Abstract
The need for an efficacious vaccine against Francisella tularensis is a consequence of its low infectious dose and high mortality rate if left untreated. This study sought to characterize a live attenuated subspecies novicida-based vaccine strain (U112ΔiglB) in an established second rodent model of pulmonary tularemia, namely the Fischer 344 rat using two distinct routes of vaccination (intratracheal [i.t.] and oral). Attenuation was verified by comparing replication of U112ΔiglB with wild type parental strain U112 in F344 primary alveolar macrophages. U112ΔiglB exhibited an LD50>107 CFU compared to the wild type (LD50 = 5×106 CFU i.t.). Immunization with 107 CFU U112ΔiglB by i.t. and oral routes induced antigen-specific IFN-γ and potent humoral responses both systemically (IgG2a>IgG1 in serum) and at the site of mucosal vaccination (respiratory/intestinal compartment). Importantly, vaccination with U112ΔiglB by either i.t. or oral routes provided equivalent levels of protection (50% survival) in F344 rats against a subsequent pulmonary challenge with ∼25 LD50 (1.25×104 CFU) of the highly human virulent strain SCHU S4. Collectively, these results provide further evidence on the utility of a mucosal vaccination platform with a defined subsp. novicida U112ΔiglB vaccine strain in conferring protective immunity against pulmonary tularemia.
Introduction
Animal models for vaccine development should 1) optimally reflect human susceptibility to the agent of interest and 2) provide similar host responses to humans. In the case of the Gram negative pathogen Francisella tularensis, the vast majority of work has been conducted in mice [1]–[9]; however, mice are highly susceptible to all subspecies of F. tularensis including the human avirulent subspecies novicida (mouse LD50<10 CFU) and the highly human virulent subspecies tularensis strain SCHU S4 (mouse LD50<10 CFU). Thus, vaccine studies performed in the mouse model are often restricted to a narrow range of challenge inocula with SCHU S4. Previous studies have determined that “white rats” may serve as a more relevant platform for vaccine studies, as these animals were found to be more resistant to F. tularensis than mice, guinea pigs, and rabbits [10], [11]. Recently, we [12] and others [13], [14] have begun to characterize the Fischer 344 (F344) rat as a potential second rodent model for pulmonary tularemia vaccine studies. We have demonstrated that F. tularensis replicates within hepatocytes and bone marrow derived macrophages and that the rat may better reflect human susceptibility to pulmonary tularemia as evidenced by the LD50 of each subspecies when administered intratracheally (i.t.) [12]. Rats challenged i.t. with human virulent subspecies holarctica and tularensis strains exhibited a mean time to death of 10 days and pulmonary LD50 values of approximately 105 and 500 CFU, respectively [12], as compared to the mouse where the LD50 of both subspecies is less than 10 CFU [4]. Moreover, rats exhibit similar susceptibility as humans to the other subspecies of F. tularensis (i.e., resistance to F. novicida and LVS) in comparison to mice [12], [13]. Thus, F344 rats may serve as a more reflective platform for evaluation of putative tularemia vaccine candidates.
To this end, a successful vaccine against a respiratory pathogen such as F. tularensis will require induction of protective mucosal immunity at the site of infection. Various routes have been exploited to determine the most effective site to stimulate mucosal and systemic immunity including ocular [15], sublingual [16]–[20], intranasal/intratracheal [5], [21]–[24], oral [25]–[29], intravaginal [30] and intrarectal [31]. For F. tularensis, the main routes of vaccination exploited have been subcutaneous [13], [32], intranasal/intratracheal [2], [3], [33], intradermal [34]–[37], and oral [27], [28], [37]. Protection against pulmonary F. tularensis challenge in animal models has demonstrated a role for both cellular and humoral arms of the immune system [38] since B cells [1], IgA and CD4+ T cells [27], [39], NK and CD8+ T cells [40] as well as IFN-γ [39], [41] and Th1 type responses have been shown by different investigators to assist in clearance of F. tularensis. To this end, protection also can be enhanced by the use of IL-12 as an adjuvant [42]. Among these vaccination routes, the oral and intranasal routes have received considerable attention for the ability to target microfold cells (M-cells), located in Peyer's patches within the gastrointestinal tract or nasal-associated lymphoid tissue (NALT) in the respiratory tract, as induction sites [43].
Given the establishment of the F344 rat model in our laboratory, we describe in this study the efficacy of F. tularensis subsp. novicida U112ΔiglB as a putative vaccine candidate by comparing two mucosal routes of vaccination (oral vs. i.t.). U112ΔiglB was generated using a targeted mutagenesis approach [44]. This mutant lacks the gene iglB, which is located within the iglABCD operon of the Francisella pathogenicity island (FPI). The FPI is comprised of 17 genes, found in duplicate copies in the highly human virulent subsp. tularensis and holarctica, and a single copy in subsp. novicida [45]. Genes within the pathogenicity island are required for intramacrophage replication, phagosomal escape, and virulence. The FPI gene iglB has been demonstrated to be part of a putative type VI secretion system in F. tularensis [46], [47]. Along with iglA, iglB forms an iglAB outer tubular structure which contracts around an iglC inner tubular structure through which secreted proteins are propelled into the host cell [47]. U112ΔiglB is attenuated both in vitro in J774 macrophages and in vivo with a LD50>107 CFU in BALB/c mice (as compared to its parental strain U112, LD50<10 CFU [48]).
In this study, we sought to characterize the immune responses generated from mucosal vaccination (i.t. or oral) with either live U112 or the defined attenuated mutant U112ΔiglB. Oral vaccination of F344 rats with either U112 or U112ΔiglB generated 50% protection against subsequent pulmonary SCHU S4 challenge. In contrast, i.t. vaccination with U112 was 100% protective while i.t. U112ΔiglB was 50% protective against pulmonary SCHU S4 challenge. Overall, these findings reaffirm previous studies suggesting that mucosal routes of immunization may be efficacious means to provide protection against pulmonary tularemia.
Materials and Methods
Ethics Statement
All animal experiments were performed in compliance with the Animal Welfare Act, the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and “Guide for the Care and Use of Laboratory Animals” published by the National Research Council. All animal work was done in accordance with the guidelines set forth by the University of Texas at San Antonio Institutional Animal Care and Use Committee (IACUC) and Institutional Biosafety Committee (IBC), who specifically approved this study under approved protocol MU031-11/11A0.
Animals
Six-week old female Fischer 344 rats were obtained from the National Cancer Institute (Frederick, MD). Animals were housed in ventilated cages in the University of Texas at San Antonio animal vivarium and received food and water ad libitum.
Bacteria
Francisella tularensis subspecies novicida strain U112 was obtained from Dr. Francis Nano, University of Victoria, Canada. F. tularensis subspecies tularensis strain SCHU S4 was obtained from the Centers of Disease Control and Prevention, Atlanta, GA. The vaccine strain U112ΔiglB was identical to that previously described [44], [48]. All strains were grown from original stocks in tryptic soy broth (TSB) or tryptic soy agar (TSA) (both obtained from BD Biosciences) supplemented with 0.1% (w/v) L-cysteine (Fisher Scientific). Dilution plating was carried out on this medium to determine titers of bacterial stocks.
Generation of primary rat macrophages
Bone marrow derived macrophages (BMDM) were obtained from four to six week old Fischer 344 rats (n = 3) sacrificed by CO2 asphyxiation followed by cervical dislocation. Femurs were collected aseptically and washed in Dulbecco's Modified Eagles Medium (DMEM; Mediatech, Fairfax, VA) containing 10% (w/v) fetal bovine serum (FBS; HyClone, Logan, UT) supplemented with L-glutamine (2 mM), (all contents together called D10) and penicillin/streptomycin (p/s; 100 U/mL and 100 µg/mL, respectively). The end of the femur was removed and pelleted marrow was flushed from the bone with 10 mL D10 plus p/s and then centrifuged. Supernatants were discarded and the marrow was washed twice with D10, resuspended in conditioned medium [DMEM containing 20% (w/v) FBS, L-glutamine, penicillin/streptomycin and 10% (w/v) supernatant from the L929 hybridoma cell line [49], placed in a 75 cm2 tissue culture flask, and incubated at 37°C in 5% CO2. After 24 hr, media containing non-adherent cells was decanted and transferred to a second flask to recover additional cells. The medium was replaced every two days until differentiation occurred, with the last two medium changes using conditioned medium without p/s. Cells were utilized 8 days after collection. Alveolar macrophages (ALVM) were isolated using a modification of the method of Engwall et. al. [50]. Briefly, rats (n = 3) were sacrificed as previously described and the dorsal aorta was severed distal to the kidneys. The chest cavity was opened to expose the lungs, and the trachea severed just below the larynx. The lungs and heart were removed and placed in 250 mL ice cold sterile PBS containing p/s for one hour. An 18-gauge 1½” plastic catheter sheath was carefully inserted in the trachea and tied in place using surgical thread. A 10 mL luer-lock syringe filled with 8 mL PBS containing p/s was attached to the catheter and the contents of the syringe gently injected into the lungs. Using the thumb and forefinger, the lungs were gently massaged, and then the injected solution was removed from the lungs using the syringe and the contents placed in a 50 mL conical tube on ice. This procedure was serially repeated using the total of 50 mL and the recovered lavage fluid was centrifuged to pellet cells. The supernatant was discarded and the cells were washed twice in 30 mL PBS with p/s, and then resuspended in 12 mL D10 with p/s, seeded into a 75 cm2 tissue culture flask, and incubated at 37°C. The medium was replaced after 24 hr, and the cells were maintained at 37°C for up to one week with media changes every 48 hr. Macrophages were collected by gentle scraping and subsequently stained to confirm differentiation with mouse anti-rat CD11b (OX-42) conjugated with AlexaFluor 647 (AF 647, AbD Serotec, Raleigh, NC) [51], mouse anti-rat CD11b (WT.5) conjugated with fluorescein isothiocyanate (FITC, BD Biosciences, San Diego, CA) [52], [53], or mouse anti-rat CD172 conjugated with phycoerythrin (PE; also designated as OX41; BD Biosciences) [51].
Fluorescent bead assay
Rat BMDM and ALVM in D10 were seeded into 6 well plates (1×106 cells/well) and allowed to adhere. Yellow-green (FITC) fluorescent (505/515 wavelength) carboxylate-modified microspheres (Invitrogen, Carlsbad, CA), 1.0 µm in diameter, were added to confluent cell monolayers at either 10 or 100 beads/cell and incubated for 2 hr to allow for phagocytosis. Extracellular beads were washed off, cells were collected by gentle scraping, and stained with either mouse anti-rat CD11b (OX-42)-AF 647 [BMDM] or mouse anti-rat CD172-PE [ALVM], and analyzed by flow cytometry [51].
Phagocytosis assay
ALVM were suspended in D10, seeded into 96-well plates (2×105 cells/well) and allowed to adhere. Confluent macrophage monolayers were infected with either WT U112 or the mutant U112ΔiglB strain (10 MOI) for 2 hr and washed twice with D10 prior to 1 hr of gentamicin treatment (20 µg/mL). Cells were washed three times with D10 and then incubated in D10 for up to 48 hr, a maximal time frame for replication of the bacteria. At indicated time points (3, 24, 48 hr post challenge), media was decanted and cells lysed with 0.2% deoxycholate solution. Intracellular bacteria were enumerated by plating serial dilutions of lysates on TSA containing cysteine.
Vaccination and Challenge
Eight to nine week old Fischer 344 rats were anesthetized with 5% (v/v) isoflurane and oxygen at 2 liters per minute using a rodent anesthesia chamber (Harvard Apparatus, Hollister, MA). Rats were placed dorsally on a surgical platform (Alpha Lab Supply, Alberquerque, NM), and a laryngoscope (Penn Century, Inc., Philadelphia, PA) was inserted to assist in securing the tongue and allowing visualization of the trachea and esophagus. Intratracheal vaccination was utilized in this study to ensure inocula reached the lungs and initiated the infectious process. In contrast to humans, inhalation by the rat does not allow for as effective lower respiratory tract infection, as the turbinates of rat nasal passages increase nasal deposition [54]). Intratracheal vaccination/challenge was achieved using a 20-gauge plastic catheter attached to a blunt-ended needle inserted into the trachea allowing delivery of 100 µL inoculum followed by approximately 300 µL air to ensure the bacteria reached the lungs. Oral vaccination was achieved with the catheter inserted into the esophagus with delivery of 300 µL inoculum followed by approximately 300 µL of air to ensure that bacteria reached the stomach. Animals were allowed to awaken, returned to their cages, and monitored daily for morbidity and mortality following vaccination and challenge. Vaccination doses (105 CFU for U112 and 107 CFU for U112ΔiglB or LVS) and the challenge doses (approximately 1×104 CFU SCHU S4) were similar for i.t. and oral routes of administration. Doses were chosen based on LD50 analyses following F344 pulmonary infection (SCHU S4 LD50 = 500 CFU and U112 LD50 = 5×106 CFU [12], and U112ΔiglB LD50>107 CFU, unpublished data). All vaccination and challenge inocula were dilution plated on TSA containing cysteine to verify doses.
Bacterial Dissemination of the Vaccine Strain
Eight to nine week old Fischer 344 rats were i.t. or orally vaccinated as described with either 105 CFU U112 or 107 CFU U112ΔiglB. At days 3, 14, and 21 following vaccination, rats (n = 3 per group for each time point) were sacrificed, and lungs, livers, and spleens were collected post-mortem. Tissues were homogenized using a tissue homogenizer (Fisher Scientific) and dilution plated on TSA plus cysteine to enumerate organ bacterial burdens.
Measurement of Antigen-Specific Cellular Responses
Eight to nine week old Fischer 344 rats (n = 3 per group) were vaccinated, rested, and monitored for 14 or 28 days. At those times, animals were euthanized and tissues (spleens, cervical lymph nodes [CLN], and mesenteric lymph nodes [MLN]) were collected from each group of animals and single cell suspensions of splenocytes and lymphocytes were prepared. Cells (106 splenocytes or 1–5×105 lymphocytes per well) were co-cultured in the presence of the following antigens: UV-inactivated U112 or U112ΔiglB bacterial cells (approximately 1 µg of protein/well), Concanavalin A (ConA, 1 µg/well) as a positive control, and the unrelated antigen hen egg white lysozyme (HEL, 1 µg/well) or medium as negative controls. Mock and U112- vaccinated groups were co-cultured with UV-U112 and the U112ΔiglB-vaccinated group was co-cultured with UV-U112ΔiglB. Doses for antigenic restimulation were based on previous titrations from selected studies in both mice and rats [12], [27], [48] and were subsequently converted to protein levels using a Bradford assay. After incubation for 72 hr at 37°C, plates were centrifuged and culture supernatants harvested for rat IFN-γ analysis by ELISA (eBioscience, San Diego, CA) per manufacturer's instructions.
Measurement of Humoral Responses
Eight to nine week old Fischer 344 rats were vaccinated i.t. or orally with either 105 CFU U112, 107 CFU U112ΔiglB, or PBS (mock-vaccinated). For serum antibody titers (n = 6 per group), rats were rested for 28 days and bled via the tail vein to obtain sera. Briefly, 96-well plates were coated and incubated overnight with 106 CFU/well UV-inactivated bacteria (U112 for PBS and U112-vaccinated groups, and U112ΔiglB for the U112ΔiglB-vaccinated group) or with 100 ng/well of the unrelated antigen HEL, all diluted in sodium bicarbonate buffer (pH 9.5). Plates were washed with PBS+0.05% tween-20 using an automated plate washer (BioTek) and blocked with PBS+10% FBS for 2 hr at room temperature. Serum was serially diluted across plates and incubated at room temperature for 2 hr. Plates were washed, incubated for 1 hr with secondary antibody (anti-rat total antibody (including IgA), IgG1, or IgG2a conjugated to horseradish peroxidase, Southern Biotech), washed a second time, and subsequently tetramethylbenzidine substrate (BD Biosciences) was added for color development. Plates were read on an ELISA plate reader (BioTek) at 630 nm. Reciprocal serum dilutions corresponding to 50% maximal binding were used to obtain titers for each animal as in previous studies [27], [48]. These values were then used to obtain means and standard deviation for each experimental group. No binding of immune serum was detected in plates coated with HEL.
For measurement of intestinal antibody responses (n = 6 per group), fresh fecal specimens (normalized to 0.1 g/rat) were collected in protease inhibitor solution (Roche Applied Science, Indianapolis, IN) on day 28. Samples were vortexed vigorously and supernatants collected by centrifugation were assayed for humoral responses (total antibody, IgM or IgA) by ELISA. For collection of bronchioalveolar lavage fluid (BALF), rats (n = 3 per group) were sacrificed and an incision made from the chin to the middle of chest to expose the trachea. A small cut was made in the trachea to allow insertion of an 18-gauge catheter (Exelint, Los Angeles, CA) which was tied in place by a small piece of suture. A 3 mL syringe filled with 1× sterile PBS was attached to the catheter and 1 mL PBS gently injected into the lungs and removed to obtain BALF. The PBS lavage was repeated a total of 3 times, and the BALF centrifuged to remove cells and the supernatant analyzed by ELISA. Due to the small amount of antibody in the BALF and fecal supernatant samples and the large dilution factor in acquiring them (from 3 washings of lungs to collect BALF and from the respective dilution of the fecal pellets in protease inhibitor cocktail), these samples were tested undiluted, and were subsequently reported as OD630 values. Means were obtained by taking the average OD630 reading of the three animals from each group, and p-values were obtained by two-way ANOVA of OD values of the 3 respective groups. IgM responses in fecal supernants were minimal (data not shown), and no responses were detected in BALF or fecal supernatants to plates coated with HEL.
Statistics
Statistical analysis was performed using SigmaStat software. The Kaplan-Meier test was used for statistical analysis of survival experiments and student's t test for determination of differences in intramacrophage replication and organ burden, as well as antibody and IFN-γ production. Data are represented as mean ± standard deviation from each group.
Results
Characterization of F344 Alveolar Macrophages
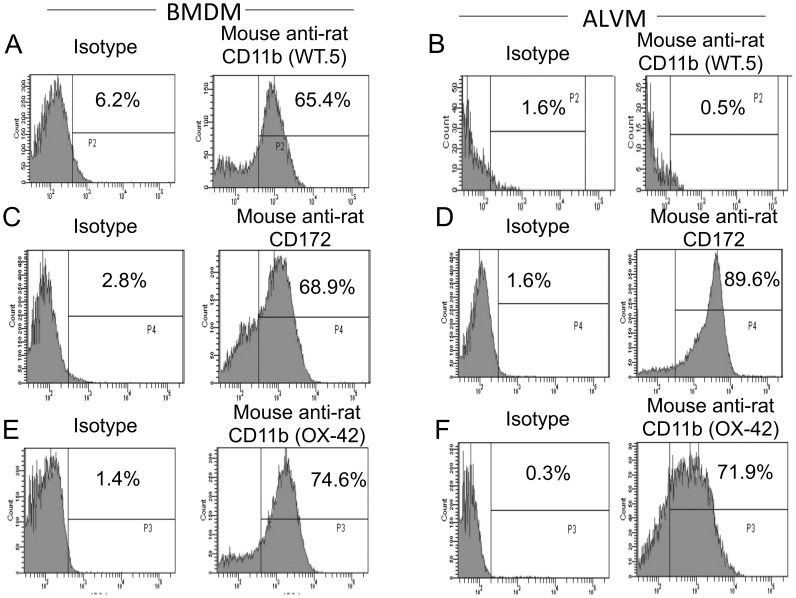
Intramacrophage replication has been used as an important tool to determine attenuation of F. tularensis vaccine strains [48]. Therefore, primary alveolar macrophages (ALVM) were utilized to determine whether U112ΔiglB was attenuated in the Fischer 344 (F344) rat. Primary ALVM were phenotypically compared with BMDM using flow cytometry [9] for the following macrophage population markers: CD11b (OX-42) (AbD Serotec, Raleigh, NC) [51], CD11b (WT.5) (BD Biosciences, San Diego, CA) [52], [53], and CD172 (also designated as OX41; BD Biosciences) [51]. As shown in Fig. 1A & B, the two types of macrophages could be distinguished by WT.5 (anti-CD11b+), with BMDM 65.4% positive while ALVM exhibited 0.5% positive staining as compared to respective isotype controls (6.2 and 1.6%, respectively). In contrast to the differential WT.5 staining, both BMDM and ALVM were positive for CD172 (68.9 and 89.6% respectively) and OX-42 (74.6 and 71.9% respectively) when compared to corresponding isotype controls (2.8 and 1.6%; 1.4 and 0.3%, respectively). Thus, F344 ALVM are a distinct population from that of BMDM as distinguished by CD172+ (high) OX-42+ (high) and WT.5− (low).
Figure 1. Flow cytometry characterization of bone marrow derived macrophages (BMDM) vs. alveolar macrophages (ALVM).
Cells were isolated from either bone marrow obtained from Fischer 344 rat femurs (panel A, C, E) or following bronchioalveolar lavage (panel B, D, F) and cultured as described (n = 3 rats for each determination). Upon reaching optimal differentiation, macrophages were stained with Alexa Fluor 647 (AF 647) conjugated mouse anti-rat CD11b (OX-42), fluorescein isothiocyanate (FITC) conjugated mouse anti-rat CD11b (WT.5), or phycoerythrin (PE) conjugated mouse anti-rat CD172 [right column] or respective isotype control [left column] and analyzed by flow cytometry. Results are representative of two independent experiments.
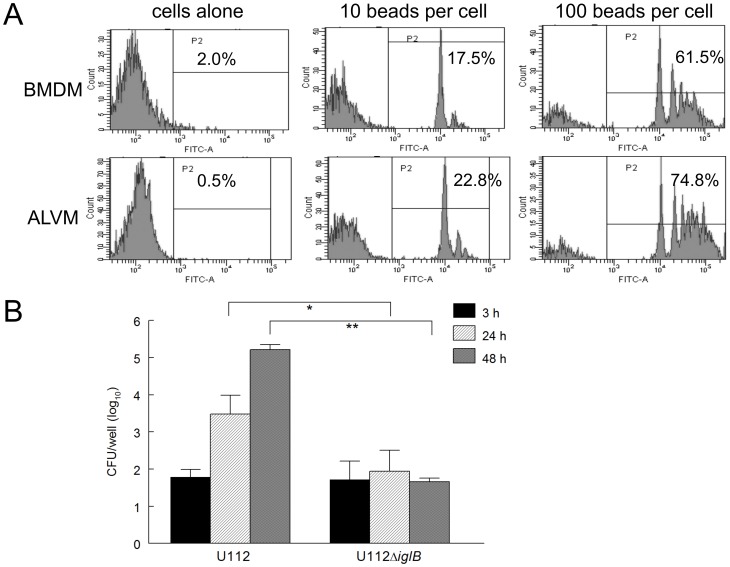
In order to assess the functionality and potential differences in phagocytosis capabilities of the two macrophage populations, the phagocytic capacity of ALVM and BMDM was evaluated using a fluorescent bead uptake assay as previously described [12]. Briefly, BMDM and ALVM (1×106 cells/well) were seeded into 6-well plates and allowed to adhere. Fluorescent labeled-beads (FITC conjugated) were added to both cell populations at either 10 or 100 beads/cell and incubated for 2 hr for uptake. Cells were washed to remove unphagocytosed beads, stained and subjected to flow cytometry analysis. These analyses (Fig. 2A) revealed that ALVM exhibited increased uptake of labeled beads compared to BMDM (22.8 vs 17.5% at 10 beads/cell and 74.8 vs. 61.5% at 100 beads/cell, respectively). There were minimal levels of FITC fluorescence observed in BMDM and ALVM cells cultured without beads (2.0 and 0.5%, respectively). Intramacrophage replication of the WT U112 and mutant U112ΔiglB in F344 ALVM cells also was compared. Alveolar macrophages (2×105 cells/well) were infected (10 MOI) for 2 hr with U112 or U112ΔiglB, and then pulsed with gentamicin for an additional 1 hr to kill any extracellular bacteria and subsequently incubated for a total of 48 hr. At defined intervals (3, 24, and 48 hr), cells were lysed with 0.2% (w/v) deoxycholate followed by dilution plating. As shown in Fig. 2B, U112 replicated robustly (∼3 logs) over the 48 hr time course. In contrast, minimal increases were observed for U112ΔiglB over 48 hr, with significant differences between U112 and U112ΔiglB at 24 and 48 hr (p<0.05 and p<0.005, respectively). These results, which were similar to intramacrophage replication in BMDM (data not shown), extend our previous findings using murine macrophages [48] and further confirm the attenuation of U112ΔiglB in F344 ALVMs.
Figure 2. The phagocytic capacity of F344 alveolar macrophages.
A) Bone marrow derived macrophages (BMDM) or alveolar macrophages (ALVM) were seeded (1×106 cells/well) into 6-well plates and allowed to adhere. Fluorescent beads were added to each cell type at either 10 or 100 beads/cell and incubated for 2 hr to allow for phagocytosis. Cells were washed to remove unphagocytosed beads and then stained for flow cytometry analysis. B) ALVM (2×105 cells/well) were seeded, allowed to adhere, and infected for 2 hr with 10 MOI of either U112 or the live attenuated defined mutant strain U112ΔiglB. Cells were subsequently treated with gentamicin for one hour to kill any remaining extracellular bacteria and incubated at 37°C for 48 hr. At defined time points (3, 24, and 48 hr), cells were lysed with 0.2% (w/v) deoxycholate and dilution plated to enumerate intracellular bacteria. Differences between U112 and U112ΔiglB at 24 and 48 hr were significant (*p<0.05 and **p<0.005, respectively). Results are representative of two separate experiments.
In vivo Growth and Dissemination of Vaccine Strain
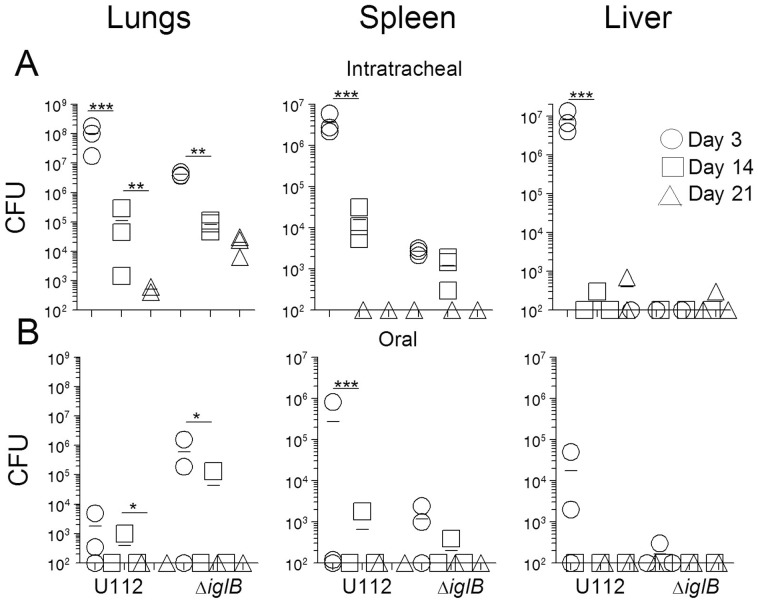
To determine the dissemination profile of U112ΔiglB and elucidate appropriate time points for analysis of immune responses from vaccination, bacterial organ burdens were determined following mucosal vaccination. Rats (n = 3 per group/time point) were vaccinated either i.t. or orally with 105 CFU U112 or 107 CFU U112ΔiglB. Animals were euthanized at days 3, 14 or 21 for collection of lungs, spleens, and livers, with organs analyzed in all animals regardless of route of vaccine administration. Organs were homogenized and dilution plated to determine bacterial burdens. As shown in Fig. 3A, significant numbers of bacteria were recovered on day 3 from the lungs of rats vaccinated i.t. with either U112 (mean = 1.0×108 CFU) or U112ΔiglB (mean = 4.0×106 CFU). Bacterial dissemination occurred in both i.t. vaccinated groups to the spleens (means of 3.6×106 and 2.7×103 CFU for U112 and U112ΔiglB groups respectively) and to the liver in the U112-vaccinated group only (8.0×105) by day 3. Bacteria were present but significantly reduced by day 14 in all organs of i.t. U112-vaccinated animals and in lungs of U112ΔiglB-vaccinated animals (* denotes p<0.05, ** denotes p<0.01, *** denotes p<0.001). Spleens and livers were cleared by day 21 with lower burdens detected in the lungs of intratracheally-vaccinated animals (limit of detection of assay = 100 CFU). In contrast, orally vaccinated groups (Fig. 3B) each had only 2 out of 3 animals with any bacterial burden on day 3. Slight dissemination from intestines to lungs (means of 1.7×103 (U112) and 5.9×105 CFU (U112ΔiglB)), spleen (2.7×105 or 1.1×103 for U112 and U112ΔiglB respectively), and liver (1.7×104 and 100 CFU for U112 and U112ΔiglB respectively) occurred by day 3. Dissemination to lungs in the orally vaccinated animals may actually have occurred from aspiration of part of the inocula and not by direct dissemination from the intestines. By day 14, negligible amounts of bacteria were recovered from all organs in the orally vaccinated groups, with a significant decrease in burden in the lungs of bothgroups (p<0.05) and in the spleens of U112-vaccinated animals (p<0.001). Bacteria were completely cleared from all organs by day 21. Regardless of route or vaccine strain, burdens were lower on days 14 and 21 as the strains are cleared from the animal.
Figure 3. In vivo dissemination of U112 and U112ΔiglB in Fischer 344 rats.
Rats were i.t. (panel A) or orally (panel B) vaccinated with either 105 CFU U112 or 107 CFU U112ΔiglB. At defined time points (days 3, 14, and 21 post vaccination), rats (n = 3 per group/time point) were euthanized for collection of lungs, spleens, and livers. Organs were homogenized and dilution plated on TSA plus cysteine to enumerate organ bacterial burdens. Significant differences in burden over the time course are noted (* denotes p<0.05, ** denotes p<0.01). Results are representative of two separate experiments.
Antigen Specific Cellular Responses Following Mucosal Vaccination
In order to compare the efficacy of two different mucosal routes of immunization with U112ΔiglB, F344 rats (n = 3 per group) were vaccinated i.t. or orally with either 105 CFU U112, 107 CFU U112ΔiglB or mock vaccinated with PBS and rested for 14 or 28 days. Cellular responses were measured at two time points because at the earlier 14 day time point spleens still showed presence of bacteria in burden assays. As spleens were cleared by day 21 (Figure 3), cellular responses also were measured at day 28 to ensure no bacteria was present in organs at this time point. Rats were euthanized as described previously for collection of spleens and draining lymph nodes. Single-cell suspensions were prepared and cultured for 72 hr in the presence of approximately 1 µg of protein from either UV-inactivated U112 (for mock and U112 vaccinated groups) or UV-inactivated U112ΔiglB (for U112ΔiglB vaccinated groups). Supernatants were analyzed by ELISA for the production of IFN-γ.
As shown in Table 1, splenocytes from rats vaccinated i.t. or orally with U112 produced significant (p<0.001) levels of antigen-specific IFN-γ (16,258±1355 and 29,840±1158 pg/mL, respectively) compared with mock-vaccinated animals at 14 days and are able to maintain IFN-γ production upon recall at 28 days (1050±28 and 146±28 pg/mL for i.t. and orally vaccinated animals, respectively). Splenocytes from rats vaccinated with U112ΔiglB also produced significant amounts of IFN-γ (2234±329 pg/mL i.t. and 151±83 pg/mL orally, p<0.01 compared to mock at day 14, albeit at lower levels than U112. Only U112ΔiglB intratracheally-vaccinated animals produced IFN-γ at day 28 (53±10 pg/mL). Rats vaccinated with U112 also produced significant (p<0.001 for both routes versus mock groups) amounts of IFN-γ (1103±99 pg/mL i.t. and 2967±7 pg/mL orally) within cervical lymph nodes (CLN) at day 14, whereas rats receiving U112ΔiglB produced minimal amounts of IFN-γ at this time point by either route of vaccine administration. A similar pattern was seen at day 14 in mesenteric lymph nodes (MLN,), with U112 i.t. vaccination generating 3715±207 pg/mL of IFN-γ (p<0.001 compared to mock) and orally resulting in 2765±31 pg/mL compared to low levels of IFN-γ produced from U112ΔiglB-primed MLN. Due to limitations in the amount of cells recovered from CLN and MLN at day 28 (1×105 cells/well at this time point compared to 5×105 cells/well at day 14) we cannot conclude that IFN-γ is not produced in the draining lymph nodes upon restimulation at day 28, however, under these conditions we did not see any IFN-γ production in our draining lymph node ELISAs at this time point. Across the board, there was negligible IFN-γ production in any cells (regardless of route or vaccine) cultured with media or with the unrelated antigen hen egg lysozyme (HEL. data not shown) and potent responses (p<0.001) comparable across all groups when tissues were cultured with the mitogen Concanavalin A.
Table 1. Antigen-specific cellular responses following mucosal vaccination.
| IFN-γ Production | DAY 14 | DAY 28 | |||||||||||
| Post | |||||||||||||
| Immunization | PBS | U112 | U112ΔiglB | PBS | U112 | U112ΔiglB | |||||||
| (pg/mL) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| I.T. | Spleen | <30 | N/A | 16258 | 1355 | 2234 | 329 | <30 | N/A | 1051 | 28 | 54 | 10 |
| CLN | <30 | N/A | 1103 | 99 | <30 | N/A | <30 | N/A | <30 | N/A | <30 | N/A | |
| MLN | 540 | 526 | 3716 | 207 | 101 | 52 | <30 | N/A | <30 | N/A | <30 | N/A | |
| Oral | Spleen | <30 | N/A | 29840 | 1158 | 151 | 83 | <30 | N/A | 146 | 28 | <30 | N/A |
| CLN | <30 | N/A | 2967 | 7 | <30 | N/A | <30 | N/A | <30 | N/A | <30 | N/A | |
| MLN | <30 | N/A | 2766 | 31 | 32 | N/A | <30 | N/A | <30 | N/A | <30 | N/A | |
Rats were vaccinated either i.t. (top panel) or orally (bottom panel) with either 105 CFU U112, 107 CFU U112ΔiglB, or mock-vaccinated with PBS, and rested for 14 or 28 days. Rats were euthanized and spleens (106 cells/well) and draining lymph nodes (CLN and MLN) collected (day 14: 5×105 cells/well and day 28: 1×105 cells/well). Single cells were cultured for 72 hr in the presence of approximately 1 µg of protein from either UV-inactivated U112 (for U112 and mock vaccinated groups), or UV-inactivated U112ΔiglB (for U112ΔiglB vaccinated group). Cells were then separated by centrifugation and supernatants were collected and assayed for IFN-γ production by ELISA (shown here in pg/mL, limit of detection is 30 pg/mL). Results are representative of two independent experiments at each time point. CLN = cervical lymph nodes, MLN = mesenteric lymph nodes, SD = standard deviation, NA = not applicable.
Humoral Responses Following Mucosal Vaccination
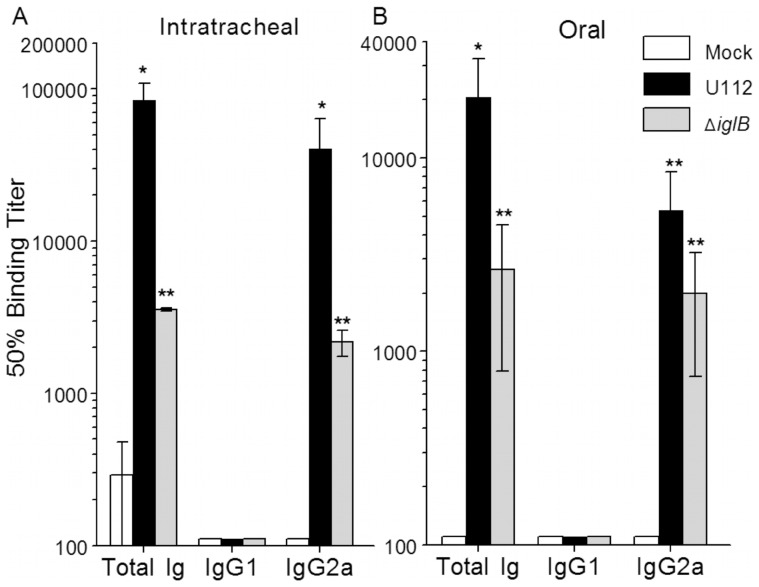
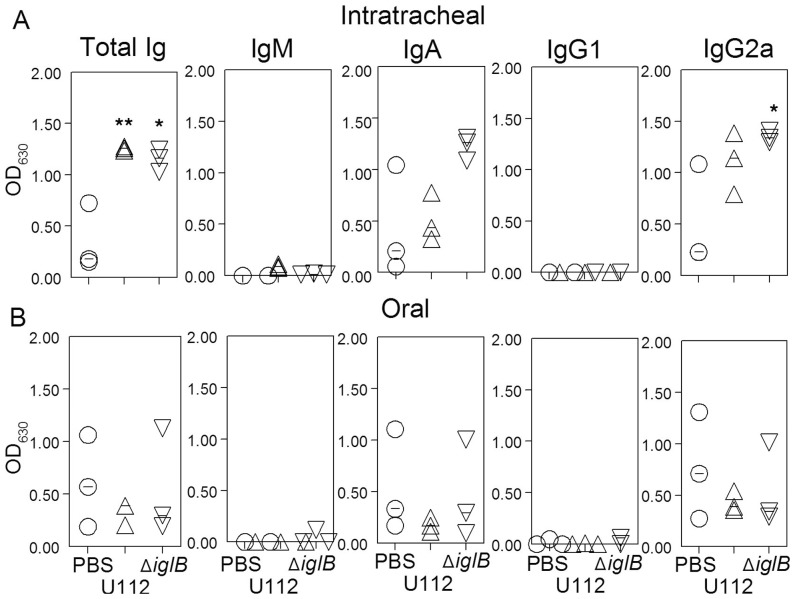
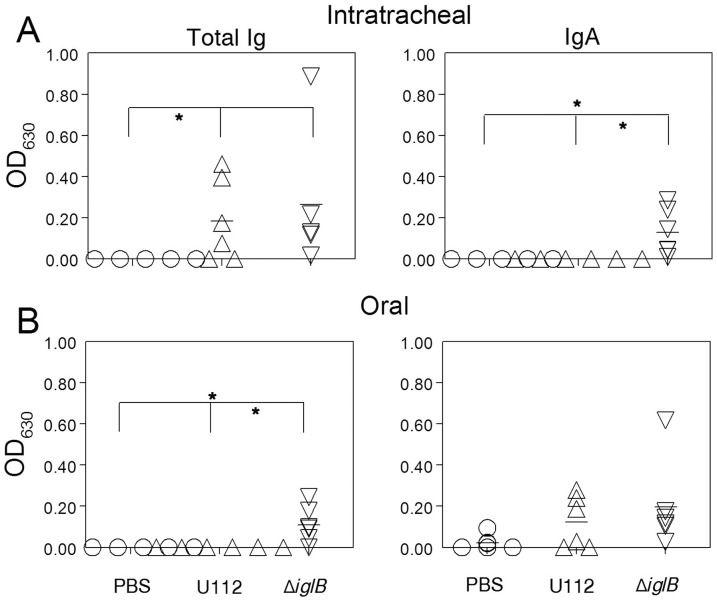
Humoral responses within the systemic and mucosal compartments were analyzed at 28 days following vaccination. Rats (n = 6 per group) were vaccinated i.t. or orally with either 105 CFU U112, 107 CFU U112ΔiglB, or mock vaccinated with PBS. Prior to bacterial challenge, animals were bled (day 28 post-vaccination) and serum analyzed by ELISA. F344 rats vaccinated i.t. with U112 exhibited significant (p<0.01) induction of total Ig and IgG2a with minimal production of IgG1 in contrast to mock-vaccinated animals (Fig. 4A). A similar profile, albeit at lower levels, was observed in rats vaccinated i.t. with U112ΔiglB (p<0.001 compared to mock-vaccinated group). A comparable but somewhat reduced antibody profile was observed following oral vaccination with the WT and mutant strains of bacteria (Fig. 4B). In all cases, there was minimal reactivity to the unrelated HEL antigen (data not shown) and negligible antibody production in mock-vaccinated animals (detection limit ∼100). Antibody responses on day 28 at the sites of vaccination also were evaluated by collection of bronchioalveolar lavage fluid (BALF) (Fig. 5) and fecal supernatants (Fig. 6) from vaccinated rats. As shown on Fig. 5A, rats vaccinated i.t. with U112 exhibited increased mean levels of total antibody, IgA and IgG2a in contrast to mock-vaccinated animals (*p<0.05, **p<0.01). Rats vaccinated i.t. with U112ΔiglB showed comparable levels of total antibody and IgG2a, and increased levels of IgA when compared with animals vaccinated with U112. In contrast, oral vaccination gave rise to lower levels of total antibody, IgA and IgG2a compared to rats primed i.t. (Fig. 5B). There was a greater induction of fecal IgA response following i.t. vaccination with U112ΔiglB than with U112 (Fig. 6A). In contrast, there were comparable levels of fecal IgA production following oral vaccination with U112 or U112ΔiglB (Fig. 6B), with minimal IgM production across all groups (data not shown). As expected, a minimal antibody response was observed in mock (PBS)-vaccinated rats.
Figure 4. Serum antibody responses following mucosal vaccination.
Animals (n = 6 per group) were vaccinated i.t. (panel A) or orally (panel B) with either 105 CFU U112, 107 CFU U112ΔiglB, or mock vaccinated with PBS and rested for 28 days. Serum was analyzed by ELISA to obtain 50% binding titers. Titers from both vaccinated groups, regardless of route, were shown to be significant compared to mock (**p<0.001, *p<0.01). Results are representative of two independent experiments.
Figure 5. Respiratory antibody response following mucosal vaccination.
Animals (n = 3 per group) were vaccinated i.t. (top panel) or orally (bottom panel) with either 105 CFU U112, 107 CFU U112ΔiglB, or mock vaccinated with PBS and rested for 28 days. Animals were euthanized to obtain bronchioalveolar lavage fluid (BALF) which was assayed by ELISA. Significant differences were observed between U112 and U112ΔiglB i.t. vaccinated and respective mock groups for total Ig and IgG2a (*p<0.05, **p<0.01). Animals primed with U112ΔiglB exhibited responses which were comparable to or higher than that observed for U112-primed rats. Horizontal lines represent mean of each group. Results are representative of two separate experiments.
Figure 6. Intestinal antibody response following mucosal vaccination.
Animals (n = 6 per group) were vaccinated i.t. (A, top panel) or orally (B, bottom panel) with either 105 CFU U112, 107 CFU U112ΔiglB, or mock vaccinated with PBS and rested for 28 days. Fecal specimens (0.1 g/rat) were collected, processed and supernatants analyzed by ELISA for total immunoglobulin response and IgA. Vaccinated animals produced higher levels of IgA and total Ig (*p<0.05) compared to the mock-vaccinated group. Animals primed with U112ΔiglB exhibited responses which were comparable to or higher than that of U112-vaccinated rats. Horizontal lines represent mean of each group. Results are representative of two independent experiments.
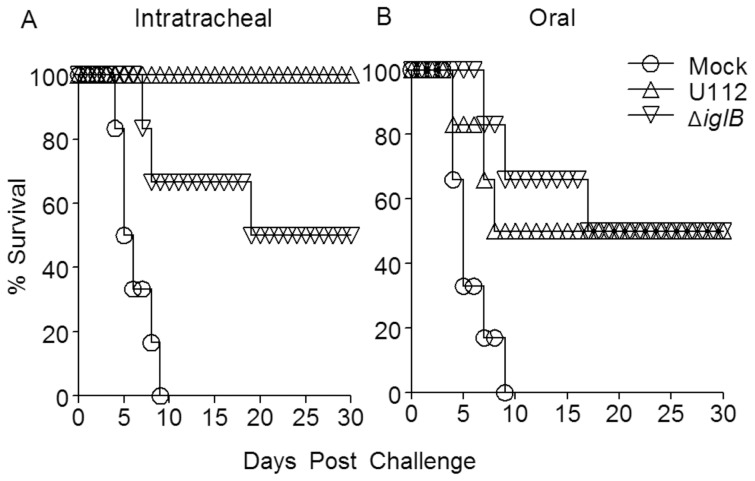
Pulmonary Protection Conferred by Mucosal Vaccination
The efficacy of both routes of vaccination in providing protection against lethal pulmonary challenge with the highly human virulent SCHU S4 strain of F. tularensis was compared (Fig. 7). Rats (n = 6 per group) were vaccinated i.t. or orally with either 105 CFU U112, 107 CFU U112ΔiglB, or mock-vaccinated with PBS and rested for 30 days prior to i.t. challenge with 1.25×104 CFU (approximately 25 LD50) of SCHU S4. As shown in Fig. 7A., i.t. vaccination with the WT strain U112 provided complete protection (100% survival, p<0.001 compared to mock-vaccinated animals) against the lethal SCHU S4 pulmonary challenge in agreement with previously published results [12], whereas animals vaccinated orally with U112 exhibited 50% survival (p<0.05 compared to mock, Fig. 7B). In contrast, regardless of route, vaccination with U112ΔiglB conferred 50% protection against subsequent pulmonary challenge (p<0.01 for i.t. group and p<0.005 for oral group compared to mock, Fig. 7). Mock-vaccinated rats succumbed to the bacterial challenge by day 10. These results, which are consistent between replicate experiments, clearly indicate the feasibility of developing a defined U112 vaccine strain and the efficacy in providing protective immunity against pulmonary tularemia.
Figure 7. Protective immunity conferred by mucosal vaccination.
Animals (n = 6 per group) were vaccinated either i.t. (panel A) or orally (panel B) with either 105 CFU U112, 107 CFU U112ΔiglB, or mock-vaccinated with PBS and rested for 30 days. Rats were challenged i.t. with 1.25×104 CFU SCHU S4 (approx. 25 LD50) and monitored daily for morbidity and mortality. Kaplan-Meier survival analysis revealed protection following immunization with U112 by both i.t. and oral routes were significant (p<0.001 and p<0.005, respectively) over mock immunization. Protection conferred by U112ΔiglB vaccination was significant over mock control (p<0.01 (i.t.) and p<0.005 (oral)). Results are representative of two independent experiments.
Discussion
Mucosal immunization has been exploited as a successful route for vaccination against a variety of pathogens that infect the respiratory and gastrointestinal tracts [21], [22], [26], [29]. The licensed intranasal vaccine FluMist has been used successfully against seasonal influenza; and oral vaccination platforms also have been effectively used against other pathogens, most notably poliovirus (Sabin vaccine), typhoid fever (Ty21a vaccine), and rotavirus [55]. In this study, we sought to analyze and compare two routes (i.t. and oral) of mucosal vaccination in the Fischer 344 rat utilizing a live attenuated vaccine strain (U112ΔiglB) that has been previously characterized in the mouse model of F. tularensis [48]. This live attenuated strain lacks the iglB gene within the iglABCD operon of the Francisella pathogenicity island (FPI), consisting of 17 genes. Two copies of the FPI are found in the highly human virulent subsp. tularensis and holarctica, whereas a single copy of the FPI is found in subsp. novicida [45]. There is >97% homology between the FPIs across subspecies, and genes of the FPI are required for intramacrophage replication, phagosomal escape, and virulence [45], [46], [56]–[61]. The iglB gene has been shown to have homology in other bacteria such as Vibrio cholerae, Salmonella enterica, and Rhizobium leguminosarum [62] and been demonstrated to be part of a type VI secretion system in other species [63], [64] as well as in F. tularensis [46], [47].
This study utilized intratracheal vaccination in the rat, which would be an impractical route of vaccination for humans, when compared to the more common intranasal route. The complex physiology of the rat respiratory system suggests that intratracheal vaccination may be a more effective mechanism to ensure the vaccination and challenge inocula reach the lungs in this animal model. In contrast, intranasal vaccination of the rat would lead to increased deposition of inocula in the nasal passages due to the complex turbinate structure which is characteristic of rodents and is absent in humans [54]. Thus, although intratracheal vaccination may be impractical for use in humans, this route may be the most effective one in the rat to compare to intranasal routes in humans.
In this study, the overall induction of antigen–specific cellular and humoral responses was lower in U112ΔiglB-immunized rats than those receiving the parental strain U112. This difference is most likely related to the level of attenuation observed for alveolar intramacrophage replication and subsequent priming of the immune system. Likewise, cellular and humoral responses were observed to be higher for U112 at the priming sites for each respective route (cervical lymph nodes [day 14] and BALF for i.t. vaccination; mesenteric lymph nodes [day 14] and intestines for oral vaccination). Additionally, antigen-specific IFN-γ was produced at distal sites at day 14, illustrating the commonality of the mucosal immune system. Our analyses revealed that both U112 and U112ΔiglB vaccinated rats exhibited a Th1-driven, systemic humoral response (IgG2a over IgG1) which differed significantly from the mouse, where mixed serum responses of both IgG1 and IgG2a isotypes is exhibited [48]. A similar polarized antibody response (high levels of total Ig, IgA, and IgG2a and minimal IgM or IgG1) was observed at the sites of priming (lung or intestinal compartment depending on vaccination route). Rats also exhibited a lower, but intact antibody response at distal mucosal sites which mirrored the cellular IFN-γ responses.
Importantly, immunization with U112ΔiglB, regardless of route, was able to provide 50% protection against subsequent pulmonary challenge with 25 LD50 (1.25×104 CFU) of the highly human virulent F. tularensis strain SCHU S4. Moreover, when U112ΔiglB was administered orally, the observed levels of protective immunity was equal to that conferred by WT U112 providing further evidence that this defined mutant strain may serve as a promising candidate for further investigation. Interestingly, our cellular responses at day 28 correlate with the survival, as the U112 i.t. group (which had 100% survival following SCHU S4 challenge) produced significantly higher amounts of IFN-γ compared to the other three vaccine treatments/routes with which comparable IFN-γ production and resulting 50% survival.
Differences in survival between the two mucosal routes following SCHU S4 challenge may be due to a variety of factors. Oral vaccination, as opposed to immunization by the intratracheal route, may involve compounding factors which could be responsible for equalizing the immune responses generated from a lower-dose vaccination with the WT U112 and higher dose vaccination with the attenuated mutant U112ΔiglB strain. For example,organisms may not survive the highly acidic pH of the stomach, or they may be lost from the digestive tract as a consequence of peristalsis and fluid flow clearing mechanisms. In contrast, intratracheal administration places organisms directly onto the mucosal surfaces of the rat lung and thus more bacteria may be retained following i.t. immunization when compared to the GI tract.
Given that LVS has been examined extensively [13], [27], [65]–[69] as the prototypic vaccine candidate, we also evaluated the efficacy of oral LVS vaccination in this model. LVS has previously been documented to provide protection by parenteral (intradermal and subcutaneous) and mucosal (intratracheal) routes in the F344 rat [13] and has a similar LD50 to U112ΔiglB within the rat model (LD50 of both strains >107 CFU by the pulmonary route). We found that oral LVS vaccination conferred complete protection against pulmonary SCHU S4 (approximately 25 LD50) challenge (Arulanandam and Signarovitz, unpublished observations).
Despite the high level of protection conferred by WT U112, this bacterium would most likely not be a successful candidate for vaccination against tularemia due to its wild-type nature and the obvious morbidity observed following vaccination of rats. Specifically, F344 rats vaccinated i.t. with 105 CFU U112 in this study were visibly stressed and ill for 7–10 days following immunization, with symptoms including ∼10% weight loss, ruffled fur, hunched posture, and periorbital porphyrin production. Such severe morbidity in immunocompetent hosts would likely prevent administration of U112 to immunocompromised individuals. In contrast, vaccination with a hundred-fold higher dose of U112ΔiglB caused no adverse effects or visible morbidity to rats, and yet this mutant was still able to induce antigen-specific cellular and humoral responses which generated protection against subsequent SCHU S4 challenge. It is likely that booster doses of this mutant strain would increase the degree of protective efficacy. These results collectively suggest the feasibility of developing targeted oral-based attenuated mutant vaccine strains for immunization against F. tularensis and provide impetus for further refinement of novicida-based vaccines, given the ease of its genetic manipulation.
To this end, U112ΔiglB is the only F. novicida-based live attenuated vaccine strain that has been shown to provide heterologous protection against pulmonary LVS and SCHU S4 challenge in the mouse model [33]. The majority of F. novicida-based putative vaccines, including other FPI mutants such as ΔiglC [5], ΔpdpB [70], and FPI regulator ΔmglA [71], have only been tested against homologous U112 challenge. The two F. novicida-based vaccines tested against SCHU S4 were non-FPI mutants, namely ΔpurF [8] and ΔpmrA [72], which exhibited no protective efficacy following pulmonary challenge, and no SCHU S4-based FPI mutant has provided protection against subsequent Type A challenge. For example, SCHU S4ΔiglC, when administered at high doses by either intradermal or oral routes, afforded no protection against subsequent pulmonary SCHU S4 challenge [6], [34]. SCHU S4ΔiglB and SCHU S4ΔiglD also demonstrated marginal protection against subsequent pulmonary challenge [73]. Levels of protection afforded by non-FPI LVS [2], [35], [74] or SCHU S4-based [6], [34], [75] mutants varied within the limited challenge dose of less than 10 to 100 CFU, illustrating the high sensitivity of the mouse in contrast to the rat, and consequent limitation of this model for vaccine efficacy studies. The success of mucosal vaccination in the F344 rat as demonstrated here and by others [12], [13] may involve microfold cells (M-cells). These cells are predominantly found in the follicle-associated epithelium (FAE) of intestinal Peyer's patches (PP), which are components of the larger intestinal GALT (gut-associated lymphoid tissue) [76], but also can be found in isolated lymphoid follicles, the appendix, and in MALT sites outside the gastrointestinal tract including the nasal passages. Furthermore, M-cells have distinctive morphological features such as a poorly organized brush border, irregular microvilli, and a thin glycocalyx suggesting that they do not play a role in intestinal digestion or absorption [76]. Importantly, M-cells can serve as antigen sampling sites and contain a distinct basal invagination in which live and non-replicating pathogens are presented to lymphocytes, dendritic cells, and macrophages [77]. Our ongoing studies include enhancing the M-cell tropism of defined F. tularensis vaccine strains, such as U112ΔiglB, to further increase vaccine efficacy and optimal protective immunity.
Acknowledgments
The authors would like to acknowledge C. Rick Lyons and Terry Wu for their assistance with the Fischer 344 rat model.
Funding Statement
This research has been performed with funding provided by the National Institutes of Health (Grant P01 AI057986) and the Army Research Office of the Department of Defense under Contract No. W911NF-11-1-0136. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Elkins KL, Bosio CM, Rhinehart-Jones TR (1999) Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Francisella tularensis live vaccine strain. Infect Immun 67: 6002–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakshi CS, Malik M, Mahawar M, Kirimanjeswara GS, Hazlett KR, et al. (2008) An improved vaccine for prevention of respiratory tularemia caused by Francisella tularensis Schu S4 strain. Vaccine 26: 5276–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baron SD, Singh R, Metzger DW (2007) Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect Immun 75: 2152–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen W, Shen H, Webb A, KuoLee R, Conlan JW (2003) Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine 21: 3690–3700. [DOI] [PubMed] [Google Scholar]
- 5. Pammit MA, Raulie EK, Lauriano CM, Klose KE, Arulanandam BP (2006) Intranasal vaccination with a defined attenuated Francisella novicida strain induces gamma interferon-dependent antibody-mediated protection against tularemia. Infect Immun 74: 2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conlan JW, Shen H, Golovliov I, Zingmark C, Oyston PC, et al. (2010) Differential ability of novel attenuated targeted deletion mutants of Francisella tularensis subspecies tularensis strain SCHU S4 to protect mice against aerosol challenge with virulent bacteria: effects of host background and route of immunization. Vaccine 28: 1824–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ireland PM, Lebutt H, Thomas RM, Oyston PC (2011) A Francisella tularensis SCHU S4 mutant deficient in {gamma}-glutamyl transferase activity induces protective immunity: characterisation of an attenuated vaccine candidate. Microbiology [DOI] [PubMed] [Google Scholar]
- 8. Quarry JE, Isherwood KE, Michell SL, Diaper H, Titball RW, et al. (2007) A Francisella tularensis subspecies novicida purF mutant, but not a purA mutant, induces protective immunity to tularemia in mice. Vaccine 25: 2011–2018. [DOI] [PubMed] [Google Scholar]
- 9. Bitsaktsis C, Rawool DB, Li Y, Kurkure NV, Iglesias B, et al. (2009) Differential requirements for protection against mucosal challenge with Francisella tularensis in the presence versus absence of cholera toxin B and inactivated F. tularensis . J Immunol 182: 4899–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Downs CM, Coriell LL, et al. (1947) Studies on tularemia; the comparative susceptibility of various laboratory animals. J Immunol 56: 217–228. [PubMed] [Google Scholar]
- 11. Olsufiev NG, Emelyanova OS, Dunayeva TN (1959) Comparative study of strains of B. tularense in the old and new world and their taxonomy. J Hyg Epidemiol Microbiol Immunol 3: 138–149. [PubMed] [Google Scholar]
- 12. Ray HJ, Chu P, Wu TH, Lyons CR, Murthy AK, et al. (2010) The Fischer 344 rat reflects human susceptibility to Francisella pulmonary challenge and provides a new platform for virulence and protection studies. PLoS One 5: e9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu TH, Zsemlye JL, Statom GL, Hutt JA, Schrader RM, et al. (2009) Vaccination of Fischer 344 rats against pulmonary infections by Francisella tularensis type A strains. Vaccine 27: 4684–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raymond CR, Conlan JW (2009) Differential susceptibility of Sprague-Dawley and Fischer 344 rats to infection by Francisella tularensis . Microb Pathog 46: 231–234. [DOI] [PubMed] [Google Scholar]
- 15. Hu K, Dou J, Yu F, He X, Yuan X, et al. (2011) An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. Vaccine 29: 1455–1462. [DOI] [PubMed] [Google Scholar]
- 16. Kweon MN (2011) Sublingual mucosa: A new vaccination route for systemic and mucosal immunity. Cytokine 54: 1–5. [DOI] [PubMed] [Google Scholar]
- 17. Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, et al. (2008) Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A 105: 1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuburu N, Kweon MN, Hervouet C, Cha HR, Pang YY, et al. (2009) Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J Immunol 183: 7851–7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang CF, Wang CC, Wu TC, Wu KG, Lee CC, et al. (2008) Neonatal sublingual vaccination with Salmonella proteins and adjuvant cholera toxin or CpG oligodeoxynucleotides induces mucosal and systemic immunity in mice. J Pediatr Gastroenterol Nutr 46: 262–271. [DOI] [PubMed] [Google Scholar]
- 20. Huang CF, Wu TC, Wu CC, Lee CC, Lo WT, et al. (2011) Sublingual vaccination with sonicated Salmonella proteins and mucosal adjuvant induces mucosal and systemic immunity and protects mice from lethal enteritis. APMIS 119: 468–478. [DOI] [PubMed] [Google Scholar]
- 21. Tanaka N, Fukuyama S, Fukuiwa T, Kawabata M, Sagara Y, et al. (2007) Intranasal immunization with phosphorylcholine induces antigen specific mucosal and systemic immune responses in mice. Vaccine 25: 2680–2687. [DOI] [PubMed] [Google Scholar]
- 22. Sabirov A, Metzger DW (2006) Intranasal vaccination of neonatal mice with polysaccharide conjugate vaccine for protection against pneumococcal otitis media. Vaccine 24: 5584–5592. [DOI] [PubMed] [Google Scholar]
- 23. Sabirov A, Metzger DW (2008) Intranasal vaccination of infant mice induces protective immunity in the absence of nasal-associated lymphoid tissue. Vaccine 26: 1566–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hara H, Mouri A, Yonemitsu Y, Nabeshima T, Tabira T (2011) Mucosal immunotherapy in an Alzheimer mouse model by recombinant Sendai virus vector carrying Abeta1-43/IL-10 cDNA. Vaccine 29: 7474–7482. [DOI] [PubMed] [Google Scholar]
- 25. Hara H, Monsonego A, Yuasa K, Adachi K, Xiao X, et al. (2004) Development of a safe oral Aβ vaccine using recombinant adeno-associated virus vector for Alzheimer's disease. J Alzheimers Dis 6: 483–488. [DOI] [PubMed] [Google Scholar]
- 26. Branger CG, Torres-Escobar A, Sun W, Perry R, Fetherston J, et al. (2009) Oral vaccination with LcrV from Yersinia pestis KIM delivered by live attenuated Salmonella enterica serovar Typhimurium elicits a protective immune response against challenge with Yersinia pseudotuberculosis and Yersinia enterocolitica . Vaccine 27: 5363–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ray HJ, Cong Y, Murthy AK, Selby DM, Klose KE, et al. (2009) Oral live vaccine strain-induced protective immunity against pulmonary Francisella tularensis challenge is mediated by CD4+ T cells and antibodies, including immunoglobulin A. Clin Vaccine Immunol 16: 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. KuoLee R, Harris G, Conlan JW, Chen W (2007) Oral immunization of mice with the live vaccine strain (LVS) of Francisella tularensis protects mice against respiratory challenge with virulent type A F. tularensis . Vaccine 25: 3781–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cazorla SI, Becker PD, Frank FM, Ebensen T, Sartori MJ, et al. (2008) Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi . Infect Immun 76: 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cha HR, Ko HJ, Kim ED, Chang SY, Seo SU, et al. (2011) Mucosa-associated epithelial chemokine/CCL28 expression in the uterus attracts CCR10+ IgA plasma cells following mucosal vaccination via estrogen control. J Immunol 187: 3044–3052. [DOI] [PubMed] [Google Scholar]
- 31. Sedgmen BJ, Meeusen EN, Lofthouse SA (2004) Alternative routes of mucosal immunization in large animals. Immunol Cell Biol 82: 10–16. [DOI] [PubMed] [Google Scholar]
- 32. Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, et al. (2005) Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun 73: 2644–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pechous R, Celli J, Penoske R, Hayes SF, Frank DW, et al. (2006) Construction and characterization of an attenuated purine auxotroph in a Francisella tularensis live vaccine strain. Infect Immun 74: 4452–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Twine S, Bystrom M, Chen W, Forsman M, Golovliov I, et al. (2005) A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect Immun 73: 8345–8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jia Q, Lee BY, Bowen R, Dillon BJ, Som SM, et al. (2010) A Francisella tularensis live vaccine strain (LVS) mutant with a deletion in capB, encoding a putative capsular biosynthesis protein, is significantly more attenuated than LVS yet induces potent protective immunity in mice against F. tularensis challenge. Infect Immun 78: 4341–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen H, Harris G, Chen W, Sjostedt A, Ryden P, et al. (2010) Molecular immune responses to aerosol challenge with Francisella tularensis in mice inoculated with live vaccine candidates of varying efficacy. PLoS One 5: e13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Conlan JW, Shen H, Golovliov I, Zingmark C, Oyston PC, et al. (2009) Differential ability of novel attenuated targeted deletion mutants of Francisella tularensis subspecies tularensis strain SCHU S4 to protect mice against aerosol challenge with virulent bacteria: Effects of host background and route of immunization. Vaccine [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Isherwood KE, Titball RW, Davies DH, Felgner PL, Morrow WJ (2005) Vaccination strategies for Francisella tularensis . Adv Drug Deliv Rev 57: 1403–1414. [DOI] [PubMed] [Google Scholar]
- 39. Powell HJ, Cong Y, Yu JJ, Guentzel MN, Berton MT, et al. (2008) CD4+ T cells are required during priming but not the effector phase of antibody-mediated IFN-γ-dependent protective immunity against pulmonary Francisella novicida infection. Immunol Cell Biol 86: 515–522. [DOI] [PubMed] [Google Scholar]
- 40. Gosselin EJ, Gosselin DR, Lotz SA (2005) Natural killer and CD8 T cells dominate the response by human peripheral blood mononuclear cells to inactivated Francisella tularensis live vaccine strain. Hum Immunol 66: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 41. Nallaparaju KC, Yu JJ, Rodriguez SA, Zogaj X, Manam S, et al. (2011) Evasion of IFN-gamma signaling by Francisella novicida is dependent upon Francisella outer membrane protein C. PLoS One 6: e18201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Duckett NS, Olmos S, Durrant DM, Metzger DW (2005) Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun 73: 2306–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kiyono H, Fukuyama S (2004) NALT- versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol 4: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu J, Zogaj X, Barker JR, Klose KE (2007) Construction of targeted insertion mutations in Francisella tularensis subsp. novicida . Biotechniques 43: 487–490, 492. [DOI] [PubMed] [Google Scholar]
- 45. Nano FE, Zhang N, Cowley SC, Klose KE, Cheung KK, et al. (2004) A Francisella tularensis pathogenicity island required for intramacrophage growth. J Bacteriol 186: 6430–6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barker JR, Chong A, Wehrly TD, Yu JJ, Rodriguez SA, et al. (2009) The Francisella tularensis pathogenicity island encodes a secretion system that is required for phagosome escape and virulence. Mol Microbiol 74: 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Bruin OM, Duplantis BN, Ludu JS, Hare RF, Nix EB, et al. (2011) The biochemical properties of the Francisella pathogenicity island (FPI)-encoded proteins IglA, IglB, IglC, PdpB and DotU suggest roles in type VI secretion. Microbiology 157: 3483–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cong Y, Yu JJ, Guentzel MN, Berton MT, Seshu J, et al. (2009) Vaccination with a defined Francisella tularensis subsp. novicida pathogenicity island mutant (ΔiglB) induces protective immunity against homotypic and heterotypic challenge. Vaccine 27: 5554–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boltz-Nitulescu G, Wiltschke C, Holzinger C, Fellinger A, Scheiner O, et al. (1987) Differentiation of rat bone marrow cells into macrophages under the influence of mouse L929 cell supernatant. J Leukoc Biol 41: 83–91. [DOI] [PubMed] [Google Scholar]
- 50. Engwall KS, Li AP (1983) Isolation and Culturing of Rat Pulmonary Alveolar Macrophages. Journal of Tissue Culture Methods 8: 91–94. [Google Scholar]
- 51. Robinson AP, White TM, Mason DW (1986) Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology 57: 239–247. [PMC free article] [PubMed] [Google Scholar]
- 52. Dijkstra CD, Dopp EA, Joling P, Kraal G (1985) The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 54: 589–599. [PMC free article] [PubMed] [Google Scholar]
- 53. Damoiseaux JG, Dopp EA, Neefjes JJ, Beelen RH, Dijkstra CD (1989) Heterogeneity of macrophages in the rat evidenced by variability in determinants: two new anti-rat macrophage antibodies against a heterodimer of 160 and 95 kd (CD11/CD18). J Leukoc Biol 46: 556–564. [DOI] [PubMed] [Google Scholar]
- 54.National Research Council (US) Committee on Animal Models for Testing Interventions Against Aerosolized Bioterrorism Agents (2006). Overcoming Challenges to Develop Countermeasures Against Aerosolized Bioterrorism Agents: Appropriate Use of Animal Models. Washington, DC: National Academies Press (US). [PubMed]
- 55. Dietrich G, Griot-Wenk M, Metcalfe IC, Lang AB, Viret JF (2003) Experience with registered mucosal vaccines. Vaccine 21: 678–683. [DOI] [PubMed] [Google Scholar]
- 56. Chong A, Wehrly TD, Nair V, Fischer ER, Barker JR, et al. (2008) The early phagosomal stage of Francisella tularensis determines optimal phagosomal escape and Francisella pathogenicity island protein expression. Infect Immun 76: 5488–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ludu JS, de Bruin OM, Duplantis BN, Schmerk CL, Chou AY, et al. (2008) The Francisella pathogenicity island protein PdpD is required for full virulence and associates with homologues of the type VI secretion system. J Bacteriol 190: 4584–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Santic M, Molmeret M, Barker JR, Klose KE, Dekanic A, et al. (2007) A Francisella tularensis pathogenicity island protein essential for bacterial proliferation within the host cell cytosol. Cell Microbiol 9: 2391–2403. [DOI] [PubMed] [Google Scholar]
- 59. Santic M, Molmeret M, Klose KE, Jones S, Kwaik YA (2005) The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell Microbiol 7: 969–979. [DOI] [PubMed] [Google Scholar]
- 60. Schmerk CL, Duplantis BN, Wang D, Burke RD, Chou AY, et al. (2009) Characterization of the pathogenicity island protein PdpA and its role in the virulence of Francisella novicida . Microbiology 155: 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barker JR, Klose KE (2007) Molecular and genetic basis of pathogenesis in Francisella tularensis . Ann N Y Acad Sci 1105: 138–159. [DOI] [PubMed] [Google Scholar]
- 62. de Bruin OM, Ludu JS, Nano FE (2007) The Francisella pathogenicity island protein IglA localizes to the bacterial cytoplasm and is needed for intracellular growth. BMC Microbiol 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, et al. (2006) A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312: 1526–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, et al. (2006) Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A 103: 1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bosio CM, Elkins KL (2001) Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect Immun 69: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dreisbach VC, Cowley S, Elkins KL (2000) Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun 68: 1988–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cole LE, Shirey KA, Barry E, Santiago A, Rallabhandi P, et al. (2007) Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect Immun 75: 4127–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Elkins KL, Cowley SC, Bosio CM (2003) Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect 5: 135–142. [DOI] [PubMed] [Google Scholar]
- 69. Salomonsson E, Kuoppa K, Forslund AL, Zingmark C, Golovliov I, et al. (2009) Reintroduction of two deleted virulence loci restores full virulence to the live vaccine strain of Francisella tularensis . Infect Immun 77: 3424–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tempel R, Lai XH, Crosa L, Kozlowicz B, Heffron F (2006) Attenuated Francisella novicida transposon mutants protect mice against wild-type challenge. Infect Immun 74: 5095–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. West TE, Pelletier MR, Majure MC, Lembo A, Hajjar AM, et al. (2008) Inhalation of Francisella novicida ΔmglA causes replicative infection that elicits innate and adaptive responses but is not protective against invasive pneumonic tularemia. Microbes Infect 10: 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mohapatra NP, Soni S, Bell BL, Warren R, Ernst RK, et al. (2007) Identification of an orphan response regulator required for the virulence of Francisella spp. and transcription of pathogenicity island genes. Infect Immun 75: 3305–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kadzhaev K, Zingmark C, Golovliov I, Bolanowski M, Shen H, et al. (2009) Identification of genes contributing to the virulence of Francisella tularensis SCHU S4 in a mouse intradermal infection model. PLoS One 4: e5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim TH, Pinkham JT, Heninger SJ, Chalabaev S, Kasper DL (2011) Genetic modification of the O-polysaccharide of Francisella tularensis results in an avirulent live attenuated vaccine. J Infect Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Qin A, Scott DW, Thompson JA, Mann BJ (2009) Identification of an essential Francisella tularensis subsp. tularensis virulence factor. Infect Immun 77: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Corr SC, Gahan CC, Hill C (2008) M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol 52: 2–12. [DOI] [PubMed] [Google Scholar]
- 77. Neutra MR, Mantis NJ, Kraehenbuhl JP (2001) Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol 2: 1004–1009. [DOI] [PubMed] [Google Scholar]