10.1105/tpc.10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Gaber RF. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1991;8:4266–4273. doi: 10.1128/mcb.11.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ. Potassium transport in corn roots. I. Resolution of kinetics into a saturable and linear component. Plant Physiol. 1982;70:1723–1731. doi: 10.1104/pp.70.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J. 1996;9:195–203. doi: 10.1046/j.1365-313x.1996.09020195.x. [DOI] [PubMed] [Google Scholar]
- Lauchli A. Translocation of inorganic solutes. Annu Rev Plant Physiol. 1972;23:197–218. [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. Contrasting roles in ion transport of two K+ channel types in root cells of Arabidopsis thaliana. Planta. 1995;197:456–464. doi: 10.1007/BF00196667. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Verlin D, Smith FA, Sanders D, Fernandez JA, Walker NA. The physiological relevance of Na+-coupled K+ transport. Plant Physiol. 1996;112:1609–1616. doi: 10.1104/pp.112.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon AR, Saccomani M, Glass ADM. Efficiency of potassium utilization by barley varieties: the role of subcellular compartments. J Exp Bot. 1985;36:1860–1876. [Google Scholar]
- Newman IA, Kochian LV, Grusak MA, Lucas WJ. Fluxes of H+ and K+ in corn roots. Characterization and stoichiometries using ion-selective microelectrodes. Plant Physiol. 1987;84:1177–1184. doi: 10.1104/pp.84.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Krapp A, Trueman LJ, Daniel-Vedele F, Fernandez E, Forde BG, Caboche M. PCR-identification of a Nicotiana plumbaginifolia cDNA homologous to the high-affinity nitrate transporters of the crnA family. Plant Mol Biol. 1997;34:265–274. doi: 10.1023/a:1005872816881. [DOI] [PubMed] [Google Scholar]
- Quintero F, Blatt M. A new family of K+ transporters from Arabidopsis that are conserved across phyla. FEBS Lett. 1997;415:206–211. doi: 10.1016/s0014-5793(97)01125-3. [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. High-affinity potassium uptake in plants. Science. 1996;273:978–979. doi: 10.1126/science.273.5277.978. [DOI] [PubMed] [Google Scholar]
- Santa-Maria GE, Rubio F, Dubcovsky J, Rodriguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9:2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman D, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Smith FA, Walker NA. Transport of potassium in Chara australis. I. A symport with sodium. J Membr Biol. 1989;108:125–137. doi: 10.1007/BF01869452. [DOI] [PubMed] [Google Scholar]
- Takaiwa F, Oono K, Sugiura M. The complete nucleotide sequence of a rice 17S rRNA gene. Nucleic Acids Res. 1984;12:5444–5448. doi: 10.1093/nar/12.13.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueman LJ, Richardson A, and Forde BG (1996) Molecular cloning of higher plant homologues of the high-affinity nitrate transporters of Chlamydomonas reinhardtii and Aspergillus nidulans. Gene 175: 223–231 [DOI] [PubMed]
- Walker DJ, Leigh RA, Miller AJ. Potassium homeostasis in vacuolate plant cells. Proc Natl Acad Sci USA. 1996a;93:10510–10514. doi: 10.1073/pnas.93.19.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NA, Sanders D, Maathuis FJM. High-affinity potassium uptake in plants. Science. 1996b;273:977–978. doi: 10.1126/science.273.5277.977. [DOI] [PubMed] [Google Scholar]
- Williams DE. The absorption of potassium as influenced by its concentration in the nutrient medium. Plant Soil. 1961;15:387–399. [Google Scholar]
- Young M, Sims AP. The potassium relations of Lemna minor L. I. Potassium uptake and plant growth. J Exp Bot. 1972;23:958–969. [Google Scholar]