Abstract
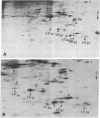
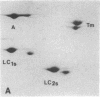
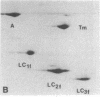
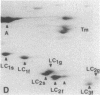
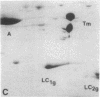
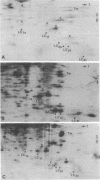
Myosin light chain synthesis has been analyzed in cultures of fast and slow muscles from chicken and quail embryos. Synthesis was assayed by [35S]methionine incorporation and two-dimensional electrophoresis of total cell extracts. Our results show that differentiated cultures of embryonic anterior latissimus dorsi and pectoral muscles synthesize proteins that comigrate on two-dimensional gels with the five myosin light chains of adult fast (pectoral) and slow (anterior latissimus dorsi) muscle. Partial proteolytic digestion and peptide analyses further confirm the identity of these proteins as adult light chains. Cultures of dividing myoblasts do not synthesize any of these fiber type isozymes, and synthesis of the isozymes is initiated at myoblast fusion. Also, myogenic clones drived from single myoblasts differentiate to synthesize these five myosin light chains, indicating that individual myoblasts have the potential to express the synthesis of all fiber type light chain isozymes. We conclude that the primary events in muscle differentiation include the initiation of synthesis of the entire set of adult fast and slow myosin light chain isozymes. The developmental and physiological implications of these results for the establishment of fiber type specificity are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bowman L. H., Emerson C. P., Jr Post-transcriptional regulation of ribosome accumulation during myoblast differentiation. Cell. 1977 Apr;10(4):587–596. doi: 10.1016/0092-8674(77)90091-5. [DOI] [PubMed] [Google Scholar]
- Buckley P. A., Konigsberg I. R. Myogenic fusion and the duration of the post-mitotic gap (G1). Dev Biol. 1974 Mar;37(1):193–212. doi: 10.1016/0012-1606(74)90179-1. [DOI] [PubMed] [Google Scholar]
- Burridge K., Bray D. Purification and structural analysis of myosins from brain and other non-muscle tissues. J Mol Biol. 1975 Nov 25;99(1):1–14. doi: 10.1016/s0022-2836(75)80154-9. [DOI] [PubMed] [Google Scholar]
- Chapman B. S., Tobin A. J. Distribution of developmentally regulated hemoglobins in embryonic erythroid populations. Dev Biol. 1979 Apr;69(2):375–387. doi: 10.1016/0012-1606(79)90298-7. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. Distribution of polymorphic forms of troponin components and tropomyosin in skeletal muscle. Nature. 1979 Apr 19;278(5706):714–718. doi: 10.1038/278714a0. [DOI] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Beckner S. K. Activation of myosin synthesis in fusing and mononucleated myoblasts. J Mol Biol. 1975 Apr 25;93(4):431–447. doi: 10.1016/0022-2836(75)90238-7. [DOI] [PubMed] [Google Scholar]
- Frearson N., Perry S. V. Phosphorylation of the light-chain components of myosin from cardiac and red skeletal muscles. Biochem J. 1975 Oct;151(1):99–107. doi: 10.1042/bj1510099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S., Hobbs A. W. Fast and slow myosin in developing muscle fibres. Nature. 1978 Jul 6;274(5666):25–29. doi: 10.1038/274025a0. [DOI] [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Masaki T., Yoshizaki C. Differentiation of myosin in chick embryos. J Biochem. 1974 Jul;76(1):123–131. doi: 10.1093/oxfordjournals.jbchem.a130536. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelloni-muller G., Ermini M., Jenny E. Myosin light chains of developing fast and slow rabbit skeletal muscle. FEBS Lett. 1976 Aug 1;67(1):68–74. doi: 10.1016/0014-5793(76)80872-1. [DOI] [PubMed] [Google Scholar]
- Roy R. K., Sreter F. A., Sarkar S. Changes in tropomyosin subunits and myosin light chains during development of chicken and rabbit striated muscles. Dev Biol. 1979 Mar;69(1):15–30. doi: 10.1016/0012-1606(79)90271-9. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Holtzer H. Fast and slow muscles in tissue culture synthesise only fast myosin. Nature. 1979 Jul 26;280(5720):323–325. doi: 10.1038/280323a0. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Pepe F. A., Holtzer H. Myosin types during the development of embryonic chicken fast and slow muscles. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4524–4527. doi: 10.1073/pnas.74.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Sreter F. A., Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971 May;68(5):946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreter F. A., Elzinga M., Mabuchi K. The Ntau-methylhistidine content of myosin in stimulated and cross-reinnervated skeletal muscles of the rabbit. FEBS Lett. 1975 Sep 1;57(1):107–111. doi: 10.1016/0014-5793(75)80163-3. [DOI] [PubMed] [Google Scholar]
- Weeds A. G. Light chains from slow-twitch muscle myosin. Eur J Biochem. 1976 Jun 15;66(1):157–173. doi: 10.1111/j.1432-1033.1976.tb10436.x. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Trentham D. R., Kean C. J., Buller A. J. Myosin from cross-reinnervated cat muscles. Nature. 1974 Jan 18;247(5437):135–139. doi: 10.1038/247135a0. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Identification of a novel form of myosin light chain present in embryonic muscle tissue and cultured muscle cells. J Mol Biol. 1978 Dec 15;126(3):415–431. doi: 10.1016/0022-2836(78)90049-9. [DOI] [PubMed] [Google Scholar]
- Yablonka Z., Yaffe D. Snythesis of polypeptides with the properties of myosin light chains direct by RNA extracted from muscle cultures. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4599–4603. doi: 10.1073/pnas.73.12.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]