Abstract
Background
Ovarian cancer stem cells are characterized by self-renewal capacity, ability to differentiate into distinct lineages, as well as higher invasiveness and resistance to many anticancer agents. Since they may be responsible for the recurrence of ovarian cancer after initial response to chemotherapy, development of new therapies targeting this special cellular subpopulation embedded within bulk ovarian cancers is warranted.
Methods
A high-throughput screening (HTS) campaign was performed with 825 compounds from the Mechanistic Set chemical library [Developmental Therapeutics Program (DTP)/National Cancer Institute (NCI)] against ovarian cancer stem-like cells (CSC) using a resazurin-based cell cytotoxicity assay. Identified sets of active compounds were projected onto self-organizing maps to identify their putative cellular response groups.
Results
From 793 screening compounds with evaluable data, 158 were found to have significant inhibitory effects on ovarian CSC. Computational analysis indicates that the majority of these compounds are associated with mitotic cellular responses.
Conclusions
Our HTS has uncovered a number of candidate compounds that may, after further testing, prove effective in targeting both ovarian CSC and their more differentiated progeny.
Keywords: High-throughput screening, Ovarian cancer, Cancer stem cells
Background
Ovarian cancer is the most lethal of gynecological cancers [1] despite its typically high initial response rate to chemotherapy [2]. Recent evidence supports the existence of ovarian cancer stem-like cells (CSC), characterized by self-renewal capacity, ability to differentiate into distinct lineages, high invasiveness and resistance to a number of anticancer agents [3-6]. Since CSC have been shown to be resistant to most current chemotherapies, the frequent recurrence of ovarian cancer is believed, at least in part, to be attributable to the existence of chemo-resistant sub-populations of cancer cells embedded within bulk tumors [6]. For this reason, there is considerable current interest in the development of new chemotherapies that can effectively target this insidious subpopulation of tumor cells [7].
Thus far, searches for compounds that may be therapeutically effective against CSC have employed two alternative strategies. One approach has been to evaluate molecules known to be inhibitory against pathways believed to be deregulated in CSC (e.g., the Hedgehog, NOTCH, PTEN/AKT and WNT/β-catenin signaling pathways) [8]. This approach has resulted in the identification of several potential therapeutic agents that are currently in clinical trials [9]. A second approach is the high-throughput screening (HTS) of CSC-enriched cell populations with libraries of potential inhibitory compounds. This approach has been productively employed to identify candidate compounds displaying cytotoxic/inhibitory effects on breast cancer [10] and glioma [11,12] CSC.
We have recently reported the isolation and characterization of ovarian CSC from an established ovarian cancer cell line OVCAR-3 [13]. These cells display a variety of features and molecular profiles characteristic of CSC previously isolated from ovarian and other cancer tissues [14-17]. Here we report the results of a high-throughput screening of 825 potential drugs (the National Cancer Institute’s “Mechanistic Set” library) [18,19] against ovarian CSC and the subsequent identification of compounds that display significant potential for future development as ovarian CSC therapeutic agents.
Methods
Cells
Spheroids were derived from OVCAR-3 cell line as previously described [13] and grown in the stem cell medium (SCM): DMEM/F12 (1:1) supplemented with 0.4% bovine serum albumin (BSA, Sigma-Aldrich, Inc. St. Louis, MO), 20 ng/mL epidermal growth factor (EGF, Invitrogen Corporation, Carlsbad, CA), 10 ng/mL basic fibroblast growth factor (bFGF, Sigma-Aldrich), 5 μg/mL insulin (Sigma-Aldrich) and 1% antibiotic-antimycotic solution (Mediatech-Cellgro, Manassas, VA) in 100 mm ultra-low attachment Petri dishes (Corning Incorporated, Corning, NY). Spheroids grown under these conditions were dissociated weekly using 0.05% trypsin-0.02% EDTA solution (Lonza, Walkerswille, MD) and sub-cultured until the amount of cells was adequate for HTS.
Compounds
The NCI Mechanistic Set was provided by the Developmental Therapeutic Program (NCI/NIH) as a set of 825 compounds plated in eleven 96-well plates (plate numbers: 4520–4530; suffix: 69). Basic information on these compounds can be retrieved from the DTP website [20] using plate number as the search parameter. These compounds were selected from 37,836 compounds in the NCI repository to represent a broad range of growth inhibition patterns in the NCI 60 cell line screen [18,19] and consequently, they likely represent a diversity of the modes of action of these compounds. Compounds were supplied by DTP as 1 mM solutions in DMSO.
HTS and data analysis
Spheroids were dissociated to single cells using trypsin; trypsin was neutralized using Soybean Trypsin Inhibitor (Life Technologies, Grand Island, NY; Catalogue # 17075029), and cells were re-suspended in SCM to a density of 50,000 cells/mL. Cells were plated into flat bottom ultra-low attachment 96 well plates (Corning, Product #3474) in a volume of 198 μL per well (200 μL of SCM for blank wells) and incubated for 24 h at 37°C and humidified atmosphere with 5% CO2. Drug dilutions were prepared as follows: the eleven supplied NCI Mechanistic Set plates were copied (4 μL of DMSO solution per well) into sterile, round bottom polypropylene 96-well plates (Corning, Product #3359) and each drug was diluted with 22 μL media (working concentrations 153.8 μM). 3 μL of diluted library were added to 198 μL of cells (4 replicated wells for each drug), which resulted in final drug concentration of 2.29 μM. The plates were incubated for 96 h at 37°C and humidified atmosphere with 5% CO2. Thereafter, 20 μL of TOX8 reagent (Sigma-Aldrich) were added to each well and after 4-h incubation fluorescence intensities were measured for each well at 560 nm (excitation) and 590 nm (emission). The resazurin (Alamar blue)-based TOX8 reagent has been previously established as a reliable method for determining cell viability/cytotoxicity of tumor spheroid cell cultures [21,22].
Fluorescence intensities for replicated wells were analyzed using Grubb’s test for detecting outliers at critical Z = 1.48 and outliers were removed from the dataset (test was applied only once for each replicated set of values). Percent growth of treated cells relative to untreated control cells was calculated from fluorescence intensities using the formula:
Other parameters recommended by Inglese et al. [23], HTS plate design, assay performance evaluation and systematic error detection are presented in Additional file 1.
Of the 793 compounds that passed our assay performance standard, 99 displayed a single outlier among 4 replicated fluorescence intensity values and these outliers were removed before % growth values were calculated.
Statistical significance of differences between fluorescence intensities of drug-treated and untreated control wells was evaluated using Welch’s t-test followed by Holm’s step-down method for multiplicity adjustment [24]. Two-sided p-values were determined by Welch’s t-test from raw fluorescence intensities corresponding to replicated treated and untreated control wells. These p-values represent probabilities that the difference between mean signal intensities for treated and control wells were obtained by chance. Holm’s procedure was applied to 793 p-values (compounds that passed assay performance test) to counteract the problem of multiple comparisons and to ascertain that the probability of falsely identifying one or more compounds as significantly affecting growth of ovarian cancer stem-like cells is not more than 10%. Thus, compounds selected by controlling for family-wise error rate (FWER) of 0.10 were classified as compounds with statistically significant effect on growth of ovarian cancer stem-like cells. Drugs with % growth 80-110% were considered as compounds with no effect on growth of ovarian cancer stem-like cells.
Results of HTS were interpreted (i) in the context of the activity of FDA-approved drugs present in the library; (ii) in comparison with the potencies of screened compounds against OVCAR-3 cell line (parental cells from which CSC were isolated), and (iii) via mapping to self-organizing maps (SOMs).
A list of 97 FDA-approved oncology drugs was built from the data on the Approved Oncology Drug Set accessible at the DTP website [25] and made available in Additional file 2.
Potencies of library compounds against OVCAR-3 cell line (Dec 2010 release) were retrieved from the DTP website [26]. If multiple GI50 values were available for the same compound, the average was taken without inclusion of default values (Note: NCI does not provide descriptive statistics (SD or SEM) for the determined GI50 values for tested cells).
All 793 library compounds that passed our assay performance criteria and selected subsets of compounds active against CSC were mapped onto SOMs using the web-based tool 3D MIND developed by the Covell group at the National Cancer Institute [27]. The SOM method represents a type of artificial neural network trained using unsupervised learning to cluster high dimensional data and project them into a low dimensional space. SOMs used in this work was generated from GI50 values for ~30,000 compounds across 60 cell lines and consists of 1,350 hexagonal clusters with 9 major cellular response categories: mitosis (M), membrane function (N), nucleic acid metabolism (S), metabolic stress and cell survival (Q), kinases/phosphatases and oxidative stress (P) and 4 unexplored regions (RFJV) [28]. The significance of differences in the distribution of CSC active subsets and all library compounds to these areas was evaluated using Fisher’s exact test with Yate’s continuity correction and the difference was considered significant for two-sided p-value < 0.01.
Determination of GI50
GI50 values (concentrations of tested agents that inhibited growth of CSC cell cultures after 96-h incubation to 50% of the untreated control) were determined for 5 compounds (NSC72961, NSC302979, NSC82116, NSC673622 and NSC243928) randomly selected from those resulting in ≤ 50% cellular growth relative to untreated controls. GI50 values were determined from concentration-response data generated by the same assay and cell system as used in our HTS. For each compound, 5 concentrations were used (15.6 nM, 62.5 nM, 250 nM, 1000 nM and 4,000 nM). Percent growth (control based normalization) was calculated as described in (Additional file 1: Table S1) and GI50 values were determined by non-linear regression of log-transformed data using a normalized response-variable slope model (GraphPad Prism 5.01; GraphPad Software, Inc.) and expressed as mean ± SEM.
Results and discussion
HTS identified over 100 compounds that significantly inhibit ovarian CSC growth
NCI’s Developmental Therapeutics Program (DTP) maintains a repository of synthetic and naturally occurring potential anticancer drugs. A sub-set of these compounds, termed the “Mechanistic Set”, represents the range of distinct growth inhibition patterns observed when the repository compounds were tested against the NCI-60 panel of cancer cell lines [18,19].
The CSC culture used in this study was derived from OVCAR-3 cells and is composed of tumor spheroids enriched with slowly proliferating self renewing stem-like cells that have been previously demonstrated to resist apoptosis after detachment from the surface (anoikis), a property known to be prerequisite for invasion and metastasis [13]. In addition, these cells have been shown to display significantly higher invasiveness, migration potential, and resistance to standard anticancer agents relative to the parental OVCAR-3 cells, as well as an ability to differentiate from a CD44-positive/mesenchymal-like phenotype to CD44-negative/epithelial like phenotype [13].
CSC were seeded in 96-well plates and allowed to proliferate for 24 h prior to exposure to the 825 compounds comprising the NCI Mechanistic Set of experimental compounds (chemical library). After 96 h of exposure, % growth of treated cells relative to untreated controls was determined. To reduce the possibility of spurious results, we conducted an assay performance test and subsequently excluded 32 compounds from our survey that were associated with unreliable results (Additional file 3). Of the remaining 793 compounds (Additional file 4), 329 (41.5%) did not display an appreciable effect on ovarian CSC growth (80-110% growth), while 161 compounds (20.3%) displayed a statistically significant effect at FWER=0.10 with 158 compounds displaying an inhibiting effect (3.3-74.1% growth) and 3 compounds displaying a stimulatory effect (135.6-158.7% growth).
In order to focus on the most inhibitory compounds, we operationally defined CSC inhibitory compounds as those displaying a ≤ 50 % cell growth relative to untreated controls. Based on these criteria, 136 of the 793 compounds (17.2%) were classified as inhibitory (range of inhibitory growth: 3.3-50.4%) (Table 1). These 136 inhibitory compounds are listed in Table 1 by a NCI compound number (NSC). A full list the various names associated with each NCI compound number is available as an ASCII file at the National Cancer Institute’s Developmental Therapeutics Program website [29].
Table 1.
List of 136 CSC-inhibitory compounds identified by HTS (%growth ≤ 50%, p-adj. ≤ 0.1) compared to GI50 of OVCAR-3 cells
| NSC | %growth | SD | p-value | p-adj (Holms) | GI50(-log 10) OVCAR-3 |
|---|---|---|---|---|---|
| 618332 |
3.3 |
4.0 |
3.73E-07 |
2.78E-04 |
5.08 |
| 219734 |
4.2 |
2.0 |
9.47E-07 |
6.88E-04 |
6.43 |
| 128305 |
4.9 |
3.4 |
8.02E-06 |
5.59E-03 |
4.34 |
| 168597 |
5.1 |
5.5 |
2.42E-06 |
1.73E-03 |
7.69 |
| 328426 |
5.3 |
3.2 |
4.64E-07 |
3.44E-04 |
7.62 |
| 143648 |
5.6 |
2.3 |
1.64E-07 |
1.23E-04 |
7.43 |
| 165563 |
6.1 |
4.4 |
3.58E-07 |
2.67E-04 |
8.05 |
| 145366 |
7.1 |
2.1 |
5.21E-08 |
4.00E-05 |
5.75 |
| 636132 |
7.3 |
3.2 |
9.47E-07 |
6.89E-04 |
4.00 |
| 323241 |
7.7 |
2.4 |
1.59E-07 |
1.20E-04 |
7.54 |
| 622732 |
8.0 |
4.9 |
4.93E-06 |
3.46E-03 |
4.90 |
| 208913 |
8.0 |
3.5 |
8.17E-07 |
5.96E-04 |
3.80 |
| 306864 |
8.0 |
3.1 |
3.86E-07 |
2.88E-04 |
5.76 |
| 4320 |
8.0 |
3.3 |
6.94E-08 |
5.31E-05 |
9.56 |
| 3053 |
8.4 |
3.9 |
1.38E-08 |
1.07E-05 |
8.54 |
| 265450 |
8.5 |
3.2 |
8.14E-08 |
6.21E-05 |
7.20 |
| 354844 |
8.6 |
6.0 |
4.34E-07 |
3.22E-04 |
7.26 |
| 164914 |
8.8 |
3.7 |
2.95E-09 |
2.32E-06 |
6.75 |
| 63701 |
8.9 |
3.4 |
1.00E-09 |
7.88E-07 |
7.46 |
| 697726 |
9.0 |
3.5 |
5.32E-09 |
4.16E-06 |
7.56 |
| 637578 |
9.1 |
2.3 |
1.04E-07 |
7.87E-05 |
8.36 |
| 614928 |
9.3 |
2.0 |
7.60E-07 |
5.56E-04 |
4.51 |
| 667467 |
9.8 |
3.9 |
3.53E-10 |
2.79E-07 |
4.00 |
| 65937 |
10.2 |
2.1 |
1.08E-07 |
8.16E-05 |
- |
| 65423 |
10.4 |
3.1 |
2.61E-10 |
2.06E-07 |
5.78 |
| 690634 |
10.8 |
2.9 |
7.52E-07 |
5.51E-04 |
7.59 |
| 24559 |
10.8 |
3.6 |
9.91E-11 |
7.86E-08 |
7.35 |
| 18268 |
10.9 |
3.4 |
2.24E-08 |
1.73E-05 |
8.41 |
| 243023 |
11.0 |
3.4 |
4.48E-06 |
3.15E-03 |
8.07 |
| 635448 |
11.1 |
5.9 |
1.19E-08 |
9.27E-06 |
7.69 |
| 7525 |
11.3 |
2.4 |
4.99E-06 |
3.49E-03 |
7.60 |
| 616232 |
11.6 |
4.0 |
3.97E-10 |
3.13E-07 |
4.00 |
| 269754 |
11.9 |
8.4 |
1.62E-04 |
1.03E-01 |
8.10 |
| 680506 |
12.4 |
5.0 |
4.85E-07 |
3.58E-04 |
- |
| 58514 |
12.5 |
3.1 |
1.34E-08 |
1.04E-05 |
9.52 |
| 353527 |
12.8 |
4.1 |
7.25E-07 |
5.32E-04 |
7.77 |
| 106408 |
12.8 |
2.4 |
1.29E-06 |
9.30E-04 |
7.34 |
| 620358 |
13.0 |
5.5 |
1.37E-05 |
9.27E-03 |
5.72 |
| 65104 |
13.2 |
4.3 |
9.57E-07 |
6.95E-04 |
8.76 |
| 325319 |
13.2 |
2.7 |
7.94E-07 |
5.80E-04 |
8.23 |
| 328166 |
13.4 |
5.4 |
4.72E-07 |
3.49E-04 |
7.35 |
| 658144 |
13.4 |
2.6 |
1.51E-08 |
1.17E-05 |
6.68 |
| 260610 |
13.9 |
5.5 |
2.48E-06 |
1.77E-03 |
6.79 |
| 631529 |
14.5 |
3.2 |
4.08E-07 |
3.04E-04 |
5.82 |
| 85236 |
14.5 |
2.5 |
8.67E-08 |
6.61E-05 |
5.82 |
| 376265 |
14.7 |
7.5 |
1.41E-05 |
9.53E-03 |
8.50 |
| 349644 |
14.9 |
4.6 |
2.95E-05 |
1.95E-02 |
7.72 |
| 89671 |
15.0 |
2.5 |
6.73E-07 |
4.95E-04 |
7.55 |
| 105808 |
15.3 |
3.8 |
1.15E-05 |
7.87E-03 |
5.96 |
| 635121 |
16.0 |
5.3 |
9.44E-06 |
6.53E-03 |
5.52 |
| 93419 |
17.1 |
7.4 |
2.92E-05 |
1.93E-02 |
6.13 |
| 268251 |
17.4 |
6.2 |
2.99E-06 |
2.12E-03 |
8.96 |
| 202000 |
17.7 |
5.0 |
3.03E-09 |
2.38E-06 |
4.05 |
| 659999 |
18.0 |
6.4 |
1.30E-08 |
1.01E-05 |
5.99 |
| 172924 |
18.5 |
3.1 |
7.81E-08 |
5.97E-05 |
6.67 |
| 673622 |
18.5 |
9.1 |
1.83E-05 |
1.23E-02 |
6.59 |
| 146604 |
19.2 |
4.6 |
2.41E-09 |
1.90E-06 |
6.65 |
| 349156 |
19.5 |
2.6 |
1.73E-06 |
1.24E-03 |
6.92 |
| 526417 |
19.7 |
3.8 |
5.38E-08 |
4.13E-05 |
9.28 |
| 400978 |
20.3 |
2.4 |
7.26E-09 |
5.67E-06 |
7.97 |
| 24817 |
20.4 |
5.8 |
2.23E-08 |
1.72E-05 |
7.67 |
| 319726 |
20.6 |
4.0 |
5.30E-09 |
4.15E-06 |
8.00 |
| 614826 |
20.8 |
5.2 |
1.41E-05 |
9.52E-03 |
6.66 |
| 679524 |
20.9 |
3.5 |
1.94E-10 |
1.54E-07 |
7.50 |
| 629301 |
20.9 |
5.5 |
2.23E-05 |
1.49E-02 |
4.80 |
| 129414 |
21.1 |
7.4 |
5.48E-05 |
3.58E-02 |
7.41 |
| 337766 |
21.4 |
5.8 |
1.20E-08 |
9.34E-06 |
6.73 |
| 7532 |
21.7 |
8.3 |
3.12E-06 |
2.21E-03 |
8.00 |
| 172946 |
21.7 |
5.6 |
1.25E-05 |
8.50E-03 |
7.46 |
| 328587 |
22.1 |
4.6 |
2.91E-09 |
2.29E-06 |
5.77 |
| 34391 |
22.2 |
4.2 |
4.20E-07 |
3.12E-04 |
5.60 |
| 153858 |
22.5 |
4.8 |
9.62E-07 |
6.97E-04 |
9.48 |
| 625483 |
22.8 |
9.7 |
2.53E-05 |
1.68E-02 |
4.66 |
| 82116 |
23.0 |
5.7 |
1.02E-07 |
7.75E-05 |
- |
| 72961 |
23.1 |
4.4 |
1.27E-07 |
9.59E-05 |
5.60 |
| 65380 |
23.6 |
6.9 |
9.38E-05 |
6.03E-02 |
7.20 |
| 85700 |
23.7 |
4.7 |
1.18E-08 |
9.20E-06 |
5.98 |
| 336628 |
23.7 |
5.9 |
2.62E-07 |
1.97E-04 |
4.77 |
| 345647 |
23.8 |
48.5 |
1.03E-05 |
7.09E-03 |
6.59 |
| 102811 |
23.9 |
5.3 |
1.99E-08 |
1.54E-05 |
6.96 |
| 669356 |
24.5 |
8.1 |
2.09E-06 |
1.49E-03 |
8.00 |
| 7521 |
24.5 |
6.9 |
9.49E-05 |
6.08E-02 |
7.52 |
| 352876 |
24.7 |
6.7 |
4.35E-05 |
2.86E-02 |
6.27 |
| 148958 |
24.9 |
6.9 |
1.03E-07 |
7.81E-05 |
3.09 |
| 613009 |
25.0 |
5.7 |
3.89E-06 |
2.75E-03 |
7.41 |
| 10447 |
25.5 |
6.6 |
3.49E-07 |
2.61E-04 |
4.65 |
| 301460 |
25.6 |
5.0 |
2.20E-06 |
1.57E-03 |
8.00 |
| 605756 |
25.8 |
6.4 |
6.31E-07 |
4.65E-04 |
5.68 |
| 407806 |
26.5 |
3.1 |
1.95E-06 |
1.40E-03 |
7.44 |
| 67574 |
27.0 |
6.0 |
1.21E-06 |
8.74E-04 |
7.68 |
| 700582 |
27.2 |
5.8 |
1.02E-07 |
7.74E-05 |
6.57 |
| 304421 |
27.9 |
4.2 |
4.98E-06 |
3.49E-03 |
6.47 |
| 521777 |
28.1 |
6.1 |
3.29E-08 |
2.53E-05 |
8.00 |
| 330500 |
29.0 |
4.8 |
6.10E-07 |
4.50E-04 |
6.05 |
| 255109 |
29.6 |
4.9 |
1.15E-05 |
7.84E-03 |
7.02 |
| 302979 |
30.4 |
10.8 |
5.65E-05 |
3.68E-02 |
5.43 |
| 33410 |
30.8 |
5.6 |
6.80E-08 |
5.21E-05 |
7.98 |
| 693632 |
30.8 |
7.4 |
2.11E-07 |
1.58E-04 |
6.44 |
| 24818 |
31.0 |
5.3 |
9.92E-06 |
6.85E-03 |
8.12 |
| 24819 |
31.1 |
4.7 |
1.14E-06 |
8.25E-04 |
8.47 |
| 52141 |
31.3 |
6.0 |
1.14E-06 |
8.24E-04 |
7.14 |
| 96932 |
31.4 |
6.9 |
6.94E-07 |
5.10E-04 |
6.77 |
| 349155 |
31.7 |
7.1 |
1.99E-05 |
1.33E-02 |
6.45 |
| 97911 |
32.4 |
7.6 |
6.23E-05 |
4.05E-02 |
5.70 |
| 243928 |
32.5 |
9.0 |
8.80E-06 |
6.11E-03 |
5.69 |
| 83265 |
34.8 |
6.7 |
4.05E-06 |
2.86E-03 |
5.70 |
| 637993 |
34.8 |
9.9 |
8.30E-05 |
5.36E-02 |
5.50 |
| 145669 |
34.9 |
6.6 |
3.26E-07 |
2.44E-04 |
7.75 |
| 157930 |
34.9 |
6.5 |
5.26E-06 |
3.68E-03 |
6.16 |
| 132791 |
35.2 |
15.3 |
1.52E-04 |
9.67E-02 |
6.96 |
| 331757 |
35.6 |
15.4 |
1.55E-04 |
9.84E-02 |
5.11 |
| 269142 |
36.0 |
10.3 |
9.02E-06 |
6.25E-03 |
6.68 |
| 1906 |
36.2 |
3.5 |
4.46E-08 |
3.43E-05 |
4.15 |
| 14229 |
37.6 |
6.1 |
9.93E-08 |
7.56E-05 |
5.55 |
| 1620 |
37.7 |
8.4 |
4.88E-06 |
3.43E-03 |
4.00 |
| 622627 |
38.4 |
11.4 |
1.82E-05 |
1.22E-02 |
5.28 |
| 215989 |
38.6 |
7.1 |
2.91E-06 |
2.07E-03 |
- |
| 13973 |
40.2 |
5.5 |
1.38E-06 |
9.94E-04 |
6.28 |
| 332598 |
40.6 |
7.4 |
2.18E-05 |
1.45E-02 |
10.38 |
| 126727 |
41.3 |
11.0 |
6.20E-05 |
4.04E-02 |
- |
| 248436 |
41.4 |
9.5 |
1.35E-05 |
9.15E-03 |
4.08 |
| 705330 |
41.6 |
10.5 |
1.35E-05 |
9.17E-03 |
5.70 |
| 166454 |
41.9 |
7.2 |
8.76E-07 |
6.39E-04 |
5.41 |
| 84074 |
42.9 |
7.3 |
1.84E-05 |
1.23E-02 |
5.46 |
| 632841 |
43.2 |
6.1 |
1.47E-06 |
1.06E-03 |
5.83 |
| 116693 |
43.6 |
5.1 |
1.86E-07 |
1.40E-04 |
5.50 |
| 375575 |
44.8 |
5.1 |
1.04E-05 |
7.14E-03 |
5.71 |
| 98904 |
45.5 |
11.5 |
1.23E-04 |
7.85E-02 |
6.05 |
| 629971 |
46.5 |
10.7 |
1.01E-05 |
6.96E-03 |
6.54 |
| 403883 |
48.3 |
7.0 |
2.37E-05 |
1.57E-02 |
4.00 |
| 267033 |
48.5 |
10.9 |
4.31E-05 |
2.84E-02 |
6.35 |
| 36437 |
48.5 |
5.5 |
8.51E-06 |
5.91E-03 |
5.23 |
| 49842 |
49.1 |
10.6 |
2.34E-05 |
1.56E-02 |
9.71 |
| 654259 |
49.8 |
9.0 |
2.57E-06 |
1.83E-03 |
7.23 |
| 182986 |
50.1 |
9.1 |
1.52E-05 |
1.02E-02 |
5.27 |
| 93739 | 50.4 | 6.5 | 1.54E-05 | 1.04E-02 | 5.40 |
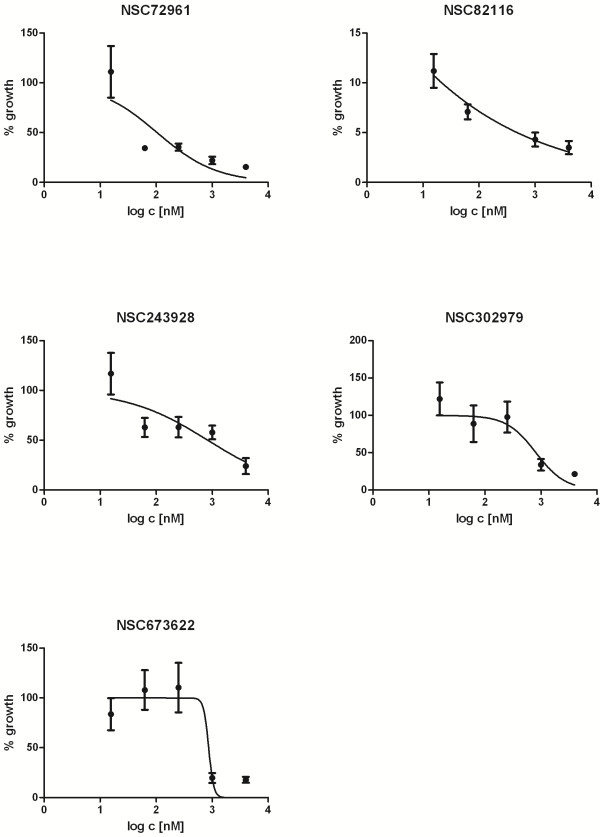
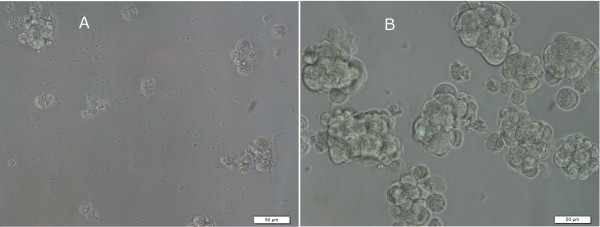
We randomly selected 5 of the 136 inhibitory compounds for confirmatory testing. In each case, the determined GI50 values were indicative of a significant inhibitory effect: NSC72961: 104.2±13.54 nM; NSC302979: 814.0±84.44 nM; NSC82116: < 1 nM; NSC673622: 865.2 nM (SEM not determined); NSC243928: 831.6±134.72 nM (Figure 1). The cellular phenotype of CSC after treatment with inhibitory compounds is consistent with cell death/apoptosis (Figure 2).
Figure 1.
Concentration-response curves for 5 library compounds identified as inhibitors of ovarian cancer stem-like cells. Curves are fitted by non-linear regression of log-transformed data using a normalized response-variable slope model. Error bars: SEM.
Figure 2.
Phenotypic effect of compound NSC72961 (8-azaadenosine) at 4 μM (96 h) on ovarian cancer stem-like cell culture. Test treatment (A); solvent control (B). Scale bar = 50 μm.
Included among the 793 compounds passing our performance test are 19 drugs previously approved by the FDA for cancer treatment (Table 2). Five of these drugs are among the 136 compounds designated as CSC-inhibitory, but none are commonly used in the treatment of epithelial ovarian cancer. On the other hand, triethylenemelamine (altretamine, NSC9706), which is used for palliative treatment of persistent or recurrent ovarian cancer [30], induced non-significant stimulation of CSC growth in our screening (Table 2).
Table 2.
Results of HTS of CSC with 19 FDA-approved oncology drugs
| NSC | Name | % growth | SD (%) | p-value | p-adj | GI50OVCAR3 (−log10) |
|---|---|---|---|---|---|---|
| 3053 |
Dactinomycin |
8.4 |
3.9 |
1.38E-08 |
1.07E-05 |
8.54 |
| 24559 |
Plicamycin |
10.8 |
3.6 |
9.91E-11 |
7.86E-08 |
7.35 |
| 67574 |
Vincristine |
27.0 |
6.0 |
1.21E-06 |
8.74E-04 |
7.68 |
| 14229 |
Mepacrine |
37.6 |
6.1 |
9.93E-08 |
7.56E-05 |
5.55 |
| 49842 |
Vinblastine |
49.1 |
10.6 |
2.34E-05 |
1.56E-02 |
9.71 |
| 125066 |
Bleomycin |
53.0 |
10.2 |
1.10E-05 |
7.54E-03 |
5.28 |
| 63878 |
Cytarabine |
76.6 |
11.0 |
1.04E-02 |
1 |
5.00 |
| 105014 |
Cladribine |
82.7 |
17.0 |
7.13E-02 |
1 |
4.59 |
| 755 |
Mercaptopurine |
95.2 |
8.4 |
2.83E-01 |
1 |
5.94 |
| 740 |
Methotrexate |
96.7 |
30.8 |
8.38E-01 |
1 |
6.69 |
| 226080 |
Rapamycin |
97.0 |
20.9 |
7.93E-01 |
1 |
8.17 |
| 296961 |
Amifostine |
99.4 |
12.0 |
9.18E-01 |
1 |
3.13 |
| 85998 |
Streptozocin |
103.7 |
28.9 |
8.10E-01 |
1 |
3.14 |
| 32065 |
Hydroxyurea |
107.4 |
14.8 |
2.38E-01 |
1 |
2.92 |
| 180973 |
Tamoxifen |
115.1 |
14.3 |
4.45E-02 |
1 |
5.23 |
| 750 |
Busulfan |
120.6 |
15.9 |
3.65E-02 |
1 |
3.60 |
| 38721 |
Mitotane |
128.2 |
47.6 |
2.68E-01 |
1 |
4.73 |
| 9706 |
Triethylenemelamine |
128.9 |
82.5 |
5.26E-01 |
1 |
4.74 |
| 45388 | Dacarbazine | 164.3 | 14.2 | 4.46E-04 | 2.68E-01 | 4.25 |
The two most CSC-inhibitory of the FDA-approved drugs, dactinomycin (NSC 3035; 8.4% cell growth) [31] and plicamycin (mithramycin A, NSC 24559; 10.8% cell growth) [32] have both been previously reported to induce programmed cell death or apoptosis by inhibiting RNA transcription. Dactinomycin is used in the treatment of several cancers including gestational trophoblastic neoplasia [33] and Wilms’ tumor [34]. Plicamycin has been used in the treatment of testicular cancer [35] and hypercalcemia associated with advanced malignancy [36]. The anti-microtubule drug vincristine (NSC24559) is used in the treatment of acute leukemias, Hodgkin lymphoma, and aggressive non-Hodgkin lymphoma, but is also included in combinations for treatment of small-cell lung cancer, breast cancers and some pediatric neoplasms [37]. Vinblastine (NSC49842) is used in the treatment of Hodgkin’s lymphoma [37], and in combination with cisplatin and bleomycin in the treatment of testicular and ovarian germ cell cancers [38]. Mepacrine (quinacrine, NSC14229), an inhibitor of NFκB [39] and topoisomerase activity [40], is primarily used as an antimalarial drug [41]. In oncology, it is most commonly used for the treatment of pleural effusions in advanced malignant diseases [42].
Most ovarian CSC growth-inhibiting compounds also inhibit the growth of more differentiated ovarian cancer cells
It has been established previously that normal stem cells are more resistant to the induction of apoptosis by radiation and cytotoxic agents than their differentiated progeny and similarly, CSC have been shown to display increased resistance to these same agents relative to the more differentiated cells that comprise the bulk of the tumor [43-45]. Indeed, it has been proposed that this dichotomy may contribute to the recurrence of cancer growth after the initial response of tumors to chemotherapeutic treatments [46]. Consistent with this view, we previously reported that several ovarian cancer drugs (e.g., NSC119875-cisplatin, NSC724770-docetaxel, NSC609699-topotecan) that are effective against ovarian cancer OVCAR-3 cells, are significantly less effective at inhibiting growth of ovarian CSC [13]. To assess if the apparent dichotomy in drug effectiveness between ovarian CSC and their more differentiated progeny is characteristic of the 136 ovarian CSC inhibitory compounds identified in our study, we sought to compare the results of our HTS with NCI’s previous testing of compounds against the OVCAR-3 cell line. In the NCI program, GI50 values (concentrations required to inhibit growth by 50%) on OVCAR-3 cells were determined for nearly all of the compounds used in our HTS. The OVCAR-3 GI50 values (expressed as -log10 GI50) for 136 of the CSC-inhibitory compounds are presented in Table 1. The data output from our HTS is relative % growth rather than GI50 values. However, since the concentration of compounds used in our HTS was 2.29 μM (see Methods), the GI50 for compounds resulting in ≤ 50% growth of CSC is predicted to be ≤ 2.29 μM or ≥ 5.64 on the -log10 scale (−log10 2.29×10-6 = 5.64). By comparing this value with the -log10 GI50 values previously determined for OVCAR-3 cells in the NCI study, we found that 73% (99/136) of the compounds that we designated as CSC inhibitory compounds are also inhibitory for OVCAR-3 cells (i.e., -log10 GI50 ≥ 5.64). This suggests that there may be a number of inhibitory compounds with the potential to target both ovarian CSC and their more differentiated progeny. Of the remaining 37 (136–99) CSC-inhibitory compounds, 5 were not previously tested on OVCAR-3. Thus, based on our criteria, we classified 32 compounds to be preferentially inhibitory for CSC (Table 3).
Table 3.
Compounds preferentially inhibitory for CSC
| NSC | Name | % growth | SD | p-adj |
|---|---|---|---|---|
| 618332 |
2,3-Dibromonaphthoquinone |
3.3 |
4.0 |
2.78E-04 |
| 128305 |
5,7-Dihydroxy-3',4'-dimethoxyflavone |
4.9 |
3.4 |
5.59E-03 |
| 636132 |
3-Cyano-N,3-bis(2-methylphenyl)-2-oxopropanamide |
7.3 |
3.2 |
6.89E-04 |
| 622732 |
N-(4-chlorophenyl)-1-methyl-1H-pyrazolo[3,4-b]quinolin-5-amine |
8.0 |
4.9 |
3.46E-03 |
| 208913 |
Ethyl 2-(((1-adamantyl(methyl)amino) carbonyl)amino)propanoate |
8.0 |
3.5 |
5.96E-04 |
| 614928 |
3,3,4,4-Tetramethyltetrahydro-2,5-furandiol |
9.3 |
2.0 |
5.56E-04 |
| 667467 |
2-Phenyl-1,4-thiazino[3,2-c]quinoline-3-thione |
9.8 |
3.9 |
2.79E-07 |
| 616232 |
Dibromodulcitol |
11.6 |
4.0 |
3.13E-07 |
| 635121 |
N'-(1-(4H-1,4-benzothiazin-2-yl)ethylidene)-2-hydroxybenzohydrazide |
16.0 |
5.3 |
6.53E-03 |
| 202000 |
(4Z)-4-[(3,4-dichlorophenyl) methylidene]-2-(furan-2-yl)-1,3-oxazol-5-one |
17.7 |
5.0 |
2.38E-06 |
| 629301 |
3,6-Dihydro-3,6-ethanocyclohepta[cd]
[1]benzofuran-10,10,11,11-tetracarbonitrile |
20.9 |
5.5 |
1.49E-02 |
| 34391 |
Cryptocyanine iodide |
22.2 |
4.2 |
3.12E-04 |
| 625483 |
1-(2-Chloro-6-fluorophenyl)-1H,3H-Thiazolo(3,4-a)benzimidazole |
22.8 |
9.7 |
1.68E-02 |
| 72961 |
8-Azaadenosine |
23.1 |
4.4 |
9.59E-05 |
| 336628 |
Merbarone |
23.7 |
5.9 |
1.97E-04 |
| 148958 |
Ftorafur |
24.9 |
6.9 |
7.81E-05 |
| 10447 |
Purpurin |
25.5 |
6.6 |
2.61E-04 |
| 302979 |
Shikoccin |
30.4 |
10.8 |
3.68E-02 |
| 637993 |
6H-Imidazo[4,5,1-de]acridin-6-one, 5-[2-(diethylamino) ethylamino]-8-methox`y-1-methyl-, dihydrochloride |
34.8 |
9.9 |
5.36E-02 |
| 331757 |
2-Anthracenecarboxamide, N-[4-(diethylamino)-1-methylbutyl]-9,10-dihydro-9,10-dioxo-, monohydrochloride |
35.6 |
15.4 |
9.84E-02 |
| 1906 |
Piperidinium piperidinedithiocarbamate |
36.2 |
3.5 |
3.43E-05 |
| 14229 |
Mepacrine |
37.6 |
6.1 |
7.56E-05 |
| 1620 |
4-[[(2-furanyl)methyl]amino]-1H-pyrazolo[3,4-D]pyrimidine |
37.7 |
8.4 |
3.43E-03 |
| 622627 |
2-(chloromethyl)-1,3-dinitro-5-(trifluoromethyl)benzene |
38.4 |
11.4 |
1.22E-02 |
| 248436 |
Platinum, dibromo(6-thioguanosine-N7,S6)-, (SP-4-3)- |
41.4 |
9.5 |
9.15E-03 |
| 166454 |
Decamine |
41.9 |
7.2 |
6.39E-04 |
| 84074 |
Phosphonium, (3-bromopropyl)triphenyl- bromide |
42.9 |
7.3 |
1.23E-02 |
| 116693 |
2,3-bis(benzoyloxy)succinic acid compound with 1,4-dimethyl-2-((4-methylphenyl)(phenyl)-l 4-sulfanyl)benzene (1:1) |
43.6 |
5.1 |
1.40E-04 |
| 403883 |
Cedran-8-ol |
48.3 |
7.0 |
1.57E-02 |
| 36437 |
Crassin acetate |
48.5 |
5.5 |
5.91E-03 |
| 182986 |
Diaziquone |
50.1 |
9.1 |
1.02E-02 |
| 93739 | Fuchsine | 50.4 | 6.5 | 1.04E-02 |
Among the 99 compounds found to be co-inhibitory for CSC and OVAR-3 cells, four have previously been FDA approved for cancer treatment (NSC3053-dactinomycin; NSC24559-plicamycin; NSC49842-vinblastine; NSC67574-vincristine) (Table 2). Only one of the previously approved cancer drugs, the NFκB-inhibitor Mepacrine (NSC14229), is included among those compounds classified as preferentially inhibitory for ovarian CSC (37.6% growth).
Computational model classifies CSC inhibitory compounds into predicted cellular response groups
Neither the mode of action nor the molecular targets of the 32 compounds classified by us as preferentially inhibitory for CSC have, as yet, been definitively determined. However, there are a variety of computational tools that can be informative in predicting the putative cellular responses to these compounds and in suggesting lines of future investigation. One such tool, developed by the Covell group at NCI [27,28,47], utilizes data from the treatment of 60 representative human cancer cell lines (the NCI-60 panel) [48] with nearly 30,000 compounds including those investigated in our HTS. These data were used to generate a self-organizing map (SOM) that visualizes the position of ~30,000 screened compounds within 9 major cellular response categories: mitosis (M), membrane function (N), nucleic acid metabolism (S), metabolic stress and cell survival (Q), kinases/phosphatases and oxidative stress (P) and 4 unexplored regions (RFJV).
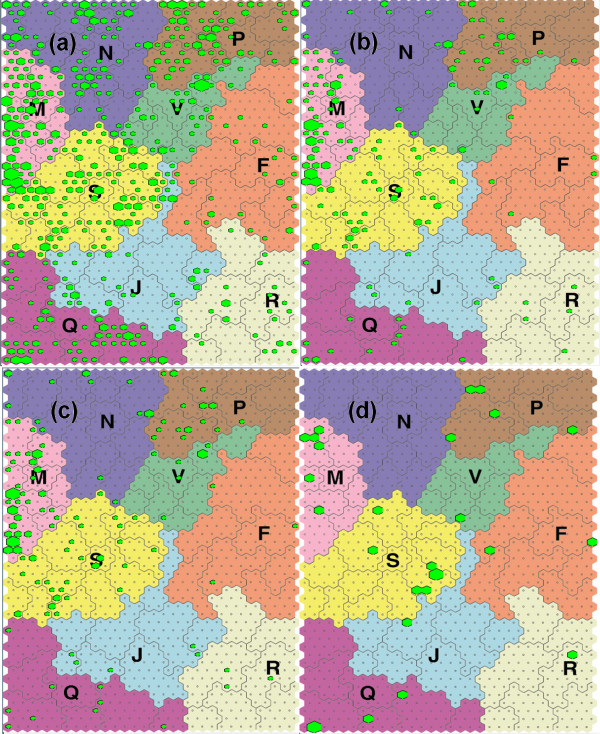
Using this SOM, we were able to visually compare the predicted cellular responses of all of our screened compounds (that passed assay performance criteria) relative to those identified as having an inhibitory effect on CSC. Shown in Figure 3 is a SOM upon which we have mapped (a) all 793 compounds used in our HTS; (b) the 136 compounds identified as inhibitors of CSC (including compounds that also inhibit OVCAR-3 cells); (c) the 99 compounds identified as co-inhibitory of CSC and OVCAR-3 cells; and (d) the 32 compounds that exert a CSC-specific inhibitory effect.
Figure 3.
Mapping of compounds used in the HTS onto SOMs. (a) all 793 evaluable compounds (b) 136 CSC inhibitory compounds; (c) 99 CSC and OVCAR-3 co-inhibitory compounds; (d) 32 CSC-specific inhibitory compounds. [mitosis (M), membrane function (N), nucleic acid metabolism (S), metabolic stress and cell survival (Q), kinases/phosphatases and oxidative stress (P) and 4 unexplored regions (RFJV)].
The results indicate that compared to all 793 evaluable compounds, the 136 identified as inhibitory for CSC are highly significantly enriched for compounds associated with M (mitotic) cellular responses (M: 36.1% vs 18.7%; p<0.0001; Figure 3b and Table 4). This region of SOM contains many compounds known to interfere with microtubule and/or actin filaments, such as taxanes, derivatives of colchicine, vinca alkaloids, rhizoxin and nocodazole [47]. This region also contains compounds associated with inhibition of the DNA polymerase pathway and is associated with the Gene Ontology (GO) terms: Mitotic checkpoint, Cytokinesis, DNA topological change, Cell cycle (Biological Processes); Nucleus, Kinetochore (Cellular Components), and DNA topoisomerase activity (Molecular Function) [28].
Table 4.
Number of compounds mapping into individual cellular response categories of SOM
| F | J | M | N | P | Q | R | S | V | |
|---|---|---|---|---|---|---|---|---|---|
|
(a) |
29 (4.2%) |
44 (6.4%) |
129 (18.7%) |
102 (14.8%) |
92 (13.3%) |
83 (12.0%) |
25 (3.6%) |
140 (20.3%) |
46 (6.7%) |
|
(b) |
5 (2.6%) |
7 (3.6%) |
70 (36.1%) |
16 (8.2%) |
25 (12.9%) |
13 (6.7%) |
5 (2.6%) |
42 (21.6%) |
11 (5.7%) |
|
(c) |
3 (1.9%) |
5 (3.1%) |
62 (38.5%) |
16 (9.9%) |
19 (11.8%) |
9 (5.6%) |
3 (1.9%) |
35 (21.7%) |
9 (5.6%) |
|
(d) |
2 (7.4%) |
1 (3.7%) |
6 (22.2%) |
0 (0%) |
5 (18.5%) |
4 (14.8%) |
1 (3.7%) |
7 (25.9%) |
1 (3.7%) |
|
p-ab |
0.3988 |
0.1653 |
<0.0001 |
0.0169 |
1.0000 |
0.0365 |
0.6537 |
0.6882 |
0.7412 |
|
p-ac |
0.2469 |
0.1326 |
<0.0001 |
0.1283 |
0.6970 |
0.0163 |
0.3325 |
0.6663 |
0.7235 |
| p-ad | Numbers too small for statistical evaluation | ||||||||
(a) all 793 evaluable compounds (b) 136 CSC inhibitory compounds; (c) 99 CSC and OVCAR-3 co-inhibitory compounds; (d) 32 CSC-specific inhibitory compounds. P-values: Fisher’s exact test for the significance of differences between proportions of compounds in a given cellular response category in 793 compound set (A) and compound subset B (p-AB), C (p-AC), or D (p-AD). [mitosis (M), membrane function (N), nucleic acid metabolism (S), metabolic stress and cell survival (Q), kinases/phosphatases and oxidative stress (P) and 4 unexplored regions (RFJV)].
The 99 compounds that were co-inhibitory for OVCAR-3 and CSC also displayed a significant enrichment for M cellular responses (M: 38.5% vs 18.7%; p<0.0001; Figure 3c and Table 4). When the 32 compounds classified as preferentially inhibitory for CSC were mapped, the enrichment for M (mitotic) cellular responses was no longer apparent (Figure 3d). Although this may be attributable to the fact that CSC are less mitotically active than cancer epithelial cells [43], the relatively small number of compounds (32) in this category precludes definitive conclusions.
Conclusion
We tested the inhibitory effect of 825 compounds (NCI Mechanistic Set) on the growth of ovarian CSC derived from a previously established epithelial ovarian cancer cell line (OVCAR-3) [13]. 158 of these compounds were found to have a significant inhibitory effect on ovarian CSC growth. The most inhibitory of these compounds (≤ 50% growth relative to controls) were designated as CSC inhibitory. Among these 136 CSC inhibitory compounds are 5 FDA-approved cancer drugs, but none of these are commonly used in the treatment of epithelial ovarian cancer. A comparison of the ovarian CSC inhibitory compounds identified in this study with compounds previously shown to be inhibitory for OVCAR-3 ovarian cancer cells revealed an unexpected 73% overlap. Computational analysis indicates that the majority of these compounds are associated with mitotic cellular responses.
While epithelial ovarian cancer is frequently responsive to current chemotherapeutic treatments, disease recurrence remains a persistent problem that has been, at least partially, attributed to the fact that ovarian CSC are resistant to standard therapies [6,13]. Our HTS has uncovered a number of candidate compounds that may, after further testing, prove effective in targeting both ovarian CSC and their more differentiated progeny.
Abbreviations
CSC: Cancer Stem Cells; DTP: Developmental Therapeutics Program; FDA: U.S. Food and Drug Administration; HTS: High Throughput Screening; NCI: National Cancer Institute; NSC: National Service Center; SOM: Self-Organizing Map.
Competing interests
All authors declare that they have no competing interests.
Authors’ contributions
RM and JM conceived the study and wrote the manuscript. RM and LW performed the HTS experiment. RM processed and analyzed the data. All authors read and approved the final manuscript.
Supplementary Material
Plate design, assay performance evaluation and systematic error detection doc.
97 drugs from the FDA-approved oncology drug set, Description: NSC – NSC identifier; CAS – CAS identifier.
Compounds from the 2 plates failing the assay performance test, Description: AP - Assay plate #; NSC – NSC identifier; %growth; SD – standard deviation of %growth; p-value – determined by Welch’st-test between treated and control wells.
Results of HTS for 793 compounds that passed the assay performance test (also shown are GI50 values (−log10) for OVCAR-3 cells), Description: AP - Assay Plate; NSC – NSC identifier; %growth; SD – standard deviation of %growth; p-value – determined by Welch’st-test between treated and control wells; rank – rank of compound according to p-value; p-adj_Holms - Holm's procedure-adjusted p-values; position – position of drug in assay plate; OVCAR-3 - GI50 values for OVCAR-3 cell line (retrieved from DTP/NCI).
Contributor Information
Roman Mezencev, Email: roman.mezencev@biology.gatech.edu.
Lijuan Wang, Email: lijuan.wang@biology.gatech.edu.
John F McDonald, Email: john.mcdonald@biology.gatech.edu.
Acknowledgements
Authors thank Dr. David G. Covell (National Cancer Institute – Frederick) for his assistance with accessing the 3D Mind tool, Vinay K. Mittal (Georgia Institute of Technology) for his help with data processing and the Developmental Therapeutics Program of NCI for providing the NCI Mechanistic Set library. The Deborah Nash Harris Endowment Fund and the Robinson Family Foundation provided support for this project.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Herzog TJ. Recurrent ovarian cancer: how important is it to treat to disease progression? Clin Cancer Res. 2004;10:7439–7449. doi: 10.1158/1078-0432.CCR-04-0683. [DOI] [PubMed] [Google Scholar]
- Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, Marks JR, Berchuck A, Murphy SK. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I, Mor G. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Charafe-Jauffret E, Birnbaum D. Targeting breast cancer stem cells: fishing season open! Breast Cancer Res. 2010;12:312. doi: 10.1186/bcr2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott SP, Wicha MS. Targeting breast cancer stem cells. Mol Oncol. 2010;4:404–419. doi: 10.1016/j.molonc.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton S, Mousa SA. Therapeutics formulated to target cancer stem cells: is it in our future? Cancer Cell Int. 2011;11:7. doi: 10.1186/1475-2867-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M, Squire JA, Smith A, Dirks P. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Visnyei K, Onodera H, Damoiseaux R, Saigusa K, Petrosyan S, De Vries D, Ferrari D, Saxe J, Panosyan EH, Masterman-Smith M, Mottahedeh J, Bradley KA, Huang J, Sabatti C, Nakano I, Kornblum HI. A molecular screening approach to identify and characterize inhibitors of glioblastoma stem cells. Mol Cancer Ther. 2011;10:1818–1828. doi: 10.1158/1535-7163.MCT-11-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mezencev R, Bowen NJ, Matyunina LV, McDonald JF. Isolation and characterization of stem-like cells from a human ovarian cancer cell line. Mol Cell Biochem. 2011;363:257–268. doi: 10.1007/s11010-011-1178-6. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Levina V, Marrangoni AM, De Marco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS One. 2008;3:e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbeck SL. Update on NCI in vitro drug screen utilities. Eur J Cancer. 2004;40:785–793. doi: 10.1016/j.ejca.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Mechanistic set information. http://dtp.nci.nih.gov/branches/dscb/mechanistic_explanation.html.
- DTP basic chemical data search. http://dtp.nci.nih.gov/dtpstandard/ChemData/index.jsp.
- Hsiao AY-C. 3D spheroid culture systems for metastatic prostate cancer dormancy studies and anti-cancer therapeutics development. Universtiy of Michigan: (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses (ID 896131060); 2011. [Google Scholar]
- Dong Y, Tan OL, Loessner D, Stephens C, Walpole C, Boyle GM, Parsons PG, Clements JA. Kallikrein-related peptidase 7 promotes multicellular aggregation via the alpha(5)beta(1) integrin pathway and paclitaxel chemoresistance in serous epithelial ovarian carcinoma. Cancer Res. 2010;70:2624–2633. doi: 10.1158/0008-5472.CAN-09-3415. [DOI] [PubMed] [Google Scholar]
- Inglese J, Shamu CE, Guy RK. Reporting data from high-throughput screening of small-molecule libraries. Nat Chem Biol. 2007;3:438–441. doi: 10.1038/nchembio0807-438. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist. 1979;6:65–70. [Google Scholar]
- Approved Oncology Drugs Set Information: A set of FDA-approved anticancer drugs to enable cancer research. http://dtp.cancer.gov/branches/dscb/oncology_drugset_explanation.html.
- DTP. http://dtp.nci.nih.gov.
- 3D MIND. http://spheroid.ncifcrf.gov/spheroid/.
- Huang R, Wallqvist A, Thanki N, Covell DG. Linking pathway gene expressions to the growth inhibition response from the National Cancer Institute's anticancer screen and drug mechanism of action. Pharmacogenomics J. 2005;5(6):381–399. doi: 10.1038/sj.tpj.6500331. [DOI] [PubMed] [Google Scholar]
- Text document with names. http://dtpsearch.ncifcrf.gov/OPEN_NAMES_FEB03.TXT.
- Wiernik PH, Yeap B, Vogl SE, Kaplan BH, Comis RL, Falkson G, Davis TE, Fazzini E, Cheuvart B, Horton J. Hexamethylmelamine and low or moderate dose cisplatin with or without pyridoxine for treatment of advanced ovarian carcinoma: a study of the Eastern Cooperative Oncology Group. Cancer Invest. 1992;10:1–9. doi: 10.3109/07357909209032783. [DOI] [PubMed] [Google Scholar]
- Fraschini A, Bottone MG, Scovassi AI, Denegri M, Risueño MC, Testillano PS, Martin TE, Biggiogera M, Pellicciari C. Changes in extranucleolar transcription during actinomycin D-induced apoptosis. Histol Histopathol. 2005;20:107–117. doi: 10.14670/HH-20.107. [DOI] [PubMed] [Google Scholar]
- Lee TJ, Jung EM, Lee JT, Kim S, Park JW, Choi KS, Kwon TK. Mithramycin A sensitizes cancer cells to TRAIL-mediated apoptosis by down-regulation of XIAP gene promoter through Sp1 sites. Mol Cancer Ther. 2006;5:2737–2746. doi: 10.1158/1535-7163.MCT-06-0426. [DOI] [PubMed] [Google Scholar]
- Lewis JL Jr. Chemotherapy of gestational choriocarcinoma. Cancer. 1972;30:1517–1521. doi: 10.1002/1097-0142(197212)30:6<1517::AID-CNCR2820300616>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Frei E 3rd. The clinical use of actinomycin. Cancer Chemother Rep. 1974;58:49–54. [PubMed] [Google Scholar]
- Kennedy BJ. Mithramycin therapy in advanced testicular neoplasms. Cancer. 1970;26:755–766. doi: 10.1002/1097-0142(197010)26:4<755::AID-CNCR2820260403>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Perlia CP, Gubisch NJ, Wolter J, Edelberg D, Dederick MM, Taylor SG 3rd. Mithramycin treatment of hypercalcemia. Cancer. 1970;25:389–394. doi: 10.1002/1097-0142(197002)25:2<389::AID-CNCR2820250217>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Ruddon RW, Ensminger WD, Maybaum J. The anticancer drugs. 2. New York: Oxford University Press; 1994. [Google Scholar]
- Gershenson DM, Morris M, Cangir A, Kavanagh JJ, Stringer CA, Edwards CL, Silva EG, Wharton JT. Treatment of malignant germ cell tumors of the ovary with bleomycin, etoposide, and cisplatin. J Clin Oncol. 1990;8:715–720. doi: 10.1200/JCO.1990.8.4.715. [DOI] [PubMed] [Google Scholar]
- Gurova KV, Hill JE, Guo C, Prokvolit A, Burdelya LG, Samoylova E, Khodyakova AV, Ganapathi R, Ganapathi M, Tararova ND, Bosykh D, Lvovskiy D, Webb TR, Stark GR, Gudkov AV. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci USA. 2005;102:17448–17453. doi: 10.1073/pnas.0508888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preet R, Mohapatra P, Mohanty S, Sahu SK, Choudhuri T, Wyatt MD, Kundu CN. Quinacrine has anticancer activity in breast cancer cells through inhibition of topoisomerase activity. Int J Cancer. 2012;130(7):1660–1670. doi: 10.1002/ijc.26158. [DOI] [PubMed] [Google Scholar]
- Van Dyke K, Lantz C, Szustkiewicz C. Quinacrine: mechanisms of antimalarial action. Science. 1970;169:492–493. doi: 10.1126/science.169.3944.492. [DOI] [PubMed] [Google Scholar]
- Koldsland S, Svennevig JL, Lehne G, Johnson E. Chemical pleurodesis in malignant pleural effusions: a randomised prospective study of mepacrine versus bleomycin. Thorax. 1993;48:790–793. doi: 10.1136/thx.48.8.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006;98:1755–1757. doi: 10.1093/jnci/djj505. [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea–a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- Rabow AA, Shoemaker RH, Sausville EA, Covell DG. Mining the National Cancer Institute's tumor-screening database: identification of compounds with similar cellular activities. J Med Chem. 2002;45:818–840. doi: 10.1021/jm010385b. [DOI] [PubMed] [Google Scholar]
- Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plate design, assay performance evaluation and systematic error detection doc.
97 drugs from the FDA-approved oncology drug set, Description: NSC – NSC identifier; CAS – CAS identifier.
Compounds from the 2 plates failing the assay performance test, Description: AP - Assay plate #; NSC – NSC identifier; %growth; SD – standard deviation of %growth; p-value – determined by Welch’st-test between treated and control wells.
Results of HTS for 793 compounds that passed the assay performance test (also shown are GI50 values (−log10) for OVCAR-3 cells), Description: AP - Assay Plate; NSC – NSC identifier; %growth; SD – standard deviation of %growth; p-value – determined by Welch’st-test between treated and control wells; rank – rank of compound according to p-value; p-adj_Holms - Holm's procedure-adjusted p-values; position – position of drug in assay plate; OVCAR-3 - GI50 values for OVCAR-3 cell line (retrieved from DTP/NCI).