Abstract
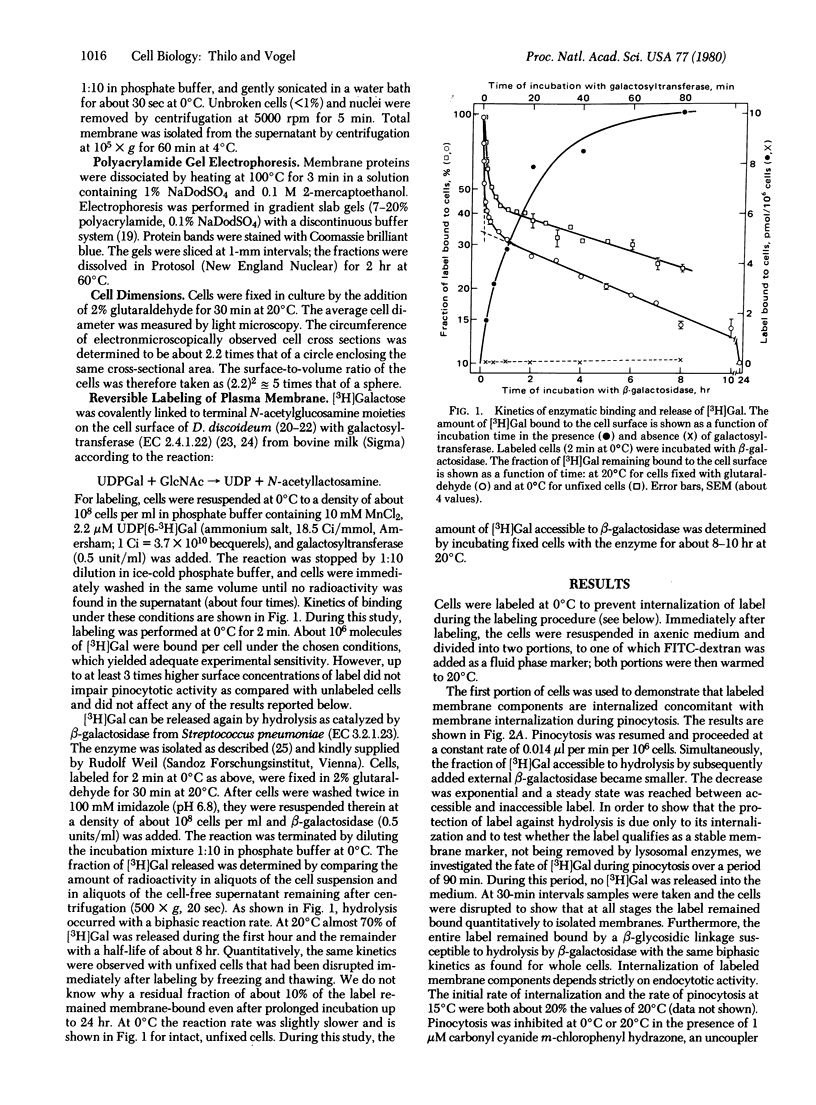
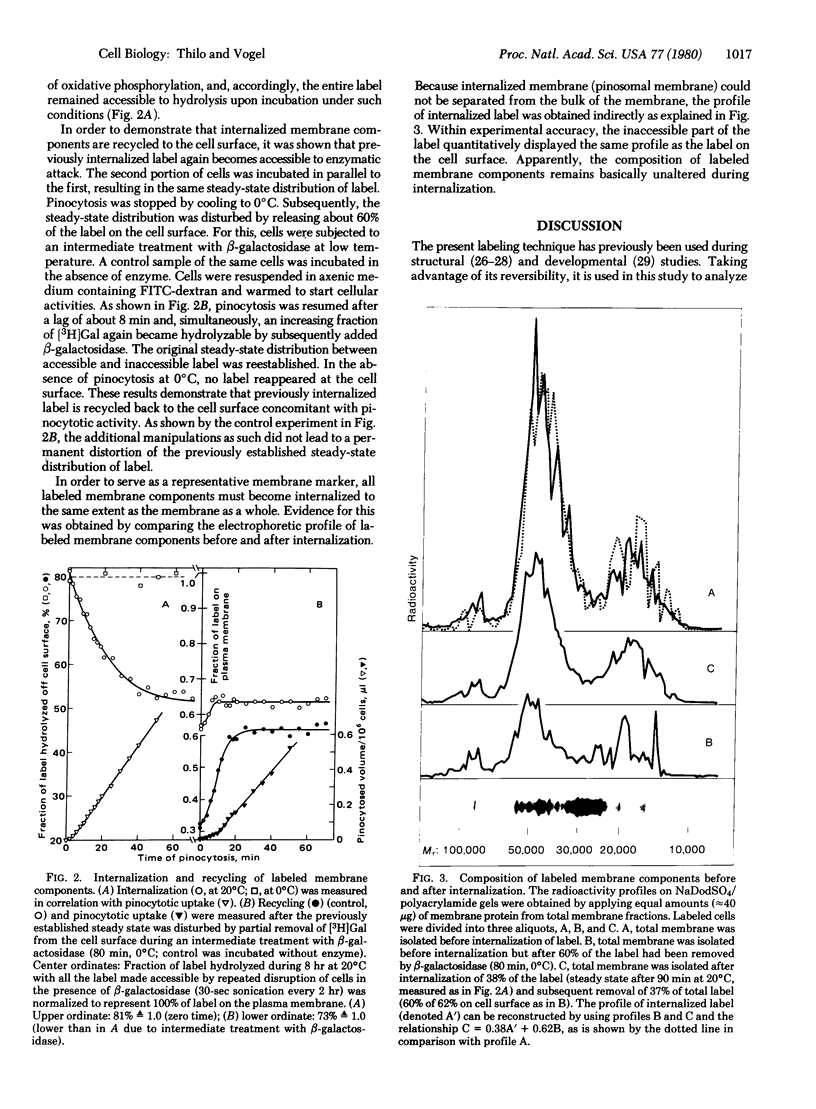
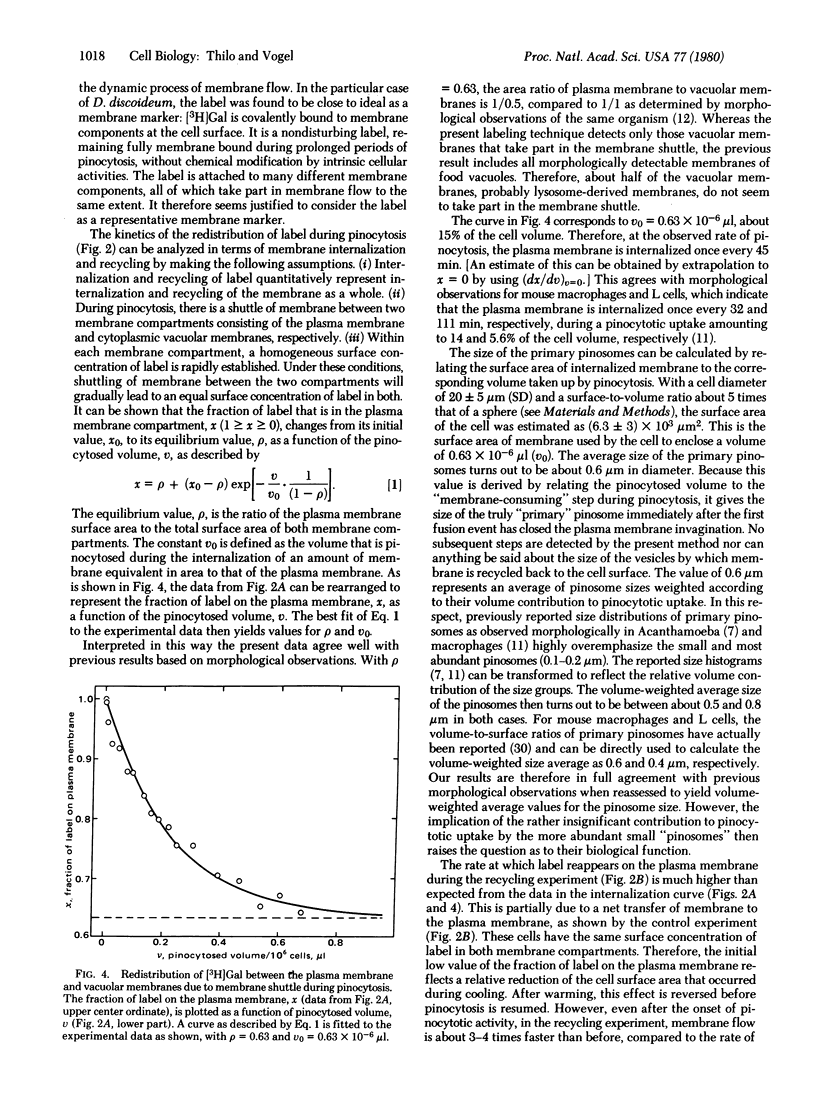
Internalization and recycling of plasma membrane during pinocytosis in Dictyostelium discoideum was analyzed quantitatively. A labeling technique was used by which [3H]galactose could be enzymatically bound to and released from the plasma membrane. Label internalized with the plasma membrane was no longer accessible to enzymatic release and could therefore be distinguished quantitatively from label remaining on the cell surface. Internalization of labeled membrane components was measured as a function of pinocytotic uptake. Direct experimental evidence for membrane recycling was obtained by demonstrating that previously internalized label reappeared at the plasma membrane. The experimental data agree with a kinetic model requiring that a shuttle of membrane between two membrane compartments leads to the same surface concentration of label in both. The two compartments consist of the plasma membrane and of cytoplasmic vacuolar membranes; their relative membrane surface areas are 1 and 0.5, respectively. One surface area equivalent of the plasma membrane is internalized during a pinocytotic uptake amounting to 15% of the cell volume. At the observed rate of pinocytosis, this occurred once every 45 min. The average size of the primary pinosomes, as weighted according to their contribution to pinocytotic uptake, was calculated to be about 0.6 microns.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowers B., Olszewski T. E. Pinocytosis in Acanthamoeba castellanii. Kinetics and morphology. J Cell Biol. 1972 Jun;53(3):681–694. doi: 10.1083/jcb.53.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes N. R., Weeks G. The purification and characterization of Dictyostelium discoideum plasma membranes. Biochim Biophys Acta. 1977 Jan 4;464(1):142–156. doi: 10.1016/0005-2736(77)90377-7. [DOI] [PubMed] [Google Scholar]
- Githens S., 3rd, Karnovsky M. L. Phagocytosis by the cellular slime mold Polysphondylium pallidum during growth and development. J Cell Biol. 1973 Sep;58(3):536–548. doi: 10.1083/jcb.58.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES R. C., JEANLOZ R. W. THE EXTRACELLULAR GLYCOSIDASES OF DIPLOCOCCUS PNEUMONIAE. I. PURIFICATION AND PROPERTIES OF A NEURAMINIDASE AND A BETA-GALACTOSIDASE. ACTION ON THE ALPHA-1-ACID GLYCOPROTEIN OF HUMAN PLASMA. Biochemistry. 1964 Oct;3:1535–1543. doi: 10.1021/bi00898a025. [DOI] [PubMed] [Google Scholar]
- Hoffman S., McMahon D. Defective glycoproteins in the plasma membrane of an aggregation minus mutant of Dictyostelium discoideum uith abnormal cellular interactions. J Biol Chem. 1978 Jan 10;253(1):278–287. [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. II. Metabolic fate of iodinated polypeptides of mouse L cells. J Cell Biol. 1975 Feb;64(2):461–479. doi: 10.1083/jcb.64.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatra B. S., Herries D. G., Brew K. Some kinetic properties of human-milk galactosyl transferase. Eur J Biochem. 1974 May 15;44(2):537–560. doi: 10.1111/j.1432-1033.1974.tb03513.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- PALADE G. E. The endoplasmic reticulum. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):85–98. doi: 10.1083/jcb.2.4.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Ryter A., de Chastellier C. Morphometric and cytochemical studies of Dictyostelium discoideum in vegetative phase. Digestive system and membrane turnover. J Cell Biol. 1977 Oct;75(1):200–217. doi: 10.1083/jcb.75.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel-Brunner H. Incorporation of galactose into blood-groups(ABH) precursor substance by lactose synthetase from human milk. Effects on cross reactivity with anti-type-14 pneumococcus serum. Eur J Biochem. 1973 Feb 15;33(1):30–35. doi: 10.1111/j.1432-1033.1973.tb02650.x. [DOI] [PubMed] [Google Scholar]
- Schindler M., Mirelman D., Schwarz U. Quantitative determination of N-acetylglucosamine residues at the non-reducing ends of peptidoglycan chains by enzymic attachment of [14C]-D-galactose. Eur J Biochem. 1976 Dec;71(1):131–134. doi: 10.1111/j.1432-1033.1976.tb11098.x. [DOI] [PubMed] [Google Scholar]
- Schneider Y. J., Tulkens P., Trouet A. Recycling of fibroblast plasma-membrane antigens internalized during endocytosis [proceedings]. Biochem Soc Trans. 1977;5(4):1164–1167. doi: 10.1042/bst0051164. [DOI] [PubMed] [Google Scholar]
- Shaper J. H., Stryer L. Accessibility of the carbohydrate moiety of membrane-boound rhodopsin to enzymatic and chemical modification. J Supramol Struct. 1977;6(3):291–299. doi: 10.1002/jss.400060302. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockem W. Pinocytose und Bewegung von Amöben. IV. Quantitative Untersuchungen zur permanenten und induzierten Endocytose von Amoeba proteus. Z Zellforsch Mikrosk Anat. 1973;136(3):433–446. [PubMed] [Google Scholar]
- Wallenfels B. Developmental and mutational changes of glycoproteins in the mouse neuronal retina: studies with bovine galactosyltransferase. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3223–3227. doi: 10.1073/pnas.76.7.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970 Sep;119(2):171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman R. A., Korn E. D. Phagocytosis of latex beads by Acanthamoeba. I. Biochemical properties. Biochemistry. 1967 Feb;6(2):485–497. doi: 10.1021/bi00854a017. [DOI] [PubMed] [Google Scholar]
- Wilhelms O. H., Lüderitz O., Westphal O., Gerisch G. Glycosphingolipids and glycoproteins in the wild-type and in non-aggregating mutant of Dictyostelium discoideum. Eur J Biochem. 1974 Oct 1;48(1):89–101. doi: 10.1111/j.1432-1033.1974.tb03746.x. [DOI] [PubMed] [Google Scholar]