Abstract
Background
Biotic stress induced by various herbivores and pathogens invokes plant responses involving different defense mechanisms. However, we do not know whether different biotic stresses share a common response or which signaling pathways are involved in responses to different biotic stresses. We investigated the common and specific responses of Arabidopsis thaliana to three biotic stress agents: Spodoptera littoralis, Myzus persicae, and the pathogen Pseudomonas syringae.
Methodology/Principal Findings
We used electrophysiology to determine the plasma membrane potential (Vm) and we performed a gene microarray transcriptome analysis on Arabidopsis upon either herbivory or bacterial infection. Vm depolarization was induced by insect attack; however, the response was much more rapid to S. littoralis (30 min −2 h) than to M. persicae (4–6 h). M. persicae differentially regulated almost 10-fold more genes than by S. littoralis with an opposite regulation. M. persicae modulated genes involved in flavonoid, fatty acid, hormone, drug transport and chitin metabolism. S. littoralis regulated responses to heat, transcription and ion transport. The latest Vm depolarization (16 h) was found for P. syringae. The pathogen regulated responses to salicylate, jasmonate and to microorganisms. Despite this late response, the number of genes differentially regulated by P. syringae was closer to those regulated by S. littoralis than by M. persicae.
Conclusions/Significance
Arabidopsis plasma membranes respond with a Vm depolarization at times depending on the nature of biotic attack which allow setting a time point for comparative genome-wide analysis. A clear relationship between Vm depolarization and gene expression was found. At Vm depolarization timing, M. persicae regulates a wider array of Arabidopsis genes with a clear and distinct regulation than S. littoralis. An almost completely opposite regulation was observed between the aphid and the pathogen, with the former suppressing and the latter activating Arabidopsis defense responses.
Introduction
Plants are attacked by a multitude of organisms, like insects, microbes and fungi, which all infer a biotic stress. As plants are sessile organisms and cannot escape their predators they have evolved diverse mechanisms to react specifically to each attacking biotroph. Chewing herbivores, like Spodoptera littoralis, consume leaves by continuously clipping off and ingesting small pieces of tissue reducing both photosynthetic capacity and biomass of fed plants [1]–[5]. Aphids, like Myzus persicae, are sap-sucking insects that remove plant nutrients, elicit plant responses that are deleterious to plant productivity and alter the mass flow of phloem contents, resulting in changes in source–sink relationships [6]–[10]. Phytopathogenic bacteria of the genus Pseudomonas colonize the leaf surfaces of plants without causing disease [11]. Pseudomonas syringae multiplies in the plant cell apoplastic intercellular spaces and remains extracellular triggering plant defenses aimed to restrict bacterial growth [12].
Upon all of these biotic interactions with plants, it is crucial to understand how plants dissect and convert these different stress signals into appropriate physiological reactions.
The earliest event that is detectable as a consequence of leaf damage is depolarization of the plasma transmembrane potential (Vm), followed by a cascade of biochemical and molecular events including protein phosphorylation, activation of signaling cascades and, eventually, gene expression and translation [12]–[17]. Both S. littoralis direct herbivory and the insect's oral secretions have been demonstrated to induce a fast mesophyll cell Vm depolarization of Arabidopsis [18] and other plant species [1], [19]–[21], whereas a significant Vm depolarization is observed at almost every stylet puncture of the plant plasmalemma during M. persicae phloem feeding [22]. In plant-pathogen interactions, Vm depolarization is a reliable early indicator of leaf hypersensitive response (HR) [23]
A variety of experimental methods have been employed to study the complex interactions of Arabidopsis and aphid herbivores, including measurements of the transcriptional responses [8], [24]–[27], whereas microarray-based genome-wide transcriptomic analyses have been performed in several plant species, including Arabidopsis thaliana, upon herbivore attack by Spodoptera spp., [28]–[31]. Although the exact nature of the systemic acquired resistance (SAR) signal in Arabidopsis after localized infection by avirulent P. syringae remains complex and has been a matter of debate [32], [33], the transcriptional changes associated with basal defense to live bacteria and the contribution of specific elicitors/effectors to the regulation of the basal defense transcriptome and other host physiological processes have been thoroughly studied [12], [34]–[36].
Although many commonly induced or suppressed defense-related genes have been identified in plants infested with chewing or phloem-feeding insects, and bacterial pathogens, there is considerable difference in the transcriptomic response of infested plants to different insects or bacteria. In the dazzling diversity of possible differential plant responses, the most difficult aspect is to assess whether a common response exists and to which extent each pathogen or herbivore differentially expresses and regulates defense response genes. Timing appears important in the interplay among the multiple plant responses to herbivores [37] and pathogenic microorganisms [38]. The aim of this work was to use a common physiological response to the herbivores M. persicae and S. littoralis and the pathogen P. syringae and, i.e. the leaf Vm depolarization, as a time point for a comparative genome-wide analysis of gene expression and regulation in Arabidopsis, when attacked by different biotic agents. The obtained results should complement other studies and provide a useful resource for future study of plant multitrophic interactions.
Results
S. littoralis, M. persicae and P. syringae induce the same strong Vm depolarization in A. thaliana leaves but at different times
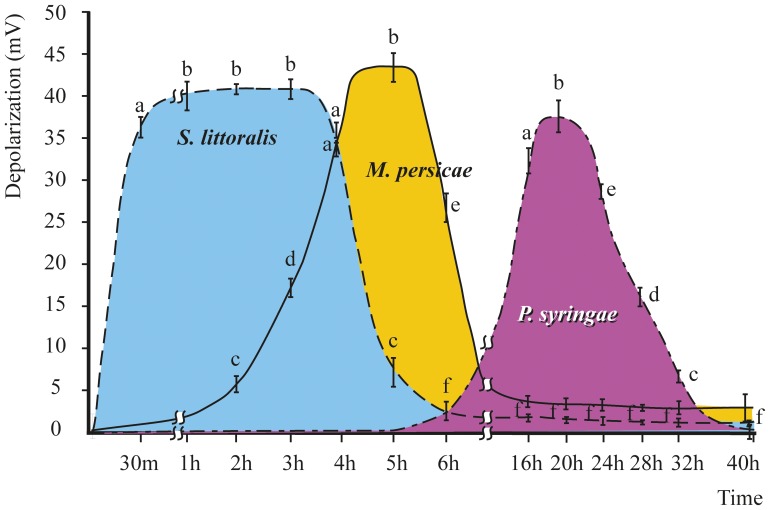
Time-course measurements of Vm in Arabidopsis showed that after S. littoralis herbivory a strong and rapid Vm depolarization (with respect to mechanical damage) occurs after a few minutes from the herbivore wound, with recovery of the Vm between 5 and 6 h (Figure 1). When Arabidopsis was fed by M. persicae, almost the same extent of Vm depolarization was observed (P>0.05), but the timing of Vm depolarization peaked between 4 and 6 h, returning near to the basal Vm value between 16 and 24 h (Figure 1). A remarkable delay in Vm depolarization was observed when Arabidopsis leaves were infected by the avirulent strain of P. syringae. Even in this case Vm depolarization was not significantly different (P>0.05) from the values observed after S. littoralis and M. persicae herbivory; however, the maximal Vm depolarization occurred between 16 and 18 h from inoculation (Figure 1). These results indicate that Arabidopsis responds to different biotic stress with a strong and transient Vm depolarization and that the timing of this event depends on the kind of biotic stress.
Figure 1. Plasma transmembrane potential (Vm) depolarization measured in Arabidopsis mesophyll leaves at different times upon herbivory by Spodoptera littoralis and Myzus persicae and infection by Pseudomonas syringae.
Chewing herbivore induces a fast Vm depolarization that lasts about 4–6 h from feeding, whereas phloem feeding induces a Vm depolarization that occurs after about 6 h from feeding. Infection by P. syringae causes a Vm depolarization about 16 h after infection. No matter the biotic stress the level of the highest Vm depolarization shows the same value (statistical significance P>0.05). For each time point at least 50 measurements were performed. The timing of Vm depolarization depends on biotic damage. Bars represent standard error, different letters indicate significant (P<0.05) differences.
Comparative gene expression at the time of Vm depolarization in Arabidopsis leaves infested by S. littoralis, M. persicae and P. syringae
Taking into account that different biotrophs, owing to their peculiar feeding or infesting behavior, can cause different amounts of damage or trigger plant responses at different time, samples for microarray analysis were taken at time points corresponding to the maximum Vm depolarization for every biotic stress.
To identify transcriptional changes in Arabidopsis, total RNA was extracted from leaves wounded for 2 h by the herbivore S. littoralis, for 5 h by the phloem feeder M. persicae and after 16 h from inoculation of the pathogen P. syringae, these timings corresponding to maximal Vm depolarization. As control for S. littoralis, leaves were mechanically damaged with a pattern wheel, whereas for M. persicae leaves were wounded with the tip of an electrophysiology microcapillary. Mechanical damage was done at the same extension as observed after herbivory. Control for P. syringae consisted of MgCl2 leaf infiltration.
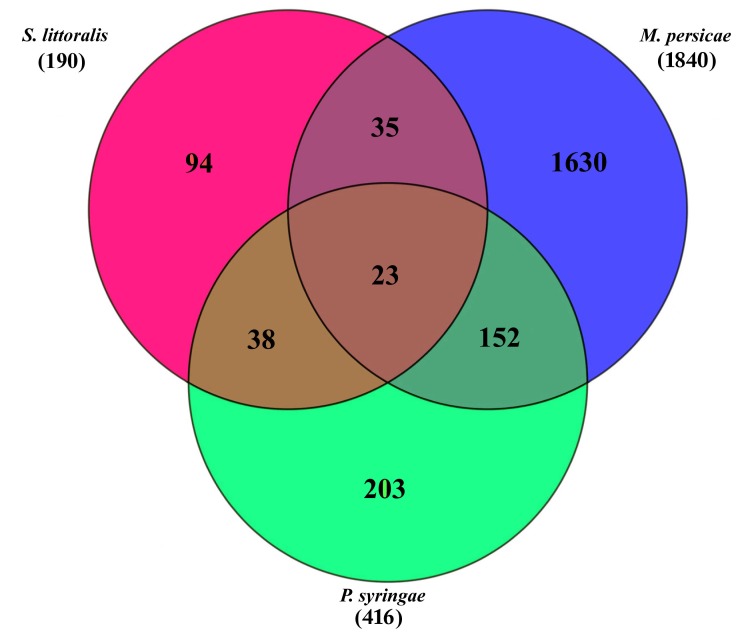
In order to evaluate robustly regulated sequences that are useful to evaluate the plant response to biotic stress, four biological replicates of each biotic stress (each consisting of several stressed leaves) were used for the gene microarray analysis. By using the stringent criteria described in material and methods (fold change ≥2, P≤0.05), out of 38,463 sequences on the Agilent spotted slide, 190 genes fulfilled these stringent criteria for S. littoralis, 1840 genes for M. persicae and 416 for P. syringae. Among these genes, 23 were commonly regulated, whereas the comparative analysis between the biotrophs revealed the presence of 35, 38 and 152 co-regulated genes in the interactions M. persicae/S. littoralis, S. littoralis/P. syringae and M. persicae/P. syringae, respectively (Figure 2).
Figure 2. Venn diagram of commonly and differentially expressed Arabidopsis genes upon herbivory by Spodoptera littoralis and Myzus persicae and infection by Pseudomonas syringae.
A few Arabidopsis commonly expressed genes are regulated upon M. persicae and S. littoralis herbivory and P. syringae infection
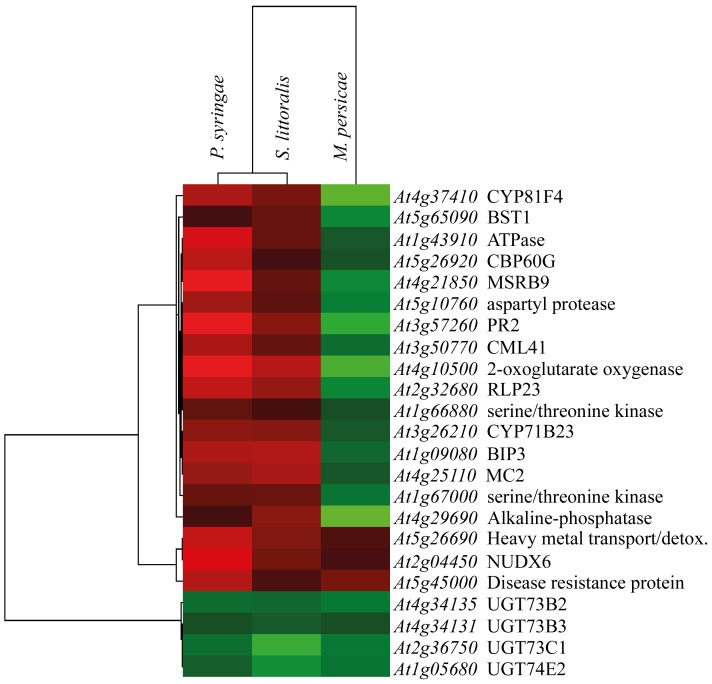
The cluster analysis of commonly regulated genes shows that at the time of Vm depolarization a common feature of Arabidopsis plants under biotic stress is a cluster of down-regulated genes including four UDP-glycosyltransferases (UGT73B2, UGT73B3, UGT73C1, UGT74E2) and a cluster of up-regulated genes made of Toll-Interleukin-Resistance (TIR-NBS-LRR class) transmembrane receptor (At5g45000), NADH pyrophosphatase (NUDX6) and heavy metal transport/detoxification superfamily protein (At5g26690) (Figure 3 and Table 1). All other commonly regulated genes were differentially expressed and the cluster analysis showed a close linkage between P. syringae and S. littoralis, indicating a common up-regulation of the remaining genes, whereas the same genes were always down-regulated by M. persicae (Table 1 and Figure 3).
Figure 3. Cluster analysis of commonly regulated genes in Arabidopsis fed by the herbivores Myzus persicae and Spodoptera littoralis and infected by Pseudomonas syringae.
M. persicae shows a lower statistical linkage with S. littoralis and P. syringae, which are linked together.
Table 1. Arabidopsis thaliana genes commonly expressed at the time of Vm depolarization upon Spodoptera littoralis (2 h) and Myzus persicae (5 h) herbivory and Pseudomonas syringae (16 h) infection.
| GO category and AGI | Short description | P. syringae | S. littoralis | M. persicae |
| Transferase and Transporter activity | ||||
| At4g34135 | flavonol 7-O-glucosyltransferase (Group D) (UGT73B2) | −3.00 | −2.82 | −3.40 |
| At4g34131 | UDP-glucosyl transferase (Group D) (UGT73B3) | −2.22 | −2.53 | −2.23 |
| At2g36750 | UDP-glucosyl transferase (Group D) (UGT73C1) | −3.08 | −6.66 | −3.40 |
| At1g05680 | UDP-glucosyltransferase (Group L) (UGT74E2) | −2.59 | −4.21 | −3.23 |
| Hydrolase activity | ||||
| At1g43910 | P-loop containing nucleoside triphosphate hydrolases superfamily protein | 8.83 | 2.90 | −2.43 |
| At3g57260 | beta 1,3-glucanase (PR2) | 14.85 | 3.94 | −6.05 |
| At4g29690 | Alkaline-phosphatase-like family protein | 2.14 | 4.06 | −14.95 |
| At5g65090 | involved in root hair morphogenesis and tip growth (BST1) | 2.09 | 2.91 | −3.89 |
| Kinase activity | ||||
| At1g66880 | protein serine/threonine kinase activity | 2.74 | 2.14 | −2.23 |
| At1g67000 | protein serine/threonine kinase activity | 2.87 | 2.97 | −3.24 |
| At2g32680 | receptor like protein 23 (RLP23) | 7.11 | 4.55 | −3.85 |
| Response to biotic stress | ||||
| At2g04450 | NADH pyrophosphatase (NUDX6) | 9.33 | 3.28 | 2.20 |
| At5g26690 | Heavy metal transport/detoxification superfamily protein | 7.42 | 3.74 | 2.34 |
| At5g45000 | Disease resistance protein (TIR-NBS-LRR class) family | 6.20 | 2.26 | 3.39 |
| At1g09080 | ATP binding protein (BIP3) | 5.76 | 6.05 | −2.84 |
| At3g26210 | cytochrome P450 (CYP71B23) | 4.15 | 3.86 | −2.43 |
| At4g37410 | cytochrome P450 (CYP81F4) | 5.82 | 3.42 | −11.95 |
| At3g50770 | calmodulin-like 41 (CML41) | 5.71 | 2.84 | −3.01 |
| At5g26920 | calmodulin-binding protein (CBP60G) | 6.61 | 3.74 | −2.30 |
| At4g10500 | 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase superfamily protein | 33.52 | 6.41 | −8.40 |
| At4g21850 | methionine sulfoxide reductase (MSRB9) | 23.57 | 2.81 | −3.91 |
| At4g25110 | type I metacaspase (MC2) | 4.55 | 5.59 | −2.42 |
| At5g10760 | aspartyl protease family protein | 5.09 | 2.61 | −3.58 |
Values are expressed as fold change with respect to controls (P<0.05). AGI, Arabidopsis Genome Initiative gene index.
Commonly expressed Arabidopsis genes upon S. littoralis and M. persicae herbivory show different regulation patterns
Most of the genes that were commonly regulated by M. persicae and S. littoralis herbivory showed opposite regulation, with many genes showing down-regulation upon M. persicae feeding and up-regulation after S. littoralis herbivory (Figure 4 and Table 2).
Figure 4. GO analysis of Arabidopsis commonly expressed genes upon Spodoptera littoralis (SL) and Myzus persicae (MP) herbivory.
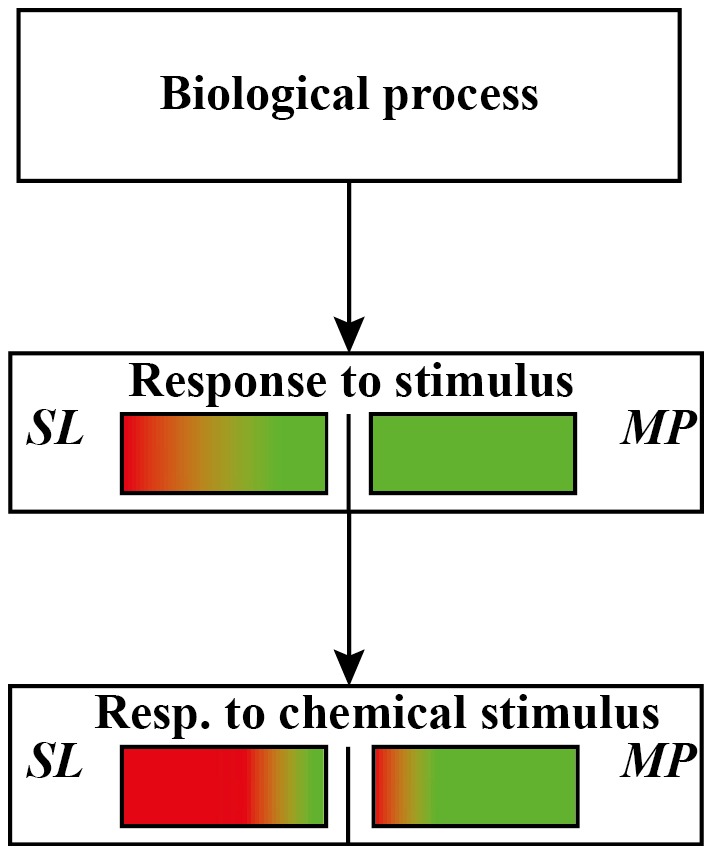
Red color indicates up-regulation, green color indicates down-regulation.
Table 2. Arabidopsis thaliana genes commonly expressed at the time of Vm depolarization upon Spodoptera littoralis (2 h) and Myzus persicae (5 h) herbivory.
| GO categories | AGI | Short description | M. persicae | S. littoralis |
| Response to stimulus | ||||
| Response to stimulus | At1g76690 | 12-oxophytodienoic acid reductase (OPR2) | −2.57 | −2.43 |
| At5g41750 | disease resistance protein (TIR-NBS-LRR class) | −2.63 | 2.63 | |
| Response to chemical stimulus | At1g66370 | MYB113 | −5.81 | 4.30 |
| At3g59220 | cupin-domain containing protein (PRN1) | −6.31 | −2.98 | |
| At3g23240 | ethylene response factor (ERF1) | −4.95 | 2.44 | |
| At5g47220 | ethylene response factor (ERF2) | −3.45 | 3.07 | |
| At1g77120 | alcohol dehydrogenase (ADH1) | 2.41 | −2.26 | |
| At5g17330 | glutamate decarboxylase (GAD1) | −2.40 | 4.12 | |
| At5g24380 | metal-phytosiderophore yellow stripe like (YSL2) | −2.11 | 2.94 | |
| Other GO categories | ||||
| Transferase and Transporter activity | At2g37870 | protease inhibitor/seed storage/lipid transfer protein (LTP) family protein | −2.83 | −3.35 |
| At3g50280 | HXXXD-type acyl-transferase family protein | −3.23 | 2.01 | |
| At5g54060 | UDP-glucose:flavonoid 3-o-glucosyltransferase (UF3GT) | −12.36 | 3.99 | |
| Hydrolase activity | At1g14890 | invertase/pectin methylesterase inhibitor superfamily protein; | 2.41 | −2.02 |
| At4g29700 | Alkaline-phosphatase-like family protein; | −2.38 | 2.62 | |
| At1g53830 | pectin methylesterase (PME2) | −4.09 | 2.36 | |
| At3g62040 | Haloacid dehalogenase-like hydrolase (HAD) superfamily protein | −5.13 | 2.35 | |
| Transcription factors | At3g48360 | essential component of the TAC1-mediated telomerase activation pathway (BT2) | 8.63 | 2.78 |
| At5g22380 | ANAC090 | −16.79 | 4.78 | |
| Response to biotic stress | At4g36850 | PQ-loop repeat family protein/transmembrane family protein | 25.48 | 5.22 |
| At3g13520 | GPI-anchored arabinogalactan peptide (AGP12) | 3.36 | −2.54 | |
| At1g77120 | alcohol dehydrogenase (ADH1) | 2.41 | −2.26 | |
| At3g30460 | zinc finger (C3HC4-type RING finger) family protein | 2.33 | −2.35 | |
| At4g01575 | serine protease inhibitor, Kazal-type family protein | 2.26 | −2.19 | |
| At5g02540 | short-chain dehydrogenase/reductase (SDR) family protein | 2.15 | −2.14 | |
| At4g37540 | LOB domain-containing protein (LBD39) | 2.01 | 2.07 | |
| At5g07460 | Methionine sulfoxide reductase (ATMSRA2) | −2.08 | 2.01 | |
| At4g24350 | phosphorylase family protein | −2.09 | 2.12 | |
| At2g25130 | armadillo/beta-catenin repeat family protein | −2.46 | −3.17 | |
| At5g53820 | Late embryogenesis abundant protein (LEA) family protein | −2.59 | −2.04 | |
| At1g59590 | ZCF37 mRNA | −2.66 | 4.10 | |
| At2g27310 | F-box family protein | −3.18 | 2.06 | |
| At2g47560 | zinc finger (C3HC4-type RING finger) family protein | −3.31 | 3.14 | |
| At1g24140 | matrixin family protein metallopeptidase activity, metalloendopeptidase activity | −3.88 | 2.52 | |
| At1g13470 | unknown protein | −2.11 | 6.16 | |
| At5g22545 | unknown protein | −2.37 | 2.84 | |
Values are expressed as fold change with respect to controls (P<0.05). AGI, Arabidopsis Genome Initiative gene index.
Genes annotated for response to stimulus showed the same expression trends between the two herbivores for OPR2 and PRN1, which were commonly down-regulated, whereas up-regulation by S. littoralis and down-regulation by M. persicae was found for ethylene response factors (ERF1, ERF2), MYB113, GAD1 and a metal-phytosiderophore (YSL2). S. littoralis down-regulated alcohol dehydrogenase (ADH1), which was up-regulated by M. persicae.
An opposite gene regulation, with up-regulation caused by S. littoralis, was also found for several genes belonging to other GO categories like transferases (UF3GT, At3g50280), hydrolases (PME2, HAD, At4g29700), transcription factors (TFs) (ANAC090) and some genes responding to biotic stress (Table 2). On the other hand, an opposite regulation, with up-regulation caused by M. persicae, was found for an invertase/pectin methylesterase inhibitor (At2g37870), a GPI-anchored arabinogalactan peptide (AGP12), ADH1, and some other genes responding to biotic stress (Table 2). A common up-regulation was found for an essential component of the TAC1-mediated telomerase activation pathway (BT2), a PQ-loop repeat family protein/transmembrane family protein (At4g36850) and methionine sulfoxide reductase (ATMSRA2). Both insects down-regulated protease inhibitor/seed storage/lipid transfer protein (At2g37870), late embryogenesis abundant protein (LEA) (At5g53820) and armadillo/beta-catenin repeat family protein (At2g25130).
Commonly expressed Arabidopsis genes upon S. littoralis herbivory and P. syringae infection show the same regulation pattern
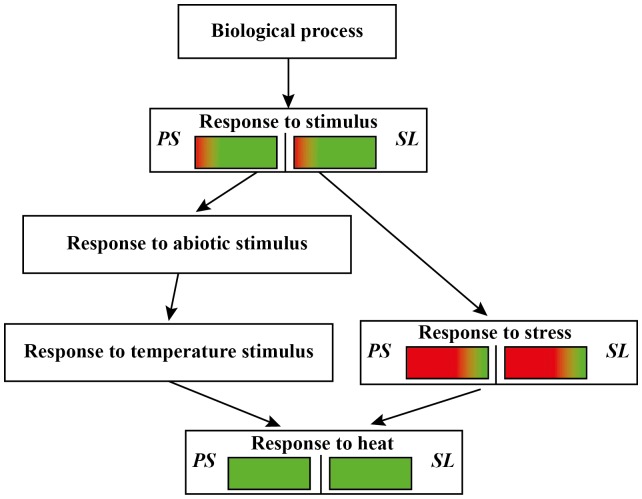
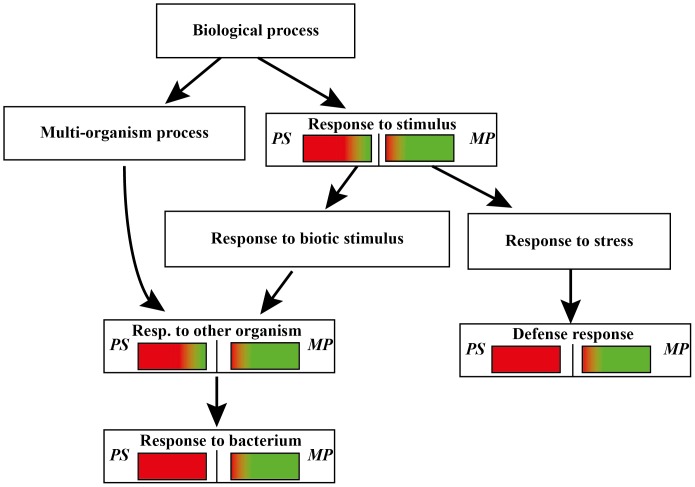
The GO analysis of genes commonly expressed in Arabidopsis upon infection of the pathogen P. syringae and the chewing herbivore S. littoralis classified 35 of the 38 annotated genes. Most of them were related to response to stimulus, leading to a common response to heat (Figure 5).
Figure 5. GO analysis of Arabidopsis commonly expressed genes upon Pseudomonas syringae (PS) infection and Spodoptera littoralis (SL) herbivory.
Red color indicates up-regulation, green color indicates down-regulation.
Several small heat shock proteins (sHSPs) (HSP17, HSP17.6II, HSP17.6A-CI, HSP17.6, HSP21, HSP23.6-MITO) and HSP70 were down-regulated by both P. syringae and S. littoralis biotic stress, as were genes involved in glutathione S-transferases (GST20, GSTU24), UDP-glycosyltransferase (UGT73B4) and a TF (ANAC102). Most of the remaining commonly expressed genes were up-regulated by both S. littoralis and P. syringae (Table 3). A remarkable up-regulation was found upon P. syringae infection for a beta-1,3-glucanase (BG3), triacylglycerol lipase (At5g24200) and genes related to ion binding and resistance (At3g57460, PCR1).
Table 3. Arabidopsis thaliana genes commonly expressed at the time of Vm depolarization upon Spodoptera littoralis (2 h) herbivory and Pseudomonas syringae (16 h) infection.
| GO categories | AGI | Short description | P. syringae | S. littoralis |
| Response to stimulus | ||||
| Response to temperature (heat) | At5g12030 | Small Heat Shock Protein (HSP17) | −12.75 | −22.62 |
| At5g12020 | Small Heat Shock Protein (HSP17.6II) | −16.00 | −10.05 | |
| At1g07400 | Small Heat Shock Protein (HSP17.6A-CI) | −5.27 | −2.60 | |
| At1g53540 | Small Heat Shock Protein (HSP17.6) | −6.06 | −18.65 | |
| At1g52560 | Small Heat Shock Protein (HSP21) | −10.12 | −36.21 | |
| At4g25200 | Small Heat Shock Protein (HSP23.6-MITO) | −15.13 | −23.74 | |
| At5g51440 | Small Heat Shock Protein (HSP23.6-MITO) | −5.46 | −3.89 | |
| At1g16030 | Heat Shock Protein (HSP70) | −4.62 | −6.24 | |
| Response to stress | At1g76680 | 12-oxophytodienoic acid reductase (OPR1) | −2.04 | −2.18 |
| At3g01080 | WRKY58 | 3.47 | 4.55 | |
| At3g45860 | cysteine-rich receptor-like protein kinase (CRK4) | 3.45 | 3.47 | |
| At4g04220 | Receptor Like Protein (RLP46) | 2.17 | 2.04 | |
| Other responses to stimulus | At3g57240 | beta-1,3-glucanase (BG3) | 26.39 | 3.20 |
| At1g77760 | nitrate reductase (NR1) | 2.24 | 5.20 | |
| At5g24530 | downy mildew resistant (DMR6) oxygenase | 4.09 | 2.71 | |
| At3g50480 | Homolog of RPW8 (HR4) | 3.71 | 2.17 | |
| At2g29480 | glutathione S-transferase (GST20) | −4.06 | −4.38 | |
| At1g17170 | glutathione S-transferase (GSTU24) | −6.05 | −5.08 | |
| At2g15490 | UDP-glycosyltransferase (UGT73B4) | −16.45 | −6.81 | |
| Other GO categories | ||||
| Hydrolase activity | At5g24200 | triacylglycerol lipase | 20.36 | 3.14 |
| Kinase activity | At1g35710 | leucine-rich repeat transmembrane protein kinase, putative | 4.34 | 3.52 |
| At5g59680 | leucine-rich repeat protein kinase, putative | 2.93 | 6.16 | |
| Transcription factors | At5g63790 | NAC family of transcription factors (ANAC102) | −2.05 | −2.16 |
| At5g01900 | WRKY62 | 9.52 | 3.31 | |
| At3g11580 | AP2/B3-like transcription factor | 6.03 | −2.08 | |
| Other response to biotic stress | At3g57460 | catalytic/metal ion binding/metalloendopeptidase/zinc ion binding | 28.85 | 3.68 |
| At1g14880 | plant cadmium resistance (PCR1) | 21.36 | 7.39 | |
| At5g39670 | calcium-binding EF hand family protein | 11.87 | 2.45 | |
| At3g47480 | calcium-binding EF hand family protein | 7.87 | 2.65 | |
| At5g55460 | protease inhibitor/seed storage/lipid transfer protein (LTP) family protein | 6.97 | 4.87 | |
| At1g10340 | ankyrin repeat family protein | 6.72 | 2.87 | |
| At4g03450 | ankyrin repeat family protein | 5.44 | 3.23 | |
| At2g47130 | short-chain dehydrogenase/reductase (SDR3) | 5.00 | 2.96 | |
| At1g23840 | unknown protein located in endomembrane system | 3.50 | 2.97 | |
| At3g61280 | unknown protein | 3.45 | 2.28 | |
| At3g48640 | unknown protein | 5.77 | 3.00 | |
| At3g61920 | unknown protein | 2.41 | −2.04 | |
Values are expressed as fold change with respect to controls (P<0.05). AGI, Arabidopsis Genome Initiative gene index.
Commonly expressed Arabidopsis genes upon M. persicae herbivory and P. syringae infection reveal a remarkable opposite regulation
The GO analysis of genes commonly expressed in Arabidopsis upon infection of the pathogen P. syringae and the phloem feeding M. persicae classified 135 genes among 152 annotated genes. Among these annotated genes, several were typical of responses to bacterium, response to other organisms, and response to stimulus (Figure 6 and Table S1)
Figure 6. GO analysis of Arabidopsis commonly expressed genes upon Pseudomonas syringae (PS) infection and Myzus persicae (MP) herbivory.
Red color indicates up-regulation, green color indicates down-regulation.
Up-regulation after both M. persicae and P. syringae biotic stress was found for genes related to pathogenesis (NIMIN-1, PCC1, ACD6, PR5, PR13), a receptor like protein (RLP38), an S-locus lectin protein kinase family protein (At1g11330), glutaredoxin (GRXS13), a gene up-regulated in response to Hyaloperonospora parasitica (LURP1), genes involved in SA and JA metabolism (ANK, BSMT1, MES9, JMT, EDS5), a NPR1/NIM1-interacting gene (NIMIN-2) and a TF (WRKY38).
A common down-regulation was found for a glucosyl transferase (UGT73B5), an ABC transporter (ABCB4) and a nodulin MtN21 family protein (At1g70260).
An opposite regulation was found for several receptor-like proteins (RLP41, RLP43), cysteine-rich receptor-like protein kinases (CRK6, CRK7, CRK23, CRK36), flg22-induced receptor-like kinase 1 (FRK1), a putative receptor serine/threonine kinase (At4g18250), a lectin receptor kinase (At5g01550), genes responding to hormone stimulus (JAZ10, GID1B and At3g12830), several transporters (GPT2, LHT7, At3g21080, ST2A, PTR3), genes coding for hydrolase activity (CWINV6, CHI, At1g54010), a kinase (MAPKKK19), a TF (WRKY55) and several other genes expressed in response to biotic stress (At1g14120, At3g49340, MSRB8, LAS1) (Table S1). Several other genes showing opposite trends are listed in Table S1.
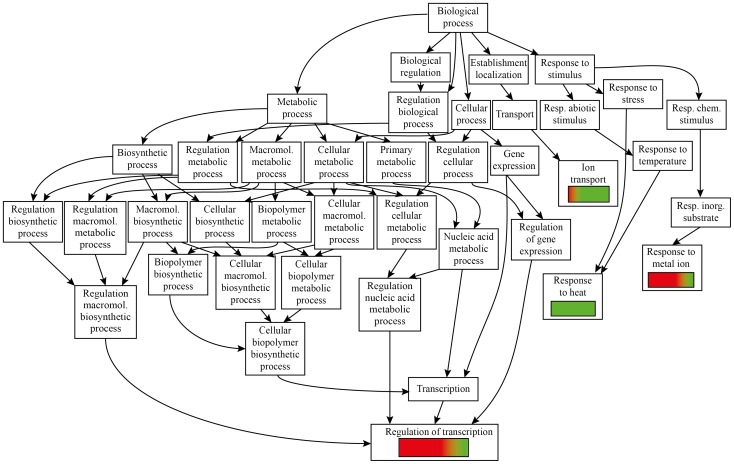
Differential gene expression in Arabidopsis upon S. littoralis herbivory reveals up-regulation of genes involved in transcription regulation and down-regulation of heat-shock proteins
Among the 94 genes specifically regulated by S. littoralis herbivory, 78 were annotated and GO analysis isolated four gene categories: genes involved in the regulation of transcription, response to heat, ion transport and response to metal ion (Figure 7)
Figure 7. GO analysis of Arabidopsis genes regulated by Spodoptera littoralis herbivory.
Red color indicates up-regulation, green color indicates down-regulation.
In the regulation of transcription category, most of the genes were up-regulated by S. littoralis herbivory, including several genes involved in the circadian clock, whereas two basic helix-loop-helix (bHLH) (At4g20970, ATBHLH096) and a MYB like DNA-binding protein (AtGT-3a) were down-regulated (Table 4).
Table 4. Arabidopsis thaliana genes differentially expressed at the time of Vm depolarization (2 h) upon Spodoptera littoralis herbivory.
| GO Category | AGI | Short description | FC (P<0.05) |
| Regulation of transcription | At4g06746 | DREB subfamily A-5 of ERF/AP2 transcription factor family (RAP2.9) | −3.17 |
| At2g22200 | DREB subfamily A-6 of ERF/AP2 transcription factor family. | 2.49 | |
| At4g20970 | basic helix-loop-helix (bHLH) family protein | −3.40 | |
| At1g72210 | ATBHLH096 | −2.48 | |
| At4g05170 | basic helix-loop-helix (bHLH) family protein | 3.19 | |
| At2g28160 | ATBHLH029 | 2.56 | |
| At3g09600 | MYB TF (LHY-CCA1-LIKE5) | 2.41 | |
| At2g21650 | MYB TF (AtRL2 - RAD-LIKE 2) | 2.09 | |
| At5g17300 | MYB TF (REVEILLE 1) | 2.94 | |
| At1g01060 | MYB TF (LATE ELONGATED HYPOCOTYL 1, LHY1) | 3.80 | |
| At2g46830 | MYB TF (CIRCADIAN CLOCK ASSOCIATED 1, CCA1) | 2.05 | |
| At5g01380 | MYB like DNA-binding protein (AtGT-3a) | −2.18 | |
| At1g69570 | Dof-type zinc finger domain-containing protein | 2.28 | |
| At3g21150 | zinc finger TF (B-box type ATBBX32) | 2.95 | |
| At1g26960 | homeodomain leucine zipper class I (HD-Zip I, ATHB23) | 2.33 | |
| response to heat | At3g46230 | 17.4 kDa class I heat shock protein (HSP17.4-CI) | −20.03 |
| At1g59860 | 17.8 kDa class I heat shock protein (HSP17.8-CI) | −8.90 | |
| At2g29500 | 17.8 kDa class I heat shock protein (HSP17.8-CI) | −4.71 | |
| At1g74310 | ClpB1, Also known as AtHsp101. | −3.25 | |
| At4g21320 | heat-stress-associated 32-kD protein | −2.20 | |
| At2g32120 | HSP70 family protein (Hsc70.1) | −3.33 | |
| At3g61190 | protein with a C2 domain that binds to BON1 (BAP1) | −2.67 | |
| At4g12400 | stress-inducible protein | −2.26 | |
| Response to metal ion | At3g56240 | copper chaperone CCH protein | 2.02 |
| At2g40300 | Ferritin-4 (FER4) | −2.49 | |
| Ion transport | At3g63380 | calcium-transporting ATPase 12, plasma membrane-type (ACA12) | −2.95 |
| At3g22910 | calcium-transporting ATPase 13, plasma membrane-type (ACA13) | −2.20 | |
| At3g56240 | CCH protein | 2.01 | |
| At2g04032 | Zinc transporter (ZIP7) | −2.24 | |
| At1g77990 | sulfate transporter | 2.40 |
Values are expressed as fold change with respect to controls (P<0.05). AGI, Arabidopsis Genome Initiative gene index.
In the response to heat category, all expressed sHSPs and HSPs were down-regulated, whereas in response to metal ion, a copper chaperone CCH protein (At3g56240) was up-regulated and a ferritin (FER4) was down-regulated (Table 4). In the ion transport category, two Ca2+−ATPase (ACA12, ACA13) and a zinc transporter (ZIP7) were down-regulated, whereas a copper (At3g56240) and a sulfate (At1g77990) transporters were up-regulated.
Table S2 reports values of all other differentially expressed genes upon S. littoralis herbivory.
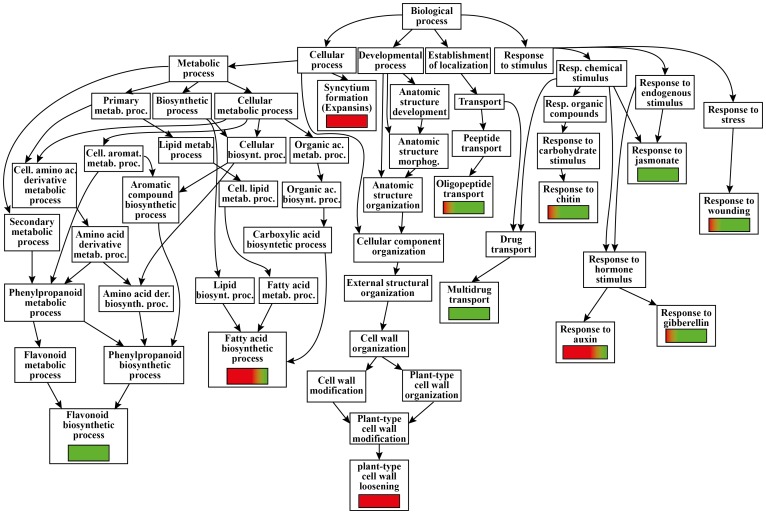
Differential gene expression in Arabidopsis leaves upon M. persicae herbivory reveals down-regulation of secondary metabolism and up-regulation of cell wall components
Among the 1630 Arabidopsis differentially expressed genes upon M. persicae herbivory, 1423 were annotated and GO analysis classified 10 gene categories (Figure 8).
Figure 8. GO analysis of Arabidopsis genes regulated by Myzus persicae herbivory.
Red color indicates up-regulation, green color indicates down-regulation.
A strong down-regulation was found for many genes related to flavonoid biosynthetic process (DFR), particularly those related to metabolism of anthocyanin/anthocyanidin (ANS, 5MAT, At1g03940, At4g22870, MYB75, MYB123), and flavonoid hydroxylation (CYP75B1, F3H) and isomerization (CFI, At5g05270). The only up-regulation were found for a chalcone-flavanone isomerase family protein (At1g53520) and a gene involved in brassinosteroid metabolic pathway (BEN1) (Table S3). Genes involved in fatty acid metabolism were all up-regulated, with the only exception of 4-coumarate-CoA ligase-like (4CLL5), that was down-regulated.
Most of genes involved in fatty acid biosynthetic process were up-regulated (particularly a Δ-9 desaturase-like 5 protein, At1g06360), whereas a strong down-regulation was found for a P450-dependent fatty acid omega-hydroxylase (At3g48520).
Genes involved in cell wall modification and loosening included 11 expansin genes, 10 of which were up-regulated by M. persicae feeding (particularly EXP8), whereas an expansin-related gene (EXLB1) was down-regulated (Table S3).
Aphid feeding regulated oligopeptide transport with many genes being either up-regulated or down-regulated. On the other hand, almost all multidrug transport-related genes belonging to the MATE efflux family were down-regulated. Aphid feeding repressed Arabidopsis TF responding to chitin, including several WRKY (WRKY18, WRKY40, WRKY48, WRKY53, WRKY60), MYB (MYB31, MYB44, MYB59), members of the NAC TF family (ANAC036, ANAC061), zinc finger proteins (CZF1, CZF1/ZFAR1, AZF2, AZF3, STZ), ethylene response factors (ERF1, RRTF1, ERF13), members of the DREB subfamily A-6 of ERF/AP2 TFs (DEAR19, At4g28140), scarecrow-like (SCL13), salt tolerance homolog (STH2), armadillo-like (At2g35930) and RING-H2 finger protein (ATL5H). A response regulator (ARR7), a U-box domain-containing protein similar to immediate-early fungal elicitor (At3g18710) and WRKY17 were up-regulated (Table S3).
Genes responding to wounding were either up-regulated (RLM3, At5g57170, 5PTASE13) or down-regulated, like a terpene synthase involved in (E)-β-ocimene synthesis (TPS03).
M. persicae also strongly regulated genes involved in action of three important hormone classes: auxins, gibberellins and JA.
Four auxin induced proteins (IAA5, IAA6, IA14, IAA5), an auxin influx transporter (AUX1), an auxin resistant gene (AXR2), 8 small auxin up-regulated (SAUR) responsive proteins (SAUR15, SAUR19, SAUR20, SAUR22, SAUR23, SAUR26, SAUR29, SAUR68), and 12 SAUR-like auxin-responsive proteins were up-regulated (Table S3). Up-regulation was also found for a IAA-amido synthetase (GH3.9) and a nucleotide diphosphate kinase (NDPK2), whereas MYB95 and a MYB responding to auxin stimulus were down-regulated.
GA-regulated proteins (particularly GASA6) and GA oxidases (GA3OX1, GA20OX1) were all up-regulated, whereas down-regulation was found for a GA-regulated protein (GASA5), a DELLA protein (RGL3), MYB7 and α-amylase (AMY1).
All genes responding to JA were down-regulated by M. persicae feeding, including genes involved in JA metabolism (OPR3, AOC3, CYP94B3), 8 JA-Zim-domain proteins (JAZ1, JAZ2, JAZ3, JAZ5, JAZ6, JAZ7, JAZ9, JAZ10), 2 JA responsive proteins (JR1, JR2), lipoxygenases (LOX3, LOX4), MYC2, MYB47, MYB74, syntaxin (SYP122) and, particularly, ribonuclease T2 (RNS1) and a thionin (THI2.1) (Table S3).
The full list of all other genes regulated by M. persicae feeding can be found in Table S4.
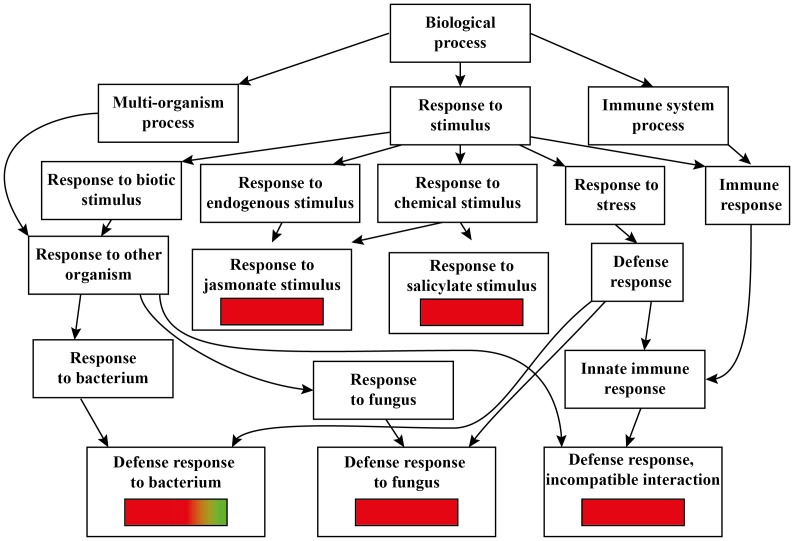
Differential gene expression in Arabidopsis leaves upon P. syringae infection shows up-regulation of defense genes including responses to JA and SA
Among the 203 genes specifically regulated by P. syringae herbivory, 186 were annotated and GO analysis identified three gene categories: defense genes in response to bacterium, to fungus and incompatible interaction, and response genes to JA and SA (Figure 9)
Figure 9. GO analysis of Arabidopsis genes regulated by Pseudomonas syringae infection.
Red color indicates up-regulation, green color indicates down-regulation.
Almost all differentially expressed genes in the defense response to bacterium were up-regulated with the only exception of a plasma membrane polypeptide (ATPCAP1) (Table 5). Up-regulation was also found for all genes responsive to fungus (At2g14560, At4g12490, At2g39200) and in incompatible interaction (PDF1.2A, AOC2), the latter two genes being also involved in responses to SA and JA (Table 5). Genes responsive to SA included two kinases (CRK9, WAK1) and a phytoalexin-deficient 4 protein (PAD4). In response to JA, up-regulation was found for vegetative storage protein (VSP1), JA-ZIM-domain protein (JAZ10), MYB50 and arginine decarboxylase (ADC2).
Table 5. Arabidopsis thaliana genes commonly expressed at the time of Vm depolarization (16 h) upon Pseudomonas syringae infection.
| GO Category | AGI | Short description | FC (P<0.05) |
| Defense response to bacterium | At5g13320 | GH3-Like Defense Gene Encodes PBS3 (GDG1) | 5.57 |
| At3g56400 | WRKY70 | 4.17 | |
| At1g70690 | plasmodesmal protein (PDLP5) | 4.12 | |
| At1g74710 | isochorismate synthase (ICS1) | 3.14 | |
| At3g49120 | Class III peroxidase (PERX34). | 2.52 | |
| At4g20260 | plasma membrane polypeptide (ATPCAP1) | −2.02 | |
| Response to salicylic acid stimulus | At4g23170 | Receptor-Like Protein Kinase (CRK9) | 2.38 |
| At1g21250 | cell wall-associated kinase (WAK1) | 2.21 | |
| At3g52430 | phytoalexin-deficient 4 protein (PAD4) | 5.08 | |
| Response to fungus | At2g14560 | unknown protein | 7.37 |
| At4g12490 | protease inhibitor/seed storage/lipid transfer protein (LTP) family protein | 6.80 | |
| At2g39200 | seven-transmembrane domain proteins specific to plants, homolog of MLO12 | 2.99 | |
| defense response, incompatible interaction | At5g44420 | ethylene- and jasmonate-responsive plant defensin. (PDF1.2A) | 2.56 |
| At3g25770 | allene oxide cyclase (AOC2) | 2.00 | |
| Response to jasmonate stimulus | At5g24780 | vegetative storage protein 1 (VSP1). | 7.41 |
| At5g13220 | Jasmonate-Zim-Domain Protein (JAZ10) | 7.26 | |
| At1g57560 | MYB50 | 4.54 | |
| At4g34710 | arginine decarboxylase (ADC2) | 2.51 |
Values are expressed as fold change with respect to controls (P<0.05). AGI, Arabidopsis Genome Initiative gene index.
The full list of all other Arabidopsis differentially expressed genes upon P. syringae infection is reported in Table S5.
Discussion
Dynamic Vm depolarization responses in Arabidopsis induced by S. littoralis, M. persicae and P. syringae are different
The primary candidate for intercellular signaling in higher plants is the stimulus-induced change in transmembrane potential (Vm) [39]. Here we showed that a common event upon biotic stress is plant Vm depolarization, which occurs at different times according to the biotroph damage. No matter the nature of the biotroph, this event occurs at the same intensity, which in Arabidopsis corresponds to a Vm depolarization of about 40 mV. We used the timing of maximal Vm depolarization as a time point to compare the genome-wide response of Arabidopsis thaliana to three known pests: two herbivores, M. persicae and S. littoralis and a pathogen, P. syringae.
Two questions arise from our results: 1) why Vm depolarization is a common event? 2) Why Vm depolarization occurrs at different times? To our knowledge this is the first report on time-course Vm variations upon either M. persicae or P. syringae, whereas more data are available on S. littoralis herbivory-induced Vm depolarization. Previous studies have demonstrated that S. littoralis herbivory triggers a calcium-induced potassium-dependent Vm depolarization in wounded tissues [40] followed by a isotropic depolarizing wave that crosses all plant tissues, from shoot to roots [18], [19], [41]–[46]. This effect was found to be strictly dependent on insect oral secretion, since both single and repeated mechanical wounding were not able to induce such response [1]. The lowering of the plant Vm is seen as a insect's strategy to reduced plant cell responses [47], thus it is conceivable to argue that the same strategy might also be used by both M. persicae and P. syringae. Elicitors are involved in Vm depolarization [48], and both M. persicae and P. syringae produce elicitors and effectors during biotic attack [16]. Furthermore, the same Vm depolarization value recorded upon all three biotic attacks suggests the occurrence of a Vm threshold to be reached for successful herbivory/infection.
The second question, why Vm polarization occurs at different times, appears to be associated with the mode of biotic damage. The fast clipping and consistent plant tissue removal by chewing herbivores evidently induces a “quantitative” response that is proportional to tissue damage; on the other hand, the stylet probing and the phloem feeding operated by the aphid induces a reduced amount of damage, that requires more time for a plant response. Finally, bacterial growth and tissue damage takes time, which appears to be proportional to Vm depolarization.
Different patterns of gene regulation responses are induced by the two insects
Our study shows that the molecular mechanism of gene regulation response to S. littoralis and M. persicae are principally different. The evidence is that M. persicae regulated almost 10-fold higher number of genes than S. littoralis did. Moreover, genes responsive to both insects were regulated mostly in opposite direction.
A remarkable and specific response to S. littoralis herbivory at the time of Vm depolarization was the down-regulation of several small heat-shock proteins (sHSP). sHSPs have a high capacity to bind non-native proteins, probably through hydrophobic interaction, and to stabilize and prevent non-native aggregation [49], [50]. Increasing data suggest a strong correlation between sHSP accumulation and plant tolerance to a wide range of stresses [51]. To our knowledge, this is the first report on sHSP down-regulation upon herbivory. Also, down-regulation of HSPs is quite rare although gene expression of sHSPs was down-regulated by SA in Arabidopsis and in tomato [52], whereas treatment by mitochondrial inhibitors and uncouplers down-regulated HSP, suggesting that mitochondrial functions are essential for sHSP synthesis [53].
With regards regulation of transcription, S. littoralis herbivory down-regulated a few genes with a regulatory role in mediating crosstalk between signaling pathways for biotic stress responses like DREB TFs [54] and two bHLH TFs. Most of the remaining TFs were up-regulated, including genes involved in the Arabidopsis circadian clock (REVEILLE 1, LHY1, CCA1 and LHY-CCA1-LIKE5) [55]–[58] and AtRL2, which is expressed in the funiculus of ovules and in embryos and is involved in the control of floral asymmetry [59]. Two zinc finger TF were also up-regulated and one of them, BBX32, has a native role in mediating gene repression to maintain dark adaptation [60]. An HD-Zip I TF, ATHB23, which is under the control of GA and other activators and is involved in establishing polarity during leaf development [61], was also up-regulated by S. littoralis herbivory. Altogether, these data indicate an effect of herbivory on the regulation of the circadian clock and the plant development.
Two members of calcium-transporting ATPase (ACA12 and ACA13), that are dramatically induced upon exposure to pathogens [62], were down-regulated by S. littoralis herbivory. Ferritin (FER4) was also down-regulated and in Arabidopsis the absence of ferritin induces higher levels of reactive oxygen species, and increased activity of enzymes involved in their detoxification [63], a condition that is commonly found upon S. littoralis herbivory [64].
The response of Arabidopsis to M. persicae phloem feeding was clearly distinct from responses to the the chewing herbivore S. littoralis. After a few hours from feeding, and at the time of maximal Vm depolarization, the aphid was able to suppress a consistent number of genes involved in flavonoid metabolism. UV-exposed plants are damaged to a lesser extent by insect herbivores than non-irradiated plants [65], and UV radiation induces the accumulation of flavonoids in Arabidopsis [66]. In general higher concentration of flavonoids constrain aphid reproduction [67]; however, the generalist M. persicae was found to perform much better than the specialist Brevicoryne brassicae, when both aphids were feeding on Brassica oleracea exposed to UV-radiation [68]. The strong down-regulation of the genes coding for anthocyanidin synthases (also known as leucoanthocyanidin dioxygenases, At4g22870 and ANS) and dihydroflavonol reductase (DRF), all involved in flavonoid synthesis [69], was correlated with the down-regulation of two MYB TFs (MYB75 and MYB123). MYB123 controls the biosynthesis of flavonoids in the seed coat of Arabidopsis and MYB5 was recently proposed to be partially redundant with MYB123 in this respect ([70] and references therein). Moreover, the up-regulation of flavonoid transferase (UF3GT) induced by S. littoralis was in contrast with the down-regulation of this gene after M. persicae feeding. These data suggest that M. persicae has the ability to suppress plant defenses based on flavonoids.
Fatty acid desaturases (FADs) introduce double bonds into the aliphatic tails of fatty acids and influence plant susceptibility to a wide variety of biotic stresses due in part to their critical role in the biosynthesis of the defense hormone JA [71]. Conversely, SA accumulation is enhanced by transient suppression of FAD [72]. Since aphid resistance has been observed in Arabidopsis and tomato mutants deficient in some FADs [73], the up-regulation of FAD-related genes upon M. persicae feeding indicates a possible aphid strategy aimed at lowering plant resistance.
Aphids have not been reported to induce detectable levels of JA, probably because of the induction of SA, which can interact antagonistically with JA signaling [26]. However, recent evidence suggests that SA accumulation may not be required for the repression of JA by whiteflies [74]. Our data show that M. persicae induction of several FADs and other genes related to fatty acid biosynthetic process was associated with a JA down-regulation. In fact, most of the genes related to JA biosynthesis (OPR2, OPR3, AOC3, CYP94B3, LOX3, LOX4) and signaling (several JAZs and JRs) were down-regulated, as were 8 out of the 12 members of the JAZ family of plant-specific proteins in Arabidopsis. These data are in agreement with a down-regulation of JA-related genes by phloem feeders [73], [74]. JAZ proteins act as indirect transcriptional repressors of JA-dependent responses and their target to be repressed is MYC2, a key transcriptional regulator of JA signaling [75], [76]. MYC2 is proposed to regulate the transcription of defense-related genes directly or indirectly through secondary TFs that mediate transcription of defense-related genes and accumulation of defense metabolites [76]. The fact that MYC2 positively regulates flavonoid biosynthesis and the down-regulation of MYC2 and several MYC2-dependent secondary TFs (e.g., CHI, ERF1) by M. persicae suggest that the aphid is able suppress plant secondary metabolism at several levels.
M. persicae feeding induced up-regulation of three families of early auxin-responsive genes, auxin/indole acetic acid (Aux/IAA), GH3, and small auxin-up RNA (SAUR), which are usually specifically induced by auxin within minutes [77]. Interestingly, two genes involved in main auxin biosynthetic pathway in Arabidopsis, YUCCA 2(At4g13260) and TAR2 (At4g24670) were up-regulated after M. persicae attack. Therefore, we can assume that up-regulation of auxin responsive genes under M. persicae attack might be linked to increased auxin concentration due to activation of auxin biosynthesis through YUCCA-TAA1 pathway [78].
M. persicae also induced several classes of expansins. Expansin activity is mainly determined by transcription, which in turn is finely regulated by phytohormones [79], [80] and increases after cell wall acidification [81]. M. persicae induction of expansins was correlated also with the up-regulation of GA metabolism. Two GA-regulated proteins (GASA5 and GASA6) showed a strong and opposite regulation. GASA5 was strongly down-regulated by M. persicae and the expression of this gene was also found to be inhibited by heat stress but unaffected by the application of exogenous SA, whereas the expression of NPR1, a key component of the SA-signaling pathway, was down-regulated by GASA5 overexpression [82]. GASA6, which was up-regulated by M. persicae, is thought to be a secreted peptide hormone precursor [83] and has also been characterized as an in vivo sugar marker gene [84]. Recently it was shown that GASA6 is severely repressed by a zinc-finger protein (AtTZF1) over-expression, a regulator connecting sugar, ABA, GA and peptide hormone responses [85]. The finding that several zinc-finger TFs were down-regulated by M. persicae correlates with the up-regulation of GASA6. The down-regulation of gene coding for α-amilase (AMY1) and DELLA (RGL3) was in line with the up-regulation of genes involved in GA synthesis.
Two groups of transporters showed opposite regulation. Many secondary metabolites are transported into cells and across the plasma membrane via endogenous membrane transporters [86]. At the time of Vm depolarization during M. persicae feeding, almost all peptide transporters were down-regulated. The peptide transporter family consists of electrochemical potential-driven transporters that catalyze uptake of their solutes by a cation-solute symport mechanism [87]. M. persicae regulated the gene expression of both yellow stripe-like (YSL) proteins and the OPTs. While YSL transporters are involved in metal homeostasis through the translocation of metal-chelates, OPT proteins play a role in plant growth and development being involved in long-distance metal distribution, nitrogen mobilization, heavy metal sequestration, and glutathione transport ([88] and references therein). MATE efflux proteins were also down-regulated by M. persicae. In Nicotiana tabacum a tonoplast jasmonate-inducible MATE transporter was found to play an important role in the nicotine translocation by acting as a secondary transporter responsible for unloading of alkaloids in the aerial parts and deposition in the vacuoles [89]. Thus, the down-regulation of secondary plant products and the suppression of JA-induced genes might be another strategy of plant defense suppression by M. persicae.
Finally, a large number of TFs expressed in response to chitin were down-regulated by M. persicae, including several WRKY, MYB and ERF.
Chitin, a polysaccharide composed of β-1-4-linked N-acetyl-d-glucosamine found in the exoskeleton of insects, has been shown or implicated as a signal in plant defense by inducing regulation of different ERF, MYB and WRKY TFs [90]–[93]. Among these TFs, WRKY53, MYB44 and RRTF1 were down-regulated by M. persicae. WRKT53 has been shown to be required to fully silence SA biosynthesis [94] and in rice its over-expression resulted in enhanced resistance to pathogens [95]. MYB44 is required for the induction of resistance to M. persicae in Arabidopsis and also affects the repression of aphid feeding activities [96], whereas several ERFs, including RRTF1, ERF1 and ERF13 were up-regulated upon chitooctaose treatment in Arabidopsis [90] or after feeding of the aphids Macrosiphum euphorbiae in tomato and Aphis gossypii in melon [97].
Common and specific response of Arabidopsis infected by P. syringae
Our results indicates that common responses between S. littoralis and P. syringae exist. We found that over 190 genes regulated by S littoralis, 32% (61 genes) were also regulated by P. syringae. Moreover, this common responsive genes were regulated in the same direction.
It is interesting to note that many genes coding for sHSPs regulated by S. littoralis herbivory were also down-regulated upon P. syringae infection but not by M. persicae. Since some HSP, like Hsp70, are essential for mediating virulence effect of virulence effector of pathogenic P. syringae (e.g., Hopl1) and play roles in basal resistance to nonpathogenic strains of P. syringae [98], [99], we might argue that down-regulation of HSP could be a common strategy for P. syringae and S. littoralis to suppress plant defense. Another common feature of S. littoralis and P. syringae was the up-regulation of EF-hand-containing proteins, which are likely to be the key transducers mediating Ca2+ action [100]. Calcium signaling has been demonstrated to play an important role in both plant-pathogen [101] and plant-insect interactions [102].
As expected, the specific response of Arabidopsis to P. syringae infection was the up-regulation of genes responding to bacterium, including several SA-related genes. GDG1, a member of the GH3-like gene family, was shown to be an important component of SA-mediated defense against P. syringae [103], whereas the transcription factor WRKY70 positively regulates basal resistance to P. syringae and is involved in SA-signaling pathway [104]. CRK9 was found to be involved in plant responses to biotic stress, like many other Arabidopsis CRKs [105]–[107], whereas Phytoalexin Deficient4 (PAD4) stimulates production of SA and other processes to limit pathogen growth and has a distinct function in the plant innate immune response [108]. Wall-Associated Kinase (WAK1) is a candidate receptor of oligogalacturonides [109] that triggers defense responses effective against fungal and bacterial pathogens and is induced by SA [110]. Moreover, SA is primarily synthesized from chorismate via isochorismate through the action of isochorismate synthase 1 (ICS1) [111], a gene required for plant defense that was also up-regulated upon P. syringae infection. Up-regulation was also found for a plasmodesmal protein (PDLP5) that was recently discovered to be essential for conferring enhanced innate immunity against bacterial pathogens in a SA-dependent manner [112].
P. syringae also differentially up-regulated genes responding to JA: JAZ10, which is a negative regulator of both JA signaling and disease symptom development [113]; vegetative storage protein1 (VSP1), which is a methyl-JA-inducible gene involved in ABA-dependent stomatal closure in Arabidopsis guard cells [114]; MYB50, that is regulated by JA [115] and ADC2, which induction has been reported for various stresses and various growth regulators including JA [116], [117]. Allene oxide cyclase (AOC2), that catalyzes an essential step in JA biosynthesis [118] and is induced by JA [119] was also up-regulated. The ethylene- and jasmonate-responsive plant defensin PDF1.2A, a known JA-responsive gene [120], was also up-regulated.
A few genes are commonly regulated by all three biotic stresses
Surprisingly only a few genes were commonly expressed at the time of Vm depolarization. In particular, four UGTs were down-regulated in all treatments. UGTs convey the transfer of glycosyl residues from activated nucleotide sugars to a wide range of acceptor molecules such as secondary metabolites, including SA and phytoalexins [121]. Down-regulated UGTs belonged mostly to group D, whose members are associated with disease resistance against some pathogens, and a member of Group L that plays an important role in local and systemic resistance in Arabidopsis [122]. A common up-regulation was found for AtNUDX6, the gene encoding ADP-ribose (Rib)/NADH pyrophosphohydrolase. AtNUDX6 is a modulator of NADH rather than ADP-Rib metabolism and significantly impacts the plant immune response as a positive regulator of NPR1-dependent SA signaling pathways [123]. The TIR domain is found in one of the two large families of homologues of plant disease resistance proteins (R proteins) [124]. The largest class of known resistance proteins includes those that contain a nucleotide binding site and leucine-rich repeat domains (NBS-LRR proteins) [125]. The commonly up-regulated gene At5g45000 is a TIR-NBS-LRR class domain-containing disease resistance protein, transmembrane receptor involved in signal transduction, defense response and innate immune response [125], [126]. Another commonly up-regulated gene was a heavy-metal-associated domain-containing protein (At5g26690) which was found in the category of SA-induced genes positively regulated by a n-butanol-sensitive pathway, implying a cross-talk between SA and phospholipase D pathways [127].
Thus, at the time of Vm depolarization a common plant responses to the two herbivores and the pathogen is activation of SA-dependent responses and the triggering of transmembrane receptors, whereas the action of all three biotrophs is the suppression of plant UGTs involved in direct defense.
An unexpected relationship was found for some Arabidopsis genes commonly up-regulated by the herbivore S. littoralis and the pathogen P. syringae and down- regulated by M. persicae. The aphid was found to suppress hydrolase and kinase plant activities, which were activated by the pathogen and the chewing herbivore. Moreover, several oxidative and calcium-related encoding genes as well as some cytochrome P450s (including CYP81F4, involved in the formation of the methoxy group in the indole ring to yield 1-methoxy-indole-3-yl-methyl glucosinolate in Arabidopsis [128]) were only suppressed by the aphid. A remarkable up-regulation was found upon S. littoralis herbivory or P. syringae infection, and the opposite regulation upon M. persicae feeding, for a 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase (At4g10500). In plants, these enzymes catalyze the molecular oxygen reduction at the Fe(II) ion binding residue, where it reacts with 2-oxoglutarate and a specific substrate through the incorporation of one atom of oxygen in each compound and have been involved in plant defense [129].
Comparison with previous transcriptome analyses
A direct comparison of our data with previous transcriptome analyses indicate similar trend of gene expression in Arabibidopsis upon herbivory by S. littoralis, with particular reference to hydrolases (beta-galactosidase, beta-1,3-glucanase, pectinesterase, xyloglucan endo-1,4-beta-D-glucanase, xyloglucan endotransglycosylase), oxido-reductases (monodehydroascorbate reductase, oxidoreductase), transcription factors (ERF/AP2, MYB, WRKY) and other genes induced by biotic stress (NAM, TIR-NBS-LRR class, RPP1, calmodulin, cysteine-rich receptor-like protein kinase). An opposite trend was found for oxophytodienoic acid reductases (OPR1), alcohol dehydrogenase (ADH), arabinogalactan-protein (AGP12), glutathione S-transferase, heat shock protein 70, and a GTP binding protein [28], [130]. However it has to be considered that timing of gene extraction was longer (3–5 h) and falling outside the timing of membrane depolarization. Feeding Arabidopsis with S. exigua for longer periods (from 8 to 48 h) showed the same gene expression only for NADP-dependent oxidoreductase and the opposite expression for lipase, 12-oxophytodienoic acid reductase and ERF1, with respect to our results [31], [131].
A comparison with M. persicae transcriptome data shows that experiments were mostly performed at longer feeding times, hence beyond the membrane depolarization effect. With respect to our results, after 36 h feeding an opposite gene expression was found for a zinc-binding family protein, a FAD-binding domain-containing protein and for syntaxin [27]. In experiment with feeding times of 48 h [31], lypoxygenase, lipase, coronatine-responsive protein, cellulose synthase, 12-oxophytodienoate reductase, 2-oxoglutarate-dependent dioxygenase, tyrosine decarboxylase, allene oxide cyclase, phenylalanine ammonia-lyase, some WRKY family transcription factors, ethylene response factor 1 (ERF-1), cytochrome B5 family protein 1, amino acid permease and branched-chain amino acid aminotransferase showed the same expression trend; whereas jacalin lectin, flavin-containing monooxygenase, O-methyltransferase, and cytochrome P450 (CYP79B2) showed an opposite trend, with respect to our results. Finally, after 72 h feeding the same gene expression trend was found for copper amine oxidase, fructose-bisphosphate aldolase, glutathione S-transferase, a cytochrome P450 [132], pathogenesis-related protein 1 precursor (PR-1), plant defensin-fusion protein (PDF1.2), superoxide dismutase (Cu-Zn), calmodulin, peroxidase, tyrosine decarboxylase [133] and a sugar transporter family protein [134]. At this timing of aphid feeding, an opposite trend of gene expression with respect to our data was found for coronatine-responsive tyrosine aminotransferase, gibberellin 20-oxidase, heat shock protein 81-1 (HSP81-1), octicosapeptide/Phox/Bem1p (PB1) domain-containing protein, purple acid phosphatase, glycosyl hydrolase family, copper amine oxidase, jacalin lectin family protein, dehydrin xero2 (XERO2), AP2 domain-containing transcription factor, ethylene-responsive element-binding protein 1 (ERF1), respiratory burst oxidase protein D (RbohD), lipoxygenase, cytochrome P450s CYP79B2 and P450 CYP83B1, IAA-amino acid hydrolase 3 and 6, glycosyl hydrolase family 1 [132] glutathione S-transferase, phenylalanine ammonia-lyase, and endotransglycosylase [133].
Finally, a comparison with literature data on Arabidopsis transcriptome after infection by P. syringae DC3000 indicates that most of the experiments were carried on at longer times of infection, with respect to the maximum depolarization time observed in our experiments. After 24 h infection, the same gene expression was still found for many genes, including a stress-responsive protein (At1g29395), leucine-rich repeat protein kinases (At1g51800, At1g51850, At1g51860), glutathione S-transferase, DC1 domain-containing protein, cytochrome P450 (CYP71A12), MLO-like protein 12 (MLO12), protein phosphatase 2C, expressed protein (At3g18250), aspartyl protease family protein, transport protein SEC61 beta 1 subunit, chitinase, protease inhibitor/seed storage/lipid transfer proteins (LTP) (At4g12490, At4g12500), protein kinase, zinc finger (CCCH-type) family protein, nodulin family protein and beta-galactosidase [135]. However, after 24 h infection, eight of the 12 JAZ genes are induced during infection in a COR-dependent manner showed an opposite gene expression [113]. Three to four days after infection several genes show the same regulation as observed in our 16 h experiment. These included cell cycle control protein-related, glutathione S-transferase, expressed protein (At1g13340), auxin-responsive GH3 family protein, cation/hydrogen exchanger (CHX17), UDP-glucoronosyl/UDP-glucosyl transferase, disease resistance protein (TIR class), AAA-type ATPase family protein, zinc finger (C3HC4-type RING finger) family protein, cytochrome P450 (CYP71A12), protease inhibitor/seed storage/lipid transfer protein (LTP), cyclic nucleotide-regulated ion channel (CNGC10) (ACBK1), calmodulin-binding protein, avirulence induced gene (AIG1), isochorismate synthase 1 (ICS1), phytoalexin-deficient 4 protein (PAD4), WRKY4, vegetative storage protein 1 (VSP1) [34] and WRKY70 [136]. In comparison to our results, an opposite regulation after 3–4 days on infection was observed for :pectinesterase family protein, O-methyltransferase 1, leucine-rich repeat protein kinase, jacalin lectin family protein, ankyrin repeat family protein, PDF1.2, DC1 domain-containing protein, pectinesterase family protein, chitinase [34], CPR5, JAR1, COI1 and PR1 [136].
Conclusions
The finding that plant plasma membranes respond with a similar Vm depolarization at times depending on the nature of biotic attack allowed us to set a time point for comparative genome-wide analysis among different pests. Our results showed that the aphid M. persicae regulates a wider array of Arabidopsis genes with a clear and distinct regulation than the chewing herbivore S. littoralis. Despite the different timing of Vm depolarization, S. littoralis and P. syringae share different commonly regulated genes, implying a relationship between Vm depolarization and gene expression. Although several commonly expressed genes between the aphid and the pathogen were present, an almost completely opposite regulation was observed, with the aphid suppressing and the pathogen activating plant defense responses.
Materials and Methods
Plant, animal and microbial material
Arabidopsis thaliana L. (Columbia 0) plants were grown from seed in a plastic pot with sterilized potting soil at 23°C and 60% humidity using daylight fluorescent tubes (120 µE m−2 s−1) with a photoperiod of 16 h. Every liter of soil contained 15 g of vermiculite and 335 g of soil. Moreover, two fertilizers, Osmocote® and Triabon® (16-8-12-4+TE), were added at a concentration of 1 g l−1 soil. All experiments were carried out using 20–22 days-old plants (phase 3) whose leaves turned out to be the most responsive to external stimuli. At least three leaves per plant were used for infestation.
The avirulent strain (Avr) of Pseudomonas syringae pv tomato DC3000 (Pst DC3000 AvrRPM1) was cultured at 28°C in NYGB medium (bactopeptone 5 g l−1, yeast extract 3 g l−1, glycerol 20 g l−1, bactoagar 15 g l−1) supplemented with rifampicin (100 µg ml−1) and kanamycin (25 µg ml−1) (all supplied by Sigma, Milan, Italy). Bacterial cultures were centrifuged at 2500 g for 15 min to recover bacteria, which were resuspended in sterile water to a final OD600 of 0.2 (equivalent to 1×108 bacteria/ml). Dilution plating was used to confirm the number of bacteria present in the inoculum.
Virus-free Myzus persicae aphids were reared on A. thaliana plants. They were allowed to grow inside Plexiglas boxes at the temperature of 23°C with a 16 h photoperiod. Only apterous aphids were used in the experiments. Prior to each experiment, at least 20 nymphs per plant were selected for the infestation.
Larvae of Spodoptera littoralis (Boisd. 1833) (Lepidoptera, Noctuidae) (supplied as egg clutches by Syngenta, Switzerland), were reared in Petri dishes at 22–24°C with a 14–16 h photoperiod and fed artificially with a diet as detailed elsewhere [1]. Only third instar larvae (at least 5 per plant) were used for herbivory and leaves were collected when 30% fed by larvae.
Plant defense responses induced by herbivores used as controls mechanical damage, which was inferred with either a pattern wheel (to mimic S. littoralis herbivore damage) or a microforged glass micropipettes (to mimic aphid stinging). P. syringae inoculation used as a control inoculation with MgCl2.
Membrane potentials
Membrane potentials were determined in leaf segments in time course experiments. The transmembrane potential (Vm) was determined with glass micropipettes as previously described [1], [19], [137]. Based on topographical and temporal determination of Vm performed previously [18] the electrode was inserted between 0.5 and 2 mm from the wounded zone, where a significant Vm depolarization was found to occur. The results of Vm are shown as the average number of at least 50 Vm measurements.
RNA extraction from Arabidopsis leaves upon insect attack and bacterial infection and cDNA synthesis
Having assessed the timing of Vm depolarization, after each experiment, leaves were collected and immediately frozen in liquid nitrogen. Samples for the evaluation of S. littoralis herbivory were sampled after 2 h from feeding, whereas samples for M. persicae phloem feeding were sampled after 5 h from feeding. Samples for the evaluation of Arabidopsis responses to P. syringae were sampled after 16 h from infiltration. Four biological replicates were run for each tested biotic attack.
One hundred mg of frozen leaves were ground in liquid nitrogen with mortar and pestle. Total RNA was isolated using the Agilent Plant RNA Isolation Mini Kit (Agilent Technologies, Santa Clara, CA, US) and RNase-Free DNase set (Qiagen, Hilden, Germany). Sample quality and quantity was checked by using the RNA 6000 Nano kit and the Agilent 2100 Bioanalyzer (Agilent Technologies) according to manufacturer's instructions. Quantification of RNA was also confirmed spectrophotometrically by using a NanoDrop ND-1000 (Thermo Fisher Scientific, Waltham, MA, US).
Gene microarray analyses (including MIAME)
Five hundred nanograms of total RNA from each treated samples, were separately reverse-transcribed into double-strand cDNAs by the Moloney murine leukemia virus reverse transcriptase (MMLV-RT) and amplified for 2 h at 40°C using the Agilent Quick Amp Labelling Kit, two-color (Agilent Technologies). Subsequently, cDNAs were transcribed into antisense cRNA and labeled with either Cy3-CTP or Cy5-CTP fluorescent dyes for 2 h at 40°C following the manufacturer's protocol. Cyanine-labeled cRNAs were purified using RNeasy Minikit (Qiagen, Hilden, Germany). Purity and dye incorporation were assessed with the NanoDrop ND-1000 UV-VIS Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, US) and the Agilent 2100 Bioanalyzer (Agilent Technologies). Then, 825 ng of control Cy3-RNAs and 825 ng of treated Cy5-RNAs were pooled together and hybridized using the Gene Expression Hybridization Kit (Agilent Technologies) onto 4×44 K Arabidopsis (v3) Oligo Microarray (Agilent Technologies), satisfying Minimum Information About a Microarray Experiment (MIAME) requirements [138].
After a 17 h incubation at 65°C and 10 rpm, microarrays were first washed with Gene Expression Wash buffer 1 for 1 min, then with Gene Expression Wash buffer 2 for 1 min, then with 100% acetonitrile for 30 s, and finally washed in the Stabilization and Drying Solution for 30 s.
Microarrays were scanned with the Agilent Microarray G2505B Scanner (with the extended dynamic range (XDR) scan mode to scan the same slide at two different levels and data were extracted and normalized from the resulting images using Agilent Feature Extraction (FE) software (v.9.5.1)
Agilent Arabidopsis (v3) Gene Expression Microarray (44 K) was used for expression profiling treatments. The microarray experiment followed a direct 2×2 factorial two-color design. For each of the treatment combinations, RNA from three independent lines was extracted and used for hybridization. For each RNA samples, four biological replicates were used. This resulted in 12 two-color arrays.
GO enrichment information for the differently expressed probe sets was performed using EasyGO (http://bioinformatics.cau.edu.cn/easygo/category_treeBrowse.html).
The microarray data is MIAME compliant and has been submitted to GEO database with Accession number pending.
Validation of microarrays
Validation of the microarray analysis was performed by quantitative real time PCR. First strand cDNA synthesis was accomplished with 1.5 µg total RNA and random primers using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, US), according to the manufacturer's instructions. Briefly, the reactions were prepared by adding 10 µl total RNA (1.5 µg), 2 µl of 10× RT Buffer, 0.8 µl of 25× dNTPs mix (100 mM), 2 µl 10× RT random primer, 1 µl of Multiscribe™ Reverse Transcriptase and nuclease-free sterile water up to 20 µl. Then the reaction mixtures were subjected to thermal incubation according to the following conditions; 25°C for 10 minutes, 37°C for 2 hours, and 85°C for 5 seconds.
All qPCR experiments were performed on a Stratagene Mx3000P Real-Time System (La Jolla, CA, USA) using SYBR green I with ROX as an internal loading standard. The reaction was performed with 25 µl of mixture consisting of 12.5 µl of 2× Maxima™ SYBR Green/ROX qPCR Master Mix (Fermentas International, Inc, Burlington, ON, Canada), 0.5 µl of cDNA and 100 nM primers (Integrated DNA Technologies, Coralville, IA, US). Controls included non-RT controls (using total RNA without reverse transcription to monitor for genomic DNA contamination) and non-template controls (water template). Specifically, PCR conditions were the following: plant cadmium resistance 1 (PCR1, At1g14880), HSP20-like chaperones superfamily (At1g52560): initial polymerase activation of 10 min at 95°C, and 40 cycles of 30 s at 95°C, 30 sec at 56°C, and 45 sec at 72°C; methionine reductase B8 (MSRB8, At4g21840), UDP-glycosyltransferase 73B4 (UGT73B4, At2g15490): initial polymerase activation of 10 min at 95°C, and 40 cycles of 15 s at 95°C, 30 sec at 59°C, and 30 sec at 72°C; germin-like protein (GR3, At5g20630), flowering promoting factor 1 (At5g10625), cytoplasmic glyceraldehyde-3-phosphate dehydrogenase, (GAPC2, At1g13440), ubiquitin specific protease 6 (UBP6, At1g51710), β-adaptin (At4g11380), elongation factor 1B alpha-subunit 2 (eEF1Balpha2, At5g19510): initial polymerase activation of 10 min at 95°C, and 40 cycles of 15 s at 95°C, 20 sec at 57°C, and 30 sec at 72°C. Fluorescence was read following each annealing and extension phase. All runs were followed by a melting curve analysis from 55 to 95°C. The linear range of template concentration to threshold cycle value (Ct value) was determined by performing a dilution series using cDNA from three independent RNA extractions analyzed in three technical replicates. All primers were designed using Primer 3 software [139]. Primer efficiencies for all primers pairs were calculated using the standard curve method [140]. Four different reference genes (cytoplasmic glyceraldehyde-3-phosphate dehydrogenase, (GAPC2, At1g13440), ubiquitin specific protease 6 (UBP6, At1g51710), β-adaptin (At4g11380) and the elongation factor 1B alpha-subunit 2 (eEF1Balpha2, At5g19510) were used to normalize the results of the real time PCR. The best of the four genes was selected using the Normfinder software [141]; the most stable gene was the elongation factor 1B alpha-subunit 2.
All amplification plots were analyzed with the MX3000P™ software to obtain Ct values. Relative RNA levels were calibrated and normalized with the level of the elongation factor 1B alpha-subunit 2 mRNA.
Primers used for real-time PCR were as follows: Plant cadmium resistance 1 (PCR1, At1g14880) forward primer 5′-GATCGAGGATCCAAATCGTG-3′, reverse primer 5′-TGTTGGGTCAAAGCACAGAG-3′; HSP20-like chaperones superfamily (At1g52560) forward primer 5′-GCTCACCTGAGGAAGACGAG-3′, reverse primer 5′-TCCGCCTTAATGTCCTCAAC-3′; methionine reductase B8 (MSRB8, At4g21840) forward primer 5′-CTAAGTTCGACTCCGGTTGC-3′, reverse primer 5′-TGGCCTAGATGTCCATCACA-3′; UDP-glycosyltransferase 73B4 (UGT73B4, At2g15490) forward primer 5′-TTGGTTGCCTAAAGGGTTTG-3′, reverse primer 5′-TCCAAAGTCGAGTTCCATCC-3′; Germin-like protein (GR3, At5g20630) forward primer 5′-CATCCTGGTGCTTCTGAGGT-3′, reverse primer 5′-GGGCCTTTCCCAGAGTTTAG-3′; flowering promoting factor 1 (At5g10625), forward primer 5′-CTAGTGGAGAACCCGAACCA-3′, reverse primer 5′-TGTTCGAGCGACGAGTAAGA-3′; elongation factor 1B alpha-subunit 2 (eEF1Balpha2, At5g19510) forward primer 5′-ACTTGTACCAGTTGGTTATGGG-3′, reverse primer 5′-CTGGATGTACTCGTTGTTAGGC-3′; ubiquitin specific protease 6 (UBP6, At1g51710) forward primer 5′-GAAAGTGGATTACCCGCTG-3′, reverse primer 5′-CTCTAAGTTTCTGGCGAGGAG-3′; cytoplasmic glyceraldehyde-3-phosphate dehydrogenase (GAPC2, At1g13440) forward primer 5′-TCAGGAACCCTGAGGACATC-3′, reverse primer 5′- CGTTGACACCAACAACGAAC-3′; β-adaptin (At4g11380) forward primer 5′- CACGAGCGTCGAATCAACTA-3′, reverse primer 5′-ATCTCGGGAGTGGGAGTTTT-3′.
Validation of microarray gene expression is shown in Table S6.
Statistical analyses
For Vm measurements, the obtained data were treated by using the stem-and-leaf function of Systat 10 in order to calculate the lower and upper hinge from the Gaussian distribution of values. The data were then filtered and the mean value was calculated along with the SE. Paired t test and Bonferroni adjusted probability were used to assess the difference between treatments and the control.
Processing and statistical analysis of the microarray data were done in R using Bioconductor package limma [142]. The raw microarray data are subjected to background subtraction and loess normalized. Agilent control probes were filtered out. The linear models implemented in limma were used for finding differentially expressed genes. Comparisons were made for each of the treatment and genotype combinations. To reduce the complexity of the analysis, technical replicates were treated as biological replicates. Benjamini and Hochberg (BH) multiple testing correction was applied. We consider genes with both BH adjusted p-value<0.05 and fold changes >2 as differentially expressed genes.
Supporting Information
Arabidopsis thaliana genes commonly expressed at the time of Vm depolarization upon Myzus persicae (5 h) herbivory and Pseudomonas syringae (16 h) infection.
(DOCX)
Other differentially regulated genes upon Spodoptera littoralis herbivory on Arabidopsis thaliana leaves.
(DOCX)
Arabidopsis thaliana genes commonly expressed at the time of Vm depolarization (5 h) upon Myzus persicae herbivory.
(DOCX)
Other genes differentially expressed upon Myzus persicae phloem feeding on Arabidopsis thaliana leaves.
(XLSX)
Other differentially expressed genes upon infection by Pseudomonas syringae on Arabidopsis thaliana leaves.
(XLSX)
Validation of microarray data.
(DOCX)
Acknowledgments
The authors are grateful to G. Poppy for providing virus-free strains of Myzus persicae, M. Delledonne for providing the avirulent strain of Pseudomonas syringae DC3000 and Syngenta Corporation, Switzerland, for providing eggs of Spodoptera littoralis. The authors thank L.S. Cucuzza for technical assistance during Vm measurements.
Funding Statement
This work was partly funded by the Doctorate School of Pharmaceutical and Biomolecular Sciences of the University of Turin, Italy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- 1. Bricchi I, Leitner M, Foti M, Mithöfer A, Boland W, et al. (2010) Robotic mechanical wounding (MecWorm) versus herbivore-induced responses: early signaling and volatile emission in Lima bean (Phaseolus lunatus L.). Planta 232: 719–729. [DOI] [PubMed] [Google Scholar]
- 2. Arimura GI, Ozawa R, Maffei ME (2011) Recent advances in plant early signaling in response to herbivory. Int J Mol Sci 12: 3723–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mithöfer A, Boland W, Maffei ME (2009) Chemical ecology of plant–insect interactions. In: Parker J, editor. Molecular aspects of plant disease resistance. Chirchester: Wiley-Blackwell, pp. 261–291.
- 4. Maffei ME, Mithöfer A, Boland W (2007) Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. Phytochemistry 68: 2946–2959. [DOI] [PubMed] [Google Scholar]
- 5. Alba JM, Glas JJ, Schimmel BC, Kant MR (2011) Avoidance and suppression of plant defenses by herbivores and pathogens. J Plant Interact 6: 221–227. [Google Scholar]
- 6.Dixon AFG (1998) Aphid ecology: An optimization approach. New York: Chapman and Hall.
- 7. Goggin FL (2007) Plant-aphid interactions: molecular and ecological perspectives. Curr Opin Plant Biol 10: 399–408. [DOI] [PubMed] [Google Scholar]
- 8. Guerrieri E, Digilio MC (2008) Aphid-plant interactions: a review. J Plant Interact 3: 223–232. [Google Scholar]
- 9. Giordanengo P, Brunissen L, Rusterucci C, Vincent C, van Bel A, et al. (2010) Compatible plant-aphid interactions: How aphids manipulate plant responses. Comp Rend Biol 333: 516–523. [DOI] [PubMed] [Google Scholar]
- 10. Pitino M, Coleman AD, Maffei ME, Ridout CJ, Hogenhout SA (2011) Silencing of Aphid Genes by dsRNA Feeding from Plants. Plos One 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jan AT, Azam M, Ali A, Haq QM (2011) Novel approaches of beneficial Pseudomonas in mitigation of plant diseases - an appraisal. J Plant Interact 6: 195–205. [Google Scholar]
- 12. Gimenez-Ibanez S, Rathjen JP (2010) The case for the defense: plants versus Pseudomonas syringae . Microbes Infect 12: 428–437. [DOI] [PubMed] [Google Scholar]
- 13. Arimura GI, Ozawa R, Maffei ME (2011) Recent advances in plant early signaling in response to herbivory. Int J Mol Sci 12: 3723–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arimura G, Maffei ME (2010) Calcium and secondary CPK signaling in plants in response to herbivore attack. Biochem Biophys Res Comm 400: 455–460. [DOI] [PubMed] [Google Scholar]
- 15. Maffei ME, Mithöfer A, Boland W (2007) Before gene expression: early events in plant-insect interaction. Trends Plant Sci 12: 310–316. [DOI] [PubMed] [Google Scholar]
- 16. Hogenhout SA, Bos JIB (2011) Effector proteins that modulate plant-insect interactions. Curr Opin Plant Biol 14: 422–428. [DOI] [PubMed] [Google Scholar]
- 17. Louis J, Lorenc-Kukula K, Singh V, Reese J, Jander G, et al. (2010) Antibiosis against the green peach aphid requires the Arabidopsis thaliana MYZUS PERSICAE-INDUCED LIPASE1 gene. Plant J 64: 800–811. [DOI] [PubMed] [Google Scholar]
- 18. Zebelo SA, Maffei ME (2012) The Ventral Eversible Gland (VEG) of Spodoptera littoralis triggers early responses to herbivory in Arabidopsis thaliana . Arthropod Plant Interact In press: DOI: 10.1007/s11829-012-9200-9. [Google Scholar]
- 19. Mohanta TK, Occhipinti A, tsbaha Zebelo S, Foti M, Fliegmann J, et al. (2012) Ginkgo biloba responds to herbivory by activating early signaling and direct defenses. Plos One 7: e32822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W (2004) Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol 134: 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arimura GI, Ozawa R, Maffei ME (2011) Recent advances in plant early signaling in response to herbivory. Int J Mol Sci 12: 3723–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Powell G, Pirone T, Hardie J (1995) Aphid stylet activities during ootyvirus acquisition from plants and an in-vitro system that correlate with subsequent transmission. Europ J Plant Pathol 101: 411–420. [Google Scholar]
- 23. Pike SM, Zhang XC, Gassmann W (2005) Electrophysiological characterization of the Arabidopsis avrRpt2-specific hypersensitive response in the absence of other bacterial signals. Plant Physiol 138: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Vos M, Cheng WY, Summers HE, Raguso RA, Jander G (2010) Alarm pheromone habituation in Myzus persicae has fitness consequences and causes extensive gene expression changes. Proceedings of the National Academy of Sciences of the United States of America 107: 14673–14678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Vos M, Jander G (2009) Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana . Plant Cell Environ 32: 1548–1560. [DOI] [PubMed] [Google Scholar]
- 26. De Vos M, Kim JH, Jander G (2007) Biochemistry and molecular biology of Arabidopsis-aphid interactions. Bioessays 29: 871–883. [DOI] [PubMed] [Google Scholar]
- 27. Couldridge C, Newbury H, Ford-Lloyd B, Bale J, Pritchard J (2007) Exploring plant responses to aphid feeding using a full Arabidopsis microarray reveals a small number of genes with significantly altered expression. Bull Ent Res 97: 523–532. [DOI] [PubMed] [Google Scholar]
- 28. Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, et al. (2004) A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 16: 3132–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, et al. (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant-Microbe Interact 18: 923–937. [DOI] [PubMed] [Google Scholar]
- 31. Bidart-Bouzat M, Kliebenstein D (2011) An ecological genomic approach challenging the paradigm of differential plant responses to specialist versus generalist insect herbivores. Oecologia 167: 677–689. [DOI] [PubMed] [Google Scholar]
- 32. Attaran E, Zeier TE, Griebel T, Zeier J (2009) Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell 21: 954–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luna E, Bruce TJ, Roberts MR, Flors V, Ton J (2012) Next-seneration systemic acquired resistance. Plant Physiol 158: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thilmony R, Underwood W, He SY (2006) Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157 : H7. Plant J 46: 34–53. [DOI] [PubMed] [Google Scholar]
- 35. Tao Y, Xie ZY, Chen WQ, Glazebrook J, Chang HS, et al. (2003) Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae . Plant Cell 15: 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wan J, Dunning FM, Bent AF (2002) Probing plant-pathogen interactions and downstream defense signaling using DNA microarrays. Funct Integr Genomics 2: 259–273. [DOI] [PubMed] [Google Scholar]
- 37. Erb M, Robert CAM, Hibbard BE, Turlings TCJ (2011) Sequence of arrival determines plant-mediated interactions between herbivores. J Ecol 99: 7–15. [Google Scholar]
- 38. de Román M, Fernández I, Wyatt T, Sahrawy M, Heil M, et al. (2011) Elicitation of foliar resistance mechanisms transiently impairs root association with arbuscular mycorrhizal fungi. J Ecol 99: 36–45. [Google Scholar]
- 39. Maffei ME, Mithöfer A, Boland W (2007) Before gene expression: early events in plant-insect interaction. Trends Plant Sci 12: 310–316. [DOI] [PubMed] [Google Scholar]
- 40. Bricchi I, Occhipinti A, Bertea CM, Zebelo SA, Brillada C, et al. (2012) Separation of early and late responses to herbivory in Arabidopsis by changing plasmodesmal function. Plant J In press [DOI] [PubMed] [Google Scholar]
- 41. Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W (2004) Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol 134: 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W (2004) Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol 134: 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maffei ME, Mithöfer A, Arimura GI, Uchtenhagen H, Bossi S, et al. (2006) Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol 140: 1022–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mithöfer A, Mazars C, Maffei ME (2009) Probing spatio-temporal intracellular calcium variations in plants. Meth Mol Biol 79–92. [DOI] [PubMed] [Google Scholar]
- 45. Arimura G, Maffei ME (2010) Calcium and secondary CPK signaling in plants in response to herbivore attack. Biochem Biophys Res Comm 400: 455–460. [DOI] [PubMed] [Google Scholar]
- 46. Arimura GI, Ozawa R, Maffei ME (2011) Recent advances in plant early signaling in response to herbivory. Int J Mol Sci 12: 3723–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maffei ME, Mithöfer A, Boland W (2007) Before gene expression: early events in plant-insect interaction. Trends Plant Sci 12: 310–316. [DOI] [PubMed] [Google Scholar]
- 48. Arimura GI, Ozawa R, Maffei ME (2011) Recent advances in plant early signaling in response to herbivory. Int J Mol Sci 12: 3723–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kotak S, Larkindale J, Lee U, von Koskull-Doering P, Vierling E, et al. (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10: 310–316. [DOI] [PubMed] [Google Scholar]
- 50. Wang WX, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9: 244–252. [DOI] [PubMed] [Google Scholar]
- 51. Sun WN, Van Montagu M, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. Biochim Biophys Acta 1577: 1–9. [DOI] [PubMed] [Google Scholar]
- 52. Pavlova EL, Rikhvanov EG, Tauson EL, Varakina NN, Gamburg KZ, et al. (2009) Effect of salicylic acid on the development of induced thermotolerance and induction of heat shock protein synthesis in the Arabidopsis thaliana cell culture. Russ J Plant Physiol 56: 68–73. [Google Scholar]
- 53. Rikhvanov EG, Gamburg KZ, Varakina NN, Rusaleva TM, Fedoseeva IV, et al. (2007) Nuclear-mitochondrial cross-talk during heat shock in Arabidopsis cell culture. Plant J 52: 763–778. [DOI] [PubMed] [Google Scholar]
- 54. Tsutsui T, Kato W, Asada Y, Sako K, Sato T, et al. (2009) DEAR1, a transcriptional repressor of DREB protein that mediates plant defense and freezing stress responses in Arabidopsis. J Plant Res 122: 633–643. [DOI] [PubMed] [Google Scholar]
- 55. Farinas B, Mas P (2011) Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. Plant J 66: 318–329. [DOI] [PubMed] [Google Scholar]
- 56. Rawat R, Schwartz J, Jones MA, Sairanen I, Cheng Y, et al. (2009) REVEILLE1, a Myb-like transcription factor, integrates the circadian clock and auxin pathways. Proc Natl Acad Sci U S A 106: 16883–16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, et al. (1998) The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 93: 1219–1229. [DOI] [PubMed] [Google Scholar]
- 58. Gendron JM, Pruneda-Paz JL, Doherty CJ, Gross AM, Kang SE, et al. (2012) Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc Natl Acad Sci U S A 109: 3167–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baxter CE, Costa MM, Coen ES (2007) Diversification and co-option of RAD-like genes in the evolution of floral asymmetry. Plant J 52: 105–113. [DOI] [PubMed] [Google Scholar]
- 60. Holtan HE, Bandong S, Marion CM, Adam L, Tiwari S, et al. (2011) BBX32, an Arabidopsis B-Box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol 156: 2109–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim YK, Son O, Kim MR, Nam KH, Kim GT, et al. (2007) ATHB23, an Arabidopsis class I homeodomain-leucine zipper gene, is expressed in the adaxial region of young leaves. Plant Cell Rep 26: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 62. Boursiac Y, Harper JF (2007) The origin and function of calmodulin regulated Ca2+ pumps in plants. J Bioenerg Biomembr 39: 409–414. [DOI] [PubMed] [Google Scholar]
- 63. Ravet K, Touraine B, Boucherez J, Briat JF, Gaymard F, et al. (2009) Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J 57: 400–412. [DOI] [PubMed] [Google Scholar]
- 64. Maffei ME, Mithöfer A, Arimura GI, Uchtenhagen H, Bossi S, et al. (2006) Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol 140: 1022–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rousseaux MC, Julkunen-Tiitto R, Searles PS, Scopel AL, Aphalo PJ, et al. (2004) Solar UV-B radiation affects leaf quality and insect herbivory in the southern beech tree Nothofagus antarctica . Oecologia 138: 505–512. [DOI] [PubMed] [Google Scholar]
- 66. Goetz M, Albert A, Stich S, Heller W, Scherb H, et al. (2010) PAR modulation of the UV-dependent levels of flavonoid metabolites in Arabidopsis thaliana (L.) Heynh. leaf rosettes: cumulative effects after a whole vegetative growth period. Protoplasma 243: 95–103. [DOI] [PubMed] [Google Scholar]
- 67. Lattanzio V, Arpaia S, Cardinali A, Di Venere D, Linsalata V (2000) Role of endogenous flavonoids in resistance mechanism of Vigna to aphids. J Agric Food Chem 48: 5316–5320. [DOI] [PubMed] [Google Scholar]
- 68. Kuhlmann F, Mueller C (2010) UV-B impact on aphid performance mediated by plant quality and plant changes induced by aphids. Plant Biol 12: 676–684. [DOI] [PubMed] [Google Scholar]
- 69. Stracke R, De Vos RC, Bartelniewoehner L, Ishihara H, Sagasser M, et al. (2009) Metabolomic and genetic analyses of flavonol synthesis in Arabidopsis thaliana support the in vivo involvement of leucoanthocyanidin dioxygenase. Planta 229: 427–445. [DOI] [PubMed] [Google Scholar]
- 70. Appelhagen I, Jahns O, Bartelniewoehner L, Sagasser M, Weisshaar B, et al. (2011) Leucoanthocyanidin dioxygenase in Arabidopsis thaliana: characterization of mutant alleles and regulation by MYB-BHLH-TTG1 transcription factor complexes. Gene 484: 62–69. [DOI] [PubMed] [Google Scholar]
- 71. Song J, Lee DE, Jung S, Kim H, Han O, et al. (2004) Characterization of transgenic rice plants expressing an Arabidopsis FAD7. Biol Plant 48: 361–366. [Google Scholar]
- 72. Singh AK, Fu dQ, El-Habbak M, Navarre D, Ghabrial S, et al. (2011) Silencing genes encoding omega-3 fatty acid desaturase alters seed size and accumulation of bean pod mottle virus in soybean. Mol Plant-Microbe Interact 24: 506–515. [DOI] [PubMed] [Google Scholar]
- 73. Avila CA, Arevalo-Soliz LM, Jia L, Navarre DA, Chen Z, et al. (2012) Loss of function of fatty acid desaturase7 in tomato enhances basal aphid resistance in a salicylate-dependent manner. Plant Physiol 158: 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang PJ, Zheng SJ, van Loon JJ, Boland W, David A, et al. (2009) Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proc Natl Acad Sci U S A 106: 21202–21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chico JM, Chini A, Fonseca S, Solano R (2008) JAZ repressors set the rhythm in jasmonate signaling. Curr Opin Plant Biol 11: 486–494. [DOI] [PubMed] [Google Scholar]
- 76. Woldemariam MG, Baldwin IT, Galis I (2011) Transcriptional regulation of plant inducible defenses against herbivores: a mini-review. J Plant Interact 6: 113–119. [Google Scholar]
- 77. Paponov IA, Paponov M, Teale W, Menges M, Chakrabortee S, et al. (2008) Comprehensive transcriptome analysis of auxin responses in Arabidopsis. Mol Plant 1: 321–337. [DOI] [PubMed] [Google Scholar]
- 78. Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, et al. (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A 108: 18512–18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho HT, Cosgrove DJ (2004) Expansins as agents in hormone action. In: Davies PJ, editor. Plant hormones: Biosynthesis, signal transduction, action. Dordrecht: Kluwer, pp. 262–281.
- 80. Downes BP, Crowell DN (1998) Cytokinin regulates the expression of a soybean beta-expansin gene by a post-transcriptional mechanism. Plant Mol Biol 37: 437–444. [DOI] [PubMed] [Google Scholar]
- 81. Sharova EI (2007) Expansins: Proteins involved in cell wall softening during plant growth and morphogenesis. Russ J Plant Physiol 54: 713–727. [Google Scholar]
- 82. Zhang S, Wang X (2011) Overexpression of GASA5 increases the sensitivity of Arabidopsis to heat stress. J Plant Physiol 168: 2093–2101. [DOI] [PubMed] [Google Scholar]
- 83. Roxrud I, Lid SE, Fletcher JC, Schmidt ED, Opsahl-Sorteberg HG (2007) GASA4, one of the 14-member Arahidopsis GASA family of small polypeptides, regulates flowering and seed development. Plant Cell Physiol 48: 471–483. [DOI] [PubMed] [Google Scholar]
- 84. Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, et al. (2006) Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J Plant Res 119: 115–123. [DOI] [PubMed] [Google Scholar]
- 85. Lin PC, Pomeranz MC, Jikumaru Y, Kang SG, Hah C, et al. (2011) The Arabidopsis tandem zinc finger protein AtTZF1 affects ABA- and GA-mediated growth, stress and gene expression responses. Plant J 65: 253–268. [DOI] [PubMed] [Google Scholar]
- 86. Stacey G, Koh S, Granger C, Becker JM (2002) Peptide transport in plants. Trends Plant Sci 7: 257–263. [DOI] [PubMed] [Google Scholar]
- 87. Hauser M, Narita V, Donhardt AM, Naider F, Becker JM (2001) Multiplicity and regulation of genes encoding peptide transporters in Saccharomyces cerevisiae . Mol Membr Biol 18: 105–112. [PubMed] [Google Scholar]
- 88. Cao J, Huang J, Yang Y, Hu X (2011) Analyses of the oligopeptide transporter gene family in poplar and grape. BMC Genom 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Morita M, Shitan N, Sawada K, Van Montagu MC, Inze D, et al. (2009) Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum . Proc Natl Acad Sci U S A 106: 2447–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Libault M, Wan J, Czechowski T, Udvardi M, Stacey G (2007) Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor. Mol Plant-Microbe Interact 20: 900–911. [DOI] [PubMed] [Google Scholar]
- 91. Son GH, Wan J, Kim HJ, Xuan CN, Chung WS, et al. (2012) Ethylene-Responsive Element-Binding Factor 5, ERF5, is involved in chitin-induced innate immunity response. Mol Plant-Microbe Interact 25: 48–60. [DOI] [PubMed] [Google Scholar]
- 92. Wan JR, Zhang SQ, Stacey G (2004) Activation of a mitogen-activated protein kinase pathway in Arabidopsis by chitin. Mol Plant Pathol 5: 125–135. [DOI] [PubMed] [Google Scholar]
- 93. Chujo T, Sugioka N, Masuda Y, Shibuya N, Takemura T, et al. (2009) Promoter analysis of the elicitor-induced WRKY gene OsWRKY53, which is involved in defense responses in rice. Biosci Biotechnol Biochem 73: 1901–1904. [DOI] [PubMed] [Google Scholar]
- 94. Wang D, Amornsiripanitch N, Dong X (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog 2: 1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chujo T, Takai R, kimoto-Tomiyama C, Ando S, Minami E, et al. (2007) Involvement of the elicitor-induced gene OsWRKY53 in the expression of defense-related genes in rice. Biochim Biophys Acta 1769: 497–505. [DOI] [PubMed] [Google Scholar]
- 96. Lü B, Sun W, Zhang S, Zhang C, Qian J, et al. (2011) HrpN(Ea)-induced deterrent effect on phloem feeding of the green peach aphid Myzus persicae requires AtGSL5 and AtMYB44 genes in Arabidopsis thaliana . J Biosci 36: 123–137. [DOI] [PubMed] [Google Scholar]
- 97. Anstead J, Samuel P, Song N, Wu CJ, Thompson GA, et al. (2010) Activation of ethylene-related genes in response to aphid feeding on resistant and susceptible melon and tomato plants. Entomol Exper Appl 134: 170–181. [Google Scholar]
- 98. Jelenska J, van Hal JA, Greenberg JT (2010) Pseudomonas syringae hijacks plant stress chaperone machinery for virulence. Proc Natl.Acad.Sci U S A 107: 13177–13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jelenska J, Yao N, Vinatzer BA, Wright CM, Brodsky JL, et al. (2007) A J domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr Biol 17: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Day IS, Reddy VS, Ali GS, Reddy A (2002) Analysis of EF-hand-containing proteins in Arabidopsis. Genome Biol 3: 0056.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Qiu Y, Xi J, Du L, Roje S, Poovaiah B (2012) A dual regulatory role of Arabidopsis calreticulin-2 in plant innate immunity. Plant J 69: 489–500. [DOI] [PubMed] [Google Scholar]
- 102. Arimura G, Maffei ME (2010) Calcium and secondary CPK signaling in plants in response to herbivore attack. Biochem Biophys Res Comm 400: 455–460. [DOI] [PubMed] [Google Scholar]
- 103. Jagadeeswaran G, Raina S, Acharya BR, Maqbool SB, Mosher SL, et al. (2007) Arabidopsis GH3-LIKE DEFENSE GENE 1 is required for accumulation of salicylic acid, activation of defense responses and resistance to Pseudomonas syringae . Plant J 51: 234–246. [DOI] [PubMed] [Google Scholar]
- 104. Hu YR, Dong QY, Yu DQ (2012) Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae . Plant Sci 185: 288–297. [DOI] [PubMed] [Google Scholar]
- 105. Wrzaczek M, Brosche M, Salojarvi J, Kangasjarvi S, Idanheimo N, et al. (2010) Transcriptional regulation of the CRK/DUF26 group of Receptor-like protein kinases by ozone and plant hormones in Arabidopsis. BMC Plant Biol 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Czernic P, Visser B, Sun WN, Savoure A, Deslandes L, et al. (1999) Characterization of an Arabidopsis thaliana receptor-like protein kinase gene activated by oxidative stress and pathogen attack. Plant J 18: 321–327. [DOI] [PubMed] [Google Scholar]
- 107. Chen KG, Du LQ, Chen ZX (2003) Sensitization of defense responses and activation of programmed cell death by a pathogen-induced receptor-like protein kinase in Arabidopsis. Plant Mol Biol 53: 61–74. [DOI] [PubMed] [Google Scholar]
- 108. Rietz S, Stamm A, Malonek S, Wagner S, Becker D, et al. (2011) Different roles of Enhanced Disease Susceptibility1 (EDS1) bound to and dissociated from Phytoalexin Deficient4 (PAD4) in Arabidopsis immunity. New Phytol 191: 107–119. [DOI] [PubMed] [Google Scholar]
- 109. Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G (2010) A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci U S A 107: 9452–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. He ZH, He DZ, Kohorn BD (1998) Requirement for the induced expression of a cell wall associated receptor kinase for survival during the pathogen response. Plant J 14: 55–63. [DOI] [PubMed] [Google Scholar]
- 111. Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2002) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 417: 571. [DOI] [PubMed] [Google Scholar]
- 112. Lee JY, Wang X, Cui W, Sager R, Modla S, et al. (2011) A Plasmodesmata-Localized Protein Mediates Crosstalk between Cell-to-Cell Communication and Innate Immunity in Arabidopsis. Plant Cell 23: 3353–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Demianski AJ, Chung KM, Kunkel BN (2012) Analysis of Arabidopsis JAZ gene expression during Pseudomonas syringae pathogenesis. Mol Plant Pathol 13: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, et al. (2011) Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiol 156: 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chen YH, Yang XY, He K, Liu MH, Li JG, et al. (2006) The MYB transcription factor superfamily of arabidopsis: Expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol Biol 60: 107–124. [DOI] [PubMed] [Google Scholar]
- 116. Perez-Amador MA, Leon J, Green PJ, Carbonell J (2002) Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in arabidopsis. Plant Physiol 130: 1454–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Armengaud P, Breitling R, Amtmann A (2004) The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol 136: 2556–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gfeller A, Dubugnon L, Liechti R, Farmer EE (2010) Jasmonate biochemical pathway. Sci Signal 3: cm3. [DOI] [PubMed] [Google Scholar]
- 119. Kubigsteltig I, Laudert D, Weiler EW (1999) Structure and regulation of the Arabidopsis thaliana allene oxide synthase gene. Planta 208: 463–471. [DOI] [PubMed] [Google Scholar]
- 120. Koornneef A, Pieterse CM (2008) Cross talk in defense signaling. Plant Physiol 146: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ross J, Li Y, Lim E, Bowles DJ (2001) Higher plant glycosyltransferases. Genome Biol 2: 3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Park HJ, Kwon CS, Woo JY, Lee GJ, Kim YJ, et al. (2011) Suppression of UDP-glycosyltransferase-coding Arabidopsis thaliana UGT74E2 gene expression leads to increased resistance to Psuedomonas syringae pv. tomato DC3000 infection. Plant Pathol J 27: 170–182. [Google Scholar]
- 123. Ishikawa K, Yoshimura K, Harada K, Fukusaki E, Ogawa T, et al. (2010) AtNUDX6, an ADP-ribose/NADH pyrophosphohydrolase in Arabidopsis, positively regulates NPR1-dependent salicylic acid signaling. Plant Physiol 152: 2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fluhr R (2001) Sentinels of disease. Plant resistance genes. Plant Physiol 127: 1367–1374. [PMC free article] [PubMed] [Google Scholar]
- 125. Meyers BC, Kozik A, Griego A, Kuang HH, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15: 809–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Meyers BC, Morgante M, Michelmore RW (2002) TIR-X and TIR-NBS proteins: two new families related to disease resistance TIR-NBS-LRR proteins encoded in Arabidopsis and other plant genomes. Plant J 32: 77–92. [DOI] [PubMed] [Google Scholar]
- 127. Krinke O, Flemr M, Vergnolle C, Collin S, Renou JP, et al. (2009) Phospholipase D activation is an early component of the salicylic acid signaling pathway in Arabidopsis cell suspensions. Plant Physiol 150: 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kai K, Takahashi H, Saga H, Ogawa T, Kanaya S, et al. (2011) Metabolomic characterization of the possible involvement of a Cytochrome P450, CYP81F4, in the biosynthesis of indolic glucosinolate in Arabidopsis. Plant Biotechnol 28: 379–385. [Google Scholar]
- 129. van Damme M, Huibers RP, Elberse J, Van den Ackerveken G (2008) Arabidopsis DMR6 encodes a putative 2OG-Fe(II) oxygenase that is defense-associated but required for susceptibility to downy mildew. Plant J 54: 785–793. [DOI] [PubMed] [Google Scholar]
- 130. Bodenhausen N, Reymond P (2007) Signaling pathways controlling induced resistance to insect herbivores in Arabidopsis. Mol Plant-Microbe Interact 20: 1406–1420. [DOI] [PubMed] [Google Scholar]
- 131. Zhang JH, Sun LW, Liu LL, Lian J, An SL, et al. (2010) Proteomic Analysis of Interactions Between the Generalist Herbivore Spodoptera exigua (Lepidoptera: Noctuidae) and Arabidopsis thaliana. Plant Mol Biol Rep 28: 324–333. [Google Scholar]
- 132. Kusnierczyk A, Winge P, Midelfart H, Armbruster WS, Rossiter JT, et al. (2007) Transcriptional responses of Arabidopsis thaliana ecotypes with different glucosinolate profiles after attack by polyphagous Myzus persicale and oligophagous Brevicoryne brassicae. J Exper Bot 58: 2537–2552. [DOI] [PubMed] [Google Scholar]
- 133. Moran PJ, Cheng YF, Cassell JL, Thompson GA (2002) Gene expression profiling of Arabidopsis thaliana in compatible plant-aphid interactions. Arch Insect Biochem Physiol 51: 182–203. [DOI] [PubMed] [Google Scholar]
- 134. Moran PJ, Thompson GA (2001) Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol 125: 1074–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Truman W, de Zabala MT, Grant M (2006) Type III effectors orchestrate a complex interplay between transcriptional networks to modify basal defence responses during pathogenesis and resistance. Plant J 46: 14–33. [DOI] [PubMed] [Google Scholar]
- 136. De-La-Peña C, Rangel-Cano A, varez-Venegas R (2012) Regulation of disease-responsive genes mediated by epigenetic factors: interaction of Arabidopsis-Pseudomonas. Mol Plant Pathol 13: 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W (2004) Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol 134: 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, et al. (2001) Minimum information about a microarray experiment (MIAME) - toward standards for microarray data. Nat Genet 29: 365–371. [DOI] [PubMed] [Google Scholar]
- 139.Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols. Totowa: Humana Press, pp. 365–386. [DOI] [PubMed]
- 140. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucl Ac Res 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- 142.Smyth GK (2005) Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry, R, Huber W, editors. Bioinformatics and computational biology solutions using R and Bioconductor. New York: Springer, pp. 397–420.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arabidopsis thaliana genes commonly expressed at the time of Vm depolarization upon Myzus persicae (5 h) herbivory and Pseudomonas syringae (16 h) infection.
(DOCX)
Other differentially regulated genes upon Spodoptera littoralis herbivory on Arabidopsis thaliana leaves.
(DOCX)
Arabidopsis thaliana genes commonly expressed at the time of Vm depolarization (5 h) upon Myzus persicae herbivory.
(DOCX)
Other genes differentially expressed upon Myzus persicae phloem feeding on Arabidopsis thaliana leaves.
(XLSX)
Other differentially expressed genes upon infection by Pseudomonas syringae on Arabidopsis thaliana leaves.
(XLSX)
Validation of microarray data.
(DOCX)