Abstract
While effects of institutional care on behavioral development have been studied extensively, effects on neural systems underlying these socioemotional and attention deficits are only beginning to be examined. The current study assessed electroencephalogram (EEG) power in 18-month-old internationally adopted, post-institutionalized children (n = 37) and comparison groups of non-adopted children (n = 47) and children internationally adopted from foster care (n = 39). For their age, post-institutionalized children had an atypical EEG power distribution, with relative power concentrated in lower frequency bands compared to non-adopted children. Both internationally adopted groups had lower absolute alpha power than non-adopted children. EEG power was not related to growth at adoption or to global cognitive ability. Atypical EEG power distribution at 18 months predicted indiscriminate friendliness and poorer inhibitory control at 36 months. Both post-institutionalized and foster care children were more likely than non-adopted children to exhibit indiscriminate friendliness. Results are consistent with a cortical hypoactivation model of the effects of early deprivation on neural development and provide initial evidence associating this atypical EEG pattern with indiscriminate friendliness. Outcomes observed in the foster care children raise questions about the specificity of institutional rearing as a risk factor and emphasize the need for broader consideration of the effects of early deprivation and disruptions in care.
Keywords: international adoption, institutionalization, EEG, indiscriminate friendliness, foster care
An early history of institutional care is associated with multiple degrees and types of adversity, which can range from prenatal risks (e.g. low birth weight) to postnatal malnutrition, medical problems, insufficient cognitive and social stimulation, and deprivation of consistent and responsive caregiver-child relationships (Gunnar, Bruce, & Grotevant, 2000; Johnson, 2000). Even in institutions providing adequate physical and medical care, caregivers usually rotate frequently and there is typically a high infant to caregiver ratio (Lee, 2000; Vorria et al., 2003; Zeanah et al., 2003). Thus, at a minimum, institutionalized children are deprived of individualized attention and consistent social relatedness from a stable, responsive caregiver, a species-typical social experience that may play a role in neurodevelopment.
The first few years of life are a period of rapid neural development, and the institutional context may shape developing neural circuitry in lasting ways that then influence the child’s functioning long after removal from the institutional environment, a process that some have described as experience-adaptive programming (Rutter, O’Connor, & the ERA Study Team, 2004). The brain may develop in ways that are adaptive within the adverse early rearing context, but due to limited plasticity these neural patterns may carry over to a new environment where they are no longer adaptive, such as an adoptive home (see for review, Marshall & Kenney, 2009). A significant number of children are removed from institutional settings around the world early in life and adopted internationally. Their heterogeneous developmental outcomes following circumscribed periods of early institutional deprivation illustrate both the resilience and the vulnerability of the developing brain.
Behavioral Correlates of Institutional Rearing
Post-institutionalized children typically demonstrate significant, often rapid gains across multiple developmental domains following adoption (Judge, 2004; Morison, Ames, & Chisholm, 1995; Van IJzendoorn & Juffer, 2006). However, those who have spent prolonged periods in institutional care early in life are at risk for enduring socioemotional and behavioral problems (Chisholm, 1998; Fisher, Ames, Chisholm, & Savoie, 1997; Groze & Ileana, 1996; Gunnar, Van Dulmen, & the International Adoption Project Team, 2007; Juffer & Van IJzendoorn, 2005; Judge, 2004; O’Connor, Bredenkamp, Rutter, & the ERA Study Team, 1999; Rutter et al., 2007; Van IJzendoorn & Juffer, 2006; Verhulst, Althaus, & Versluis-den Bieman, 1992; Zeanah et al., 2009). Indiscriminate friendliness, which is characterized by difficulty maintaining social boundaries and may include a lack of wariness toward strangers, a willingness to leave with a stranger, and a disinhibited tendency to socially engage strangers, is a disconcerting and persistent social problem observed in some post-institutionalized children (Chisholm, 1998). High levels of indiscriminate friendliness were present in 69% of children assessed while they were still living in a Romanian institution (Zeanah, Smyke, & Dumitrescu, 2002).
In a significant minority of post-institutionalized children, indiscriminately friendly behavior persists for many years after leaving the institution and being placed in a family (e.g. Bruce, Tarullo, & Gunnar, 2009; Chisholm, 1998; Hodges & Tizard, 1989; O’Connor et al., 1999; Rutter et al., 2004, 2007). Indeed, Rutter et al. (2007) recently reported that at age 11, about two fifths of Romanian post-institutionalized children in the United Kingdom who had been adopted between 6 months and 4 years of age continued to exhibit symptoms of indiscriminate friendliness. While indiscriminate friendliness has been conceptualized as an attachment disorder, several studies suggest that it is unrelated to attachment status (Zeanah et al., 2002) and that it often persists even among those post-institutionalized children who have formed a secure attachment with an adoptive parent (Marvin & O’Connor, 1999).
Emerging evidence suggests that difficulty regulating attention is associated with indiscriminate friendliness and other adverse socioemotional and behavioral outcomes in post-institutionalized children. Post-institutionalized children have a well-established vulnerability to long-term problems with attention regulation and inhibitory control (Gunnar et al., 2007; Hodges & Tizard, 1989; Hoksbergen, Ter Laak, Van Dijkum, Rijk, & Stoutjesdijk, 2003; Kadlec & Cermak, 2002; Kreppner et al., 2001; Lin, 2003; Roy, Rutter, & Pickles, 2000, 2004; Zeanah et al., 2009). Among children with a history of institutional rearing, those who are inattentive and hyperactive are more likely to demonstrate disinhibited social behaviors (Roy et al., 2004). Bruce et al. (2009) reported that indiscriminate friendliness correlated with poorer attention regulation and inhibitory control, but not with attachment-related behaviors. Indiscriminate friendliness may reflect insensitivity to social cues, and children with poorer regulatory abilities may be more prone to miss social cues and engage in disinhibited behaviors that violate social boundaries.
Neural Correlates of Institutional Rearing
The persistence of socioemotional and attention deficits years after adoption suggests a role for early social experience in the development of neural circuitry relevant to socioemotional behavior regulation, attention regulation, and inhibitory control. However, while effects of institutional care on behavioral development have been studied extensively, effects on neural systems underlying these deficits are only beginning to be examined. Chugani et al. (2001) took a first step toward addressing this gap in the literature with their study of the neural metabolism of post-institutionalized Romanian children. Using positron emission tomography (PET), they observed significantly reduced glucose metabolism in prefrontal and temporal structures, areas of the brain implicated in emotion regulation, inhibitory control, and higher-level attention processes. The same research group recently published a study documenting white matter structural abnormalities in post-institutionalized Eastern European children (Eluvathingal et al., 2006). Diffusion tensor imaging tractography (DTI) revealed that the white matter tract connecting anterior temporal and frontal regions was underdeveloped. Abnormalities of this pathway have been associated with executive function problems (Nakamura et al., 2005; Nestor et al., 2004), which is noteworthy in light of the behavior problems and high levels of impulsivity that characterized the post-institutionalized group reported by Eluvathingal et al.
In a series of eletrophysiological studies, researchers involved in the Bucharest Early Intervention Project assessed a large sample of young, currently institutionalized Romanian children (Marshall, Fox, & the Bucharest Early Intervention Project Core Group, 2004; Marshall, Reeb, Fox, Nelson, & Zeanah, 2008; Moulson, Fox, Zeanah, & Nelson, 2009; Moulson, Westerland, Fox, Zeanah, & Nelson, 2009; Parker, Nelson, & the Bucharest Early Intervention Project Core Group, 2005). When presented with facial recognition (Moulson, Westerland, et al., 2009) and facial emotion processing tasks (Moulson, Fox, et al., 2009; Parker et al., 2005), institutionalized children had smaller amplitudes in several components of the event related potential (ERP) response, indicating pervasive cortical hypoactivation in response to faces (Moulson, Westerland, et al., 2009).
Marshall et al. (2004) recorded baseline EEG from the same institutionalized sample, who were between five and 31 months of age at assessment. The institutionalized children showed a greater concentration of EEG power in lower frequencies (theta) compared to Romanian children reared in their birth families. This difference in relative power distribution was widespread, occurring bilaterally in frontal, parietal, and occipital scalp regions. In family-reared, typically developing children, the relative concentration of power in low frequencies decreases with age, a developmental trend which may derive from normative neurodevelopmental changes such as neuronal growth and myelination (John, Ahn, Prichep, Trepetin, Brown, & Kaye, 1980; Marshall, Bar-Haim, & Fox, 2002; Matousek & Petersen, 1973). The power distribution in institutionalized children may signal either damage to or delay in normal brain development (Marshall et al., 2004). Power has different functional correlates depending on the frequency bands. Power in higher frequencies indicates faster processing and a more alert state. Thus, the concentration of power in lower frequencies for the institutionalized children can be interpreted as hypoactivation (Marshall et al., 2004). Given the normative developmental changes in power distributions, an excess of low frequency power compared to age-matched non-adopted peers can also be seen as suggestive of delayed maturation.
The neurophysiological processes through which a dearth in environmental stimulation would lead to cortical hypoactivation have not been determined (Moulson, Fox, et al., 2009). However, there is little reason to expect that deficits in stimulation are limited to early institutional rearing. Indeed, several studies have reported that children in Central and South America who experienced extreme poverty and other psychosocial risk factors early in life had relative power concentrated in lower frequencies compared to middle class children (Harmony, Alvarez, Pascual, Ramos, Marosi, & Diaz de Leon, 1988; Harmony, Marcosi, Diaz de Leon, Becker, & Fernandez, 1990; Otero, 1994; Otero, 1997; Otero, Pliego-Rivero, Fernandez, & Ricardo, 2003). To assess further whether cortical hypoactivation is associated specifically with institutional rearing or extends to other populations that experience early deprivation and disruption in care, the current study includes a comparison group of children internationally adopted from foster care settings.
A randomly selected subset of the children from Marshall et al.’s sample were removed from the institutional environment and placed in therapeutic foster homes between 7 and 33 months of age (mean 23 months; Marshall et al., 2008). Because this was a planned intervention, these foster homes had a number of qualities that were not necessarily present in the foster care experienced by the children in the current study. Specifically, the therapeutic foster parents in the Bucharest Early Intervention Program were trained to respond sensitively and responsively to the children, licensed, paid equitable salaries, and supported by frequent contact with social workers (Zeanah et al., 2003; 2006). In a follow-up at 42 months of age, the children who had been in foster care for at least 24 months showed a concentration of power in higher frequencies (specifically, relative alpha power) compared to children who were still institutionalized (Marshall et al., 2008), suggesting some mitigating effects of the foster care intervention on patterns of cortical hypoactivation. However, children who had been in foster care for less than 24 months did not differ from the currently institutionalized group (Marshall et al., 2008), indicating that the hypoactivation pattern persists for some time following removal from the institutional environment.
The hypoactivation pattern observed by Marshall et al. (2004) in institutionalized children mirrors the characteristic distribution seen in children with attention and behavior problems. The association of elevated relative theta power with attention problems is extensively documented, with a literature that spans over 70 years (e.g. Jasper, Solomon, & Bradley, 1938; see for review, Barry, Clarke, & Johnstone, 2003). This pattern is particularly pronounced in children who have symptoms of both hyperactive-impulsive behavior and inattention. A causal relationship between elevated theta power and attention problems has been suggested, such that Attention Deficit Hyperactivity Disorder (ADHD) results from cortical hypoactivation (e.g. Satterfield, Cantwell, & Satterfield, 1974). Some authors have referred to this model as the hypoarousal model (Barry, Clarke, & Johnstone, 2003). Atypical power distribution reliably differentiates children with attention problems from typically developing children (Barry et al., 2003; Chabot & Serfontein, 1996; Mann, Lubar, Zimmerman, Miller, & Muenchen, 1992). The persistence of an atypical power distribution for at least two years following removal from the institutional setting (Marshall et al., 2008) could suggest a neural basis for the enduring attention problems and behavior disturbances observed in some post-institutionalized children.
If these neural abnormalities were to persist across development, that finding would lend support to the sensitive period model, and indeed would suggest that there may be a sensitive period for the aspects of neural development underlying typical developmental changes in EEG power distribution, such that plasticity begins to decline within the first few years of life. Another potential explanation of the data is the maturational lag model, which has been proposed as one possibility to explain this pattern of atypical EEG power distribution, both in the ADHD literature (see for review, Barry et al., 2003) and by Marshall et al. (2004) with regard to their institutionalized sample. The maturational lag model posits that an atypical power distribution does not reflect brain damage or a permanent deficit in functioning, but rather a delay in the normal developmental progression to increased concentration of EEG power in higher frequencies. If this hypothesis is correct, electrophysiological abnormalities should fade over time. Evidence so far is mixed. A longitudinal study of impoverished children who were experiencing ongoing environmental deprivation found that abnormalities in frontal power distribution became less pronounced from the toddler years to age 6, although they remained significant (Otero et al., 2003). In typical development, ERP components show an age-related decrease in amplitude and latency from infancy to early childhood (Nelson & Luciana, 1998), but currently institutionalized children do not show this pattern (Parker et al., 2005). If the post-institutionalized children in the current study do not show an atypical power distribution a few months after adoption, that would tend to support a maturational lag model (although further studies would be needed assessing the same children both before and after adoption). If, on the other hand, the post-institutionalized children do show the atypical power distribution, that could reflect either a longer maturational lag or a sensitive period. The maturational lag model is difficult to conclusively reject, as the rate of catch-up is not specified.
To further investigate the impact of early social deprivation on brain development, the aims of the current study were (1) to ascertain if the pattern of elevated relative theta power and lower absolute alpha power that has been observed in currently institutionalized children (Marshall et al., 2004) would extend to internationally adopted post-institutionalized children and (2) to determine if EEG power distribution would predict the behavioral outcomes of indiscriminate friendliness and inhibitory control deficits. Baseline EEG was recorded from 18-month-old internationally adopted post-institutionalized children and relative and absolute power were assessed. It was expected that EEG power would be concentrated in lower frequency bands for the post-institutionalized children compared to non-adopted children reared with their birth parents. Following the methods of prior developmental studies of EEG (Clarke, Barry, McCarthy, & Selikowitz, 2001a; Marshall et al., 2004; Somsen, van’t Klooster, van der Molen, van Leeuwen, & Licht, 1997), both relative and absolute EEG power were assessed. Relative power, which quantifies the proportion of total power in a given frequency band, has the advantage of not being biased by individual anatomical factors that affect absolute power, such as skull thickness; however, absolute power can assist in the interpretation of observed relative power differences between groups (Marshall et al., 2004). An additional comparison group of children internationally adopted from foster care was included to help disentangle the effects of institutional rearing in particular from more general effects of abandonment and early care disruptions. At 36 months of age, children participated in a follow-up visit to assess indiscriminate friendliness and inhibitory control. Measures of growth at the time of adoption and nonverbal cognitive ability at 18 months were obtained to rule out malnutrition and global cognitive delays as third variables that could underlie an association between EEG power at 18 months and behavioral outcomes at 36 months. It was hypothesized that a concentration of EEG power in lower frequency bands at 18 months would predict greater indiscriminate friendliness and lower scores on inhibitory control at 36 months.
Method
Participants
The participants were 143 infants who were 18 to 20 months of age when first assessed, most of whom met criteria for one of three groups. The non-adopted group (n = 47; 39 female) were born and raised in their families in the United States. The adopted groups differed in terms of age at adoption and time spent in institutional or family care (see Table 1). The post-institutionalized group (n = 37; 33 female) had spent at least 75% of their lives prior to adoption in institutions and no more than 2 months in family based care, and at the time of adoption were at least 10 months old and less than 18 months old; the foster care group (n = 39; 15 female) had spent at least 75% of their lives prior to adoption in a family-based setting (e.g. foster care or relative care) and no more than 2 months in institutional care, and at the time of adoption were less than 18 months old. Eight adopted children (6 female) did not meet criteria for any of the groups because they had received a mix of family-based care and institutional care or because care history was unknown, so that neither type of care was known to account for 75% of their preadoption care history. Another 12 children (4 female) had spent at least 75% of their lives prior to adoption in institutions and no more than 2 months in family based care, but were excluded from the post-institutionalized group because they were under 10 months old at the time of adoption. These 20 children were not included in analyses of group differences, but were included in analyses of the total sample to more accurately reflect the diverse care histories of internationally adopted children. Four children (2 post-institutionalized, 1 foster care, one non-adopted) were later diagnosed with a medical problem related to prenatal development (e.g. Fetal Alcohol Spectrum Disorder) or a genetic abnormality. These 4 children were excluded from all analyses. All of the adopted children were born outside of the United States and were adopted by families living in the Midwestern United States.
Table 1.
Adoption group characteristics
| A. Country of origin | ||||
|---|---|---|---|---|
| Country | Post- Institutionalized† N=37 |
Foster Care† N=39 |
Early-Adopted Post- Institutionalized N=12 |
Combination of Care Type N=8 |
| N (% of group) | N (% of group) | N (% of group) | N (% of group) | |
| China | 26 (70.3) | 5 (12.8) | 0 (0) | 1 (12.5) |
| Russia | 7 (19.4) | 0 (0) | 1 (8.3) | 0 (0) |
| Ukraine | 2 (5.4) | 0 (0) | 0 (0) | 0 (0) |
| Guatemala | 1 (2.7) | 5 (12.8) | 0 (0) | 1 (12.5) |
| Korea | 0 (0) | 27 (69.2) | 0 (0) | 3 (37.5) |
| Columbia | 0 (0) | 0 (0) | 9 (75.0) | 1 (12.5) |
| Ethiopia | 0 (0) | 0 (0) | 1 (8.3) | 1 (12.5) |
| Nepal | 0 (0) | 0 (0) | 1 (8.3) | 1 (12.5) |
| Unknown | 1 (2.7) | 2 (5.1) | 0 (0) | 0 (0) |
| B. Age at adoption, history of care, and sex | ||||
|---|---|---|---|---|
| Post- Institutionalized† |
Foster Care† | Early-Adopted Post- Institutionalized |
Combination of Care Type |
|
| M (SD; Range) | M (SD; Range) | M (SD; Range) | M (SD; Range) | |
| Age at Adoption in Months*** | 12.0 (1.9; 10–17)a | 7.6 (3.1; 4–15)b | 3.5 (1.4; 2–7) c | 8.8 (3.7; 5–15) d |
| Months in Institutional Care*** | 11.5 (1.7; 10–16) a | 0.5 (0.5; 0–1) b | 3.5 (1.4; 2–7) c | 3.8 (2.0; 2–8) c |
| Months in Family Care Pre-adoption*** | 0.3 (0.9; 0–4) a | 7.0 (3.3; 3–15)b | 0 (0; 0–0) a | 4.3 (2.2; 2–7) b |
| Percent Female*** †† | 89.2% a | 38.5% b | 33.3% b | 75.0% a |
These two groups were included in group analyses, along with the non-adopted group. Early-Adopted Post-Institutionalized and Combination of Care were excluded from group analyses due to small sample size, but were included in analyses of the full sample to better represent the diverse care histories of adopted children.
The non-adopted group was 83.0% female and did not differ from the Post-Institutionalized or Combination of Care groups in sex ratio.
p < .001
The adopted groups differed in terms of country of origin (see Table 1) because at the time these children were born, their countries of origin generally had either a foster care system or an institutional system in place to care for wards of the state. As a result, as Table 1 shows, there was very little overlap in nationality across the adopted groups. Type of care was largely determined by which system the child’s country of origin had in place, rather than any factors specific to the child. The post-institutionalized and foster care groups also differed significantly from each other in age at adoption (see Table 1), because countries using foster care tend to have procedures in place that permit international adoption when children are younger (Gunnar et al., 2000). Thus, age at adoption needed to be considered in the analyses. The non-adopted children were predominantly female, to approximate the gender ratio in the post-institutionalized group. However, the foster care group did not have this gender ratio because every available and willing participant who met criteria for this group was included in the study.
Procedures
The children in the internationally adopted groups were recruited from a registry which includes over 3,000 internationally adopted children. Children were included on the registry after their parents returned a postcard expressing a willingness to participate in research. Parents who adopted from two large adoption agencies handling international adoption were sent a letter soliciting participation in this registry. The non-adopted children were recruited from a department-maintained participant list of children whose parents indicated an interest in research. Parents of all children born in the metropolitan area were solicited for this department-maintained list through a mailing received soon after the child’s birth. When children were 18–20 months of age, they participated in a laboratory session that lasted approximately 60 to 90 minutes. When children were 36–38 months of age, their parents were contacted and invited to bring the children back for a 90 minute follow-up laboratory session. Both studies were approved by the university institutional review board, and parents gave informed consent prior to each study. Parents were present for the entire session and were advised that they were free to end the session at any time and to decline any aspect of the protocol. At each laboratory visit, parents were given a $5 gift card as a token of appreciation for their participation. Children received a small toy at the 18 month visit and a prize bag of small toys and stickers at the 36 month visit.
Measures
General deprivation
To assess the duration and degree of deprivation encountered prior to adoption, parents of the internationally adopted children completed a questionnaire during the 18-month laboratory session about their children’s early care experiences. The length of time in any type of institutional care (e.g. hospital, baby home, orphanage) was used to index the duration of the institutional care. Two risk indices were created following the methods of Bruce et al. (2009). Parents reported on prenatal risks they knew or suspected their child to have experienced, including prenatal exposure to alcohol or drugs, prenatal malnourishment, and premature birth. These three prenatal risk factors correlated significantly and were summed to create a prenatal risk index (range 0–3). Similarly, parents reported on early care risks they knew or suspected their child to have experienced, including physical abuse, physical neglect, social neglect, three or more living arrangements prior to adoption, and belonging to a minority group discriminated against in the child’s country of origin. These 5 early care risk factors correlated significantly and were summed to create an early care risk index (possible range 0–5, actual range 0–3). These indices are imperfect measures, as they come from adoptive parents who may or may not have access to accurate information about their children’s preadoption history.
As an additional indirect measure of general deprivation prior to adoption, parents reported their children’s height, weight, and head circumference at their first post-adoption doctor’s visit. These growth measurements were indexed to World Health Organization (WHO) growth norms, yielding z scores for height, weight, and head circumference. For each measure, children one or more standard deviations below WHO norms were compared to the rest of the sample.
Electrophysiological recording
At the 18-month visit, electroencephalogram activity (EEG) was recorded from 16 sites: frontal, central, temporal, parietal, and occipital scalp regions (fp1, fp2, fz, f3, f4, f7, f8, cz, c3, c4, t3, t4, p3, p4, o1, and o2). All sites were referenced to the vertex (cz) during data collection and later re-referenced to an average reference. Baseline EEG was recorded for 6 minutes while the child was seated in a high chair next to the parent. To keep the child quiet and focused with reduced motor activity, the experimenter blew bubbles, showed the child a rotating Ferris wheel toy, and performed silent puppet shows. The EEG was recorded by placing a stretch Lycra cap on the child’s head which contained electrodes in the 10/20 placement system pattern. Small amounts of abrasive and conducting gels were inserted into the electrode sites of interest, and a blunt wooden end of a cotton swab was used to gently abrade the scalp. Electrode impedances were measured and accepted if below 5K ohms. Additional abrading was sometimes necessary if impedances were above 5K ohms. Amplifiers were set so that output of the signal was 50 uV peak to peak, with a gain of 10,000. The signal was digitized at 512 samples per second to prevent aliasing from affecting the data. Data was collected using the continuous recording mode of ERP-W, re-referenced to an average reference, and exported for analysis in software by James Long Company. The EEG data were then scored for artifact due to eye movement or motor activity. Epochs containing amplitudes larger than 175 uV were considered to reflect motor artifact and eliminated from analysis. Eye blinks were identified in the fp1 channel, which is sensitive to eye movement, and these epochs were also eliminated from analysis. The data were analyzed with a Discrete Fourier Transform (DFT) using a Hanning window of 1 s with a 50% overlap. The mean number of usable overlapping epochs was 348.66 (SD 151.74, range 85–689) out of a possible 720. Absolute power was computed for the 3–5 hz (theta), 6–9 hz (alpha), and 10–18 hz (beta) bands, and expressed in microvolts squared.
EEG data then were exported for statistical analysis. To normalize the distribution, a natural log (ln) transformation was used. Relative power in each band was computed for each electrode site as the ratio of the absolute power in that band to the sum of 3–5 hz, 6–9 hz, and 10–18 hz absolute power. Mean relative and absolute power in each band was computed as the arithmetic average of relative or absolute power in that band at each EEG collection site. EEG data were analyzed for 119 children (83.2%). Missing data was due to parental or child refusal of EEG collection, technical difficulties, or data that could not be analyzed due to excessive artifact. There were no group differences in the likelihood of having usable EEG data.
Nonverbal cognitive ability
At the 18-month visit, the visual reception subscale of the Mullen Scales of Early Learning was used to assess nonverbal cognitive ability (Table 2). A nonverbal measure was selected so as not to be confounded by duration of exposure to the English language. The Mullen, which is widely used to screen for developmental difficulties (Bradley-Johnson, 2001), yields standardized T-scores calculated from age-based norms.
Table 2.
Descriptive statistics for behavioral measures in the post-institutionalized, foster care, and non-adopted groups
| Measure | Post-Institutionalized Group |
Foster Care Group | Non-Adopted Group |
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| Mullen Visual Reception Subscale T-score at 18 months*** | 41.00(6.90) a | 48.24(8.35) b | 50.00(10.13) b |
| Dinky Toys | |||
| Composite | −0.06 (.59) | −0.17 (.67) | 0.14 (0.86) |
| Latency to first transgression (secs) * | 10.71 (8.07) | 9.19 (9.41) a | 15.62 (10.53) b |
| Frequency of transgressions | 1.16 (0.85) | 1.48 (0.96) | 1.33 (2.00) |
| % exhibiting worst transgression (grab) | 38.90% | 34.47% | 38.23% |
| Gift Task | |||
| Composite | .10 (.83; −1.11–1.44) | −.18 (.87; −1.61–1.44) | .01 (.87; −1.24–1.44) |
| Latency to first transgression (secs) | 21.61 (23.04) | 20.50 (22.64) | 19.78 (22.95) |
| Frequency of transgressions | 1.94 (1.63) | 3.00 (2.33) | 2.33 (1.98) |
| % exhibiting worst transgression (turns body) | 44.40% | 59.10% | 44.40% |
| DSA Task | |||
| Number of boundary violations (personal questions/comments to stranger, initiates physical contact with stranger)* | 0.54 (1.21) a | 0.42 (0.83) | 0.00 (0.00) b |
| Latency to first verbal initiation to stranger (secs) | 191.15 (193.75) | 211.25 (186.56) | 224.57 (182.36) |
| Frequency of verbal initiations to stranger (in first 5 mins) | 8.23 (8.85) | 4.78 (6.28) | 5.54 (8.09) |
p < .05
p = .001
Indiscriminately friendly behavior
At the 36-month visit, parent and child participated in an observational measure to assess children’s tendency to engage in indiscriminately friendly behavior with unfamiliar adults (Bruce et al., 2009; adapted from Tizard & Rees, 1975). The child was provided with a picture book and the parent was seated on the opposite side of the room with some paperwork. An unfamiliar female adult (i.e. stranger) then entered the room and introduced herself. The unfamiliar adult sat quietly in the corner of the room for 1 minute before inviting the child to play with some toys. After providing the toys, the stranger sat quietly for 4 minutes. During this time, the stranger responded briefly to any initiations made by the child but did not act to maintain the interactions. After this period, the stranger invited the child to play with her and proceeded to engage with the child for 4 minutes in a more typical fashion. Each interaction was videotaped and coded by a trained coder. The coder was not informed of group membership, but the child’s racial characteristics relative to their parents’ (all of whom were Caucasian) likely provided information about adoption status in some cases. Descriptive statistics for all variables from this task are shown in Table 2. Although coders recorded latency to the child’s first verbal initiation and frequency of verbal initiations to the unfamiliar adult, these measures were not included in the indiscriminate friendliness measure due to concerns that they would tap the temperamental construct of behavioral inhibition and identify children who were simply temperamentally exuberant. Instead, we constructed the indiscriminate friendliness score based on behaviors that were clearly developmentally inappropriate for this age group and tapped the underlying construct of violation of social boundaries. These behaviors included asking a personal question or making a personal comment, or initiating non-incidental physical contact, all of which reflect symptom criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR, 2000) for Reactive Attachment Disorder, Disinhibited Type. As these behaviors were relatively low frequency, each child received a dichotomous score (0, 1) for whether they engaged in any indiscriminately friendly behaviors toward the unfamiliar adult, including asking a personal question or making a personal comment (n=6), or initiating non-incidental physical contact (n=11). This dichotomous score was the measure of indiscriminate friendliness used in subsequent analyses. It should be noted that among children who exhibited indiscriminately friendly behaviors, the number of incidences ranged from 1 to 6. Interrater reliability was calculated on 20% of the videotapes. Cohen’s k coefficients were 1.00 for indiscriminately friendly behaviors and .94 for verbal initiations.
Delay of gratification
Two delay of gratification tasks were included in the 36-month assessment, the dinky toys task and the gift task (Kochanska, Murray, & Coy, 1997). For the dinky toys task, the child was asked to select a toy from a box full of prizes by verbally indicating the selection, without touching or pointing to the prize. The task was videotaped and coded for worst transgression (ranging from no transgression to pointing at, touching, or grabbing a toy), latency to first transgression, and frequency of transgressions. If there were no transgressions, the latency score was the total length of the task (60 seconds). The dinky toys task was presented three times during the session and the codes were averaged across the three trials. For the gift task, the child was instructed not to peek while a prize was being wrapped. It, too, was coded for worst transgression (no transgression, peeking by turning head only, or peeking by turning body), latency to the first transgression, and frequency of transgressions (Table 2). Interrater reliability between two trained coders was computed for 20% of the videotapes. Cohen’s k coefficients ranged from .81 to .92. Latency, worst transgression, and frequency of transgression within each task were highly correlated (r = .51–.85, p < .001), and were standardized and averaged. The composite measures from the dinky toy and gift tasks were significantly correlated, r = .35, p = .001, and were averaged to yield one composite measure of delay of gratification.
Analysis Plan
Following the methods of Marshall et al. (2004), repeated-measures analyses of variance were used to examine group differences in EEG absolute power and relative power. To parallel the Marshall et al. study, which compared currently institutionalized and non-adopted children, analyses compared the post-institutionalized and non-adopted children. Wherever there were significant group differences, the foster care group was then added to the model to assess whether the group difference could be specifically attributed to a history of institutional rearing. Given the variable gender ratios across groups, gender was considered as a potential covariate. Children who did not have any usable EEG data were excluded from analyses. Missing data for individual EEG channels was imputed via the single-imputation method in SPSS, drawing on the covariance matrix of all available data. The percentage of data imputed for each channel was: fp1: 5.0%, fp2: 13.4%, fz: 1.7%, f3: 2.5%, f4: 1.7%, f7: 1.7%, f8: 1.7%, c3: 2.5%, c4: 2.5%, t3: 4.2%, t4: 3.4%, p3: 7.6%, p4: 4.2%, o1: 3.4%, o2: 5.9%. There were 100 children with EEG data who met criteria for a group (30 post-institutionalized, 29 foster care, and 41 non-adopted children). A repeated-measures ANOVA with relative 3–5 hz (theta) power as the dependent variable, hemisphere and region as the within-subjects factors, and group as the between-subjects factor was run to assess the primary hypothesis that relative theta power would be elevated in the post-institutionalized group. To assess the specificity of this EEG difference and to aid in interpretation of this result, additional repeated-measures ANOVAs of absolute and relative power were run separately for each frequency band: 3–5 hz (theta), 6–9 hz (alpha), and 10–18 hz (beta). The frequency bands were defined based on guidelines established in research with typically developing infants of this age range (Marshall et al., 2002). For each ANOVA, group (post-institutionalized vs. non-adopted) was the between-subjects factor, and the within-subject factors were hemisphere (left, right) and scalp region (frontal, central, parietal, occipital, temporal). Where there were group differences, the analysis was expanded to include the foster care group, with follow-up analyses employing Bonferroni corrections. Main effects of group and interactions of group with hemisphere and scalp region were examined. For EEG measures on which the post-institutionalized children differed from non-adopted children, associations with general deprivation variables explored the influence of duration and severity of deprivation. Specifically, for all the adopted children (including those who did not meet criteria for any group), mean absolute or relative power was regressed on age at adoption and the prenatal and early care risk indices. For all the adopted children, t-tests compared mean absolute or relative power in children one or more standard deviations below WHO means for height, weight, and head circumference with children who were average or above average on these growth measures. To assess whether EEG measures related to general cognitive ability, mean absolute and relative power were correlated with nonverbal cognitive ability (the Mullen visual reception subscale) for all children (including those who did not meet criteria for any group).
Group differences in indiscriminately friendly behavior and delay of gratification were examined using chi-square and t-tests. For all children, repeated-measures ANOVAs were conducted in each band with the absolute and relative power measures that showed significant group differences at 18 months as the dependent variables, hemisphere and region as the within-subjects factors, and indiscriminately friendly behavior (presence vs. absence) as the between-subjects factor. Similarly, for all children, repeated-measures ANOVAs of these same absolute and relative power measures were conducted with a median split of delay of gratification as the between-subjects factor. Associations of indiscriminately friendly behavior with general deprivation variables and nonverbal cognitive ability also were examined for all children.
Results
Gender
For the total sample, there were no gender differences in mean absolute or relative EEG power in any frequency band; in the prenatal or early care risk indices; or on any of the behavioral measures. Thus, gender was not included as a covariate in subsequent analyses.
Group Differences in Absolute and Relative Power
Descriptive data for mean EEG power by group and frequency are shown in Table 3.
Table 3.
Mean relative and absolute power by frequency band in the post-institutionalized, foster care, and non-adopted groups at 18 months of age
| Measure | Post-Institutionalized Group n = 30 M(SD) |
Foster Care Group n = 29 M(SD) |
Non-Adopted Group n = 41 M(SD) |
|---|---|---|---|
| Relative Theta | 0.442 (0.050)* | 0.437 (0.032) | 0.422 (0.027) |
| Relative Alpha | 0.340 (0.023) | 0.345 (0.019) | 0.349 (0.020) |
| Relative Beta | 0.215 (0.051) | 0.216 (0.032) | 0.225 (0.025) |
| Absolute Theta | 3.94 (0.59) | 3.81 (0.40) | 4.05 (0.33) |
| Absolute Alpha | 3.13 (0.63)* | 3.06 (0.50)* | 3.40 (0.43) |
| Absolute Beta | 2.08 (0.66) | 1.97 (0.51) | 2.23 (0.33) |
Relative power expressed as a proportion of total power. Absolute power expressed in microvolts squared.
Mean difference from non-adopted group, p < .05
Relative theta power
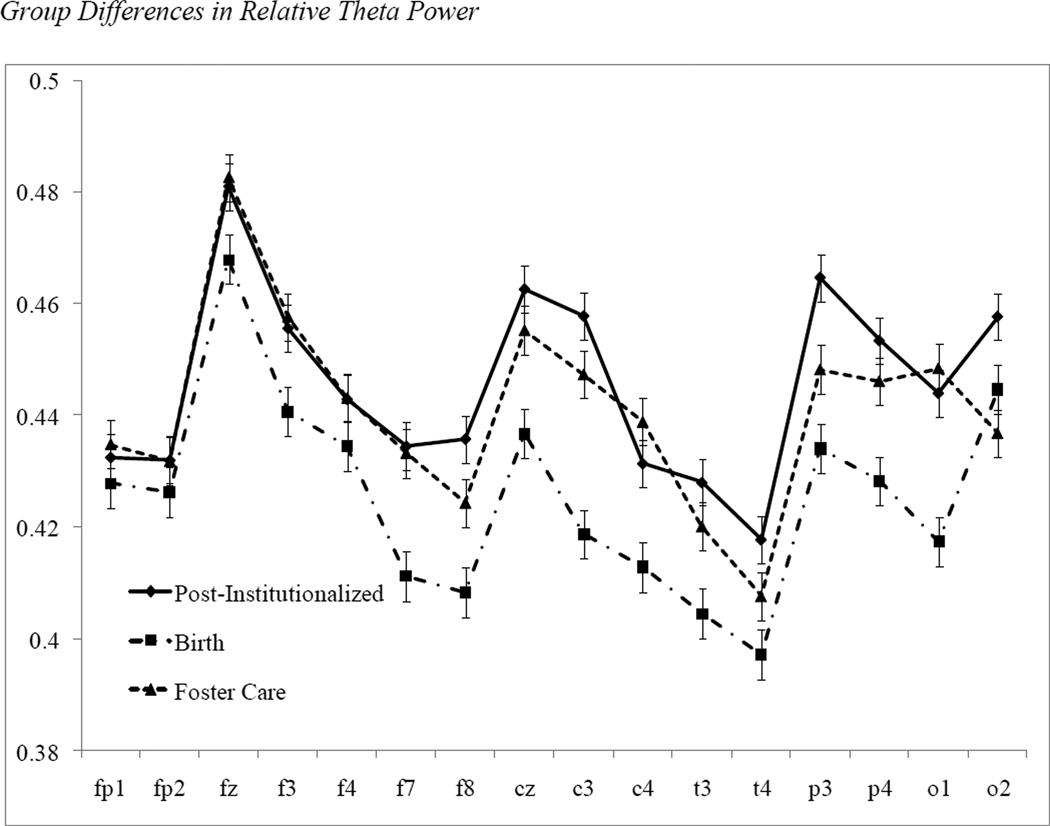
There was a main effect for group in the repeated-measures ANOVA for relative theta power, F(1, 69) = 5.14, p < .05, partial η2 = .069, with the post-institutionalized children having higher relative theta power than the non-adopted children. When the foster care children were added to the analysis, the main effect for group remained significant, F(2, 97) = 3.33, p < .05, partial η2 = 0.064, but Bonferroni follow-up analyses indicated that only the post-institutionalized and non-adopted children differed significantly from each other, with the mean relative theta power for the foster care children intermediate to the other two groups. There were no significant interactions of hemisphere or scalp region with group. The means by group for relative theta power are depicted in Figure 1.
Figure 1.
Relative theta power in the post-institutionalized, foster care, and non-adopted groups. Frontal, central, temporal, parietal, and occipital electrode sites are on the x axis. The measure on the y axis is theta power as a proportion of the total power across all frequency bands.
Absolute theta power
There were no group differences in absolute theta power, F(1, 69) = 0.94, ns, and no significant interactions.
Relative alpha power
There were no group differences in relative alpha power, F(1, 69) = 2.86, ns. Within-subjects contrasts with Greenhouse-Geisser correction did reveal a significant Hemisphere × Scalp Region × Group interaction, F(2.67, 184.48) = 2.98, p < .05, partial η2 = .041, which persisted when foster care children were added to the analysis. Follow-up analyses indicated that there was a significant Hemisphere × Scalp Region interaction in the non-adopted group only, F(2.26, 90.41) = 5.82, p < .01. ANOVAs conducted in each scalp region separately indicated that in the frontal region, there was greater relative alpha power in the left hemisphere for the non-adopted group, F(2, 39) = 28.06, p < .001. For the other regions, there was no hemispheric difference in relative alpha power.
Absolute alpha power
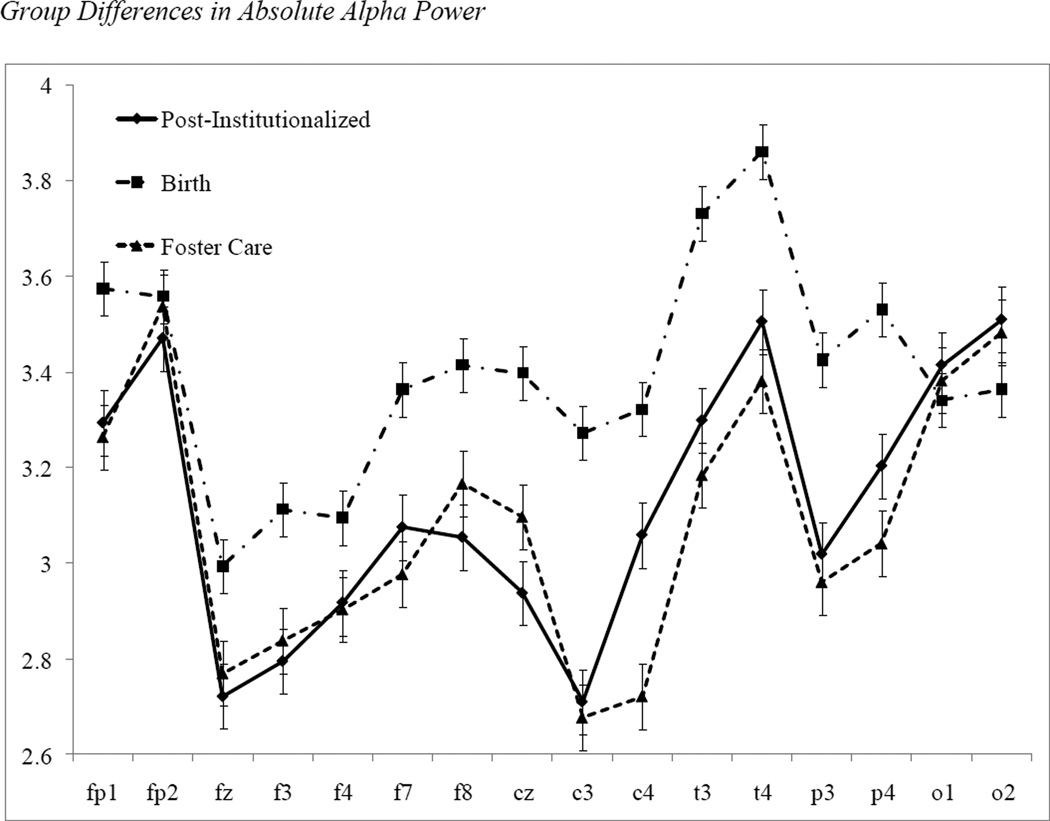
In the repeated-measures ANOVA for absolute alpha power, there was again a main effect for group, F(1, 69) = 4.65, p < .05, partial η2 = .063, with the post-institutionalized children having lower absolute alpha power than the non-adopted children. A within-subjects test with Greenhouse-Geisser correction indicated a Scalp Region × Group interaction, F(2.59, 178.95) = 2.94, p < .05, partial η2 = .041. When the foster care children were included in the repeated-measure analysis of absolute alpha power, the main effect for group remained significant, F(2, 97) = 4.36, p < .05, partial η2 = .083. Follow-up analysis with Bonferroni correction indicated that the foster care group was significantly lower in absolute alpha power than the non-adopted children. The Scalp Region × Group interaction persisted, F(5.89, 285.43) = 3.15, p < .01, partial η2 = .061. Follow-up repeated measures ANOVAs conducted within each scalp region separately indicated a significant group difference within the temporal scalp region, F(4, 192) = 4.31, p < .01, partial η2 = .082, with Bonferroni tests showing that the non-adopted children had higher bilateral temporal alpha power than both the post-institutionalized and foster care children. There was also a main effect of group for the central scalp region, F(4, 192) = 6.65, p < .001, partial η2 = .122, with Bonferroni tests indicating that the non-adopted children had higher bilateral central power than the foster care children and higher left central power than the post-institutionalized children. The parietal scalp region showed the same pattern, with a significant group difference, F(4, 192) = 2.92, p <.05, partial η2 = .057, explained by the non-adopted children having higher bilateral parietal power than the foster care children and higher left parietal power than the post-institutionalized children. There were no group differences in the frontal or occipital scalp regions in absolute alpha power. Figure 2 illustrates group differences in absolute alpha power.
Figure 2.
Absolute alpha power in the post-institutionalized, foster care, and non-adopted groups. Frontal, central, temporal, parietal, and occipital electrode sites are on the x axis. The measure on the y axis is the log transformation of absolute alpha power in microvolts squared.
Relative beta power
There was no main effect for group in relative beta power, F(1, 69) = 1.23, ns. Within-subjects contrasts with Greenhouse-Geisser correction did reveal a significant Scalp Region × Group interaction, F(2.80, 193.35) = 2.95, p < .05, partial η2 = .041, which persisted when the foster care children were included in the analysis. To explore the Scalp Region × Group interaction, follow-up repeated measures ANOVAs were conducted within each scalp region. There was a group difference in the central scalp region, F(4, 192) = 3.44, p = .01, partial η2 = .067. In Bonferroni post-hoc analyses, the non-adopted children had significantly higher left central relative beta power compared to post-institutionalized children. There were no other regional group differences.
Absolute beta power
There was no main effect for group in absolute beta power, F(1, 69) = 1.44, ns. However, Greenhouse-Geisser-corrected within-subjects tests identified a significant Scalp Region × Group interaction for absolute beta power, F(2.68, 184.92) = 3.70, p < .05, partial η2 = .051, which again persisted when foster care children were included in the analysis. Follow-up repeated measures ANOVAs within each scalp region separately revealed a significant group difference in the central scalp region, F(4, 192) = 5.32, p < .001, partial η2 = .100, which Bonferroni-corrected follow-up analyses indicated was due to greater bilateral central beta power in the non-adopted children compared to the foster care children. Similarly, the non-adopted children had greater bilateral parietal beta power than the foster care children, F(4, 192) = 2.57, p < .05, partial η2 = .051. The non-adopted children also had higher left temporal beta power than both the foster care and post-institutionalized children, F(4, 192) = 2.93, p < .05, partial η2 = .058. There were no significant group differences in the frontal or occipital scalp regions.
The pattern of results across frequency bands is summarized in Table 4.
Table 4.
Group differences in EEG power: Main effects and interactions
| Measure | Main Effect | Scalp Region × Group |
Scalp Region × Hemisphere × Group |
|---|---|---|---|
| Relative Theta | PI > NA | -- | -- |
| Relative Alpha | -- | -- | X |
| Relative Beta | -- | C3: NA > PI | -- |
| Absolute Theta | -- | -- | -- |
| Absolute Alpha | NA > PI, FC | C3: NA > PI, FC | -- |
| C4: NA > FC | |||
| T3: NA > PI, FC | |||
| T4: NA > PI, FC | |||
| P3: NA > PI, FC | |||
| P4: NA > FC | |||
| Absolute Beta | -- | C3: NA > FC | -- |
| C4: NA > FC | |||
| T3: NA > PI, FC | |||
| P3: NA > FC | |||
| P4: NA > FC |
PI = post-institutionalized; FC = foster care; NA = non-adopted
General Deprivation and Relative Theta and Absolute Alpha Power
Mean relative theta power was regressed on age at adoption and the prenatal and early care risk indices. This model was not significant, F(3, 70) = 0.07, ns. This analysis was repeated for mean absolute alpha power, and again the model was not significant, F(3, 70) = .08, ns. Being one or more standard deviations below WHO 2007 means for height, weight, or head circumference at first post-adoption doctor’s visit was not associated with relative theta power or with absolute alpha power. It should be noted that the adopted children were quite close to WHO growth norms at first post-adoption doctor’s visit: median z scores were −0.13 for weight, −0.12 for height, and 0.07 for head circumference.
Nonverbal Cognitive Ability and Relative Theta and Absolute Alpha Power
Nonverbal cognitive ability at 18 months was not correlated with mean relative theta power or with mean absolute alpha power.
Group Differences in Indiscriminately Friendly Behavior
Foster care and post-institutionalized children were more likely than non-adopted children to exhibit at least one instance of indiscriminately friendly behavior, χ2(2, 85) = 7.96, p < .05, Cramer’s V = .306. Indiscriminately friendly behaviors were observed in 19.2% of the post-institutionalized children (1–6 instances) and 20.8% of the foster care children (1–4 instances). None of the non-adopted children exhibited any indiscriminately friendly behaviors.
Although the groups did not differ in frequency or latency of verbal initiations, among the internationally adopted kids, those who exhibited indiscriminately friendly behavior also had more verbal initiations to the unfamiliar adult, t (59) = 4.91, p < .001, r2 = .290, and had a shorter latency to first verbalization to the experimenter, t(62) = 7.36, p < .001, r2 = .466.
General Deprivation and Indiscriminate Friendliness
Indiscriminate friendliness did not vary by age at adoption, nor was it related to the prenatal or early care risk indices. Children with a history of institutional care or foster care were equally likely to exhibit indiscriminately friendly behavior. Being one or more standard deviations below WHO 2007 means for height, weight, or head circumference at first post-adoption doctor’s visit did not predict indiscriminately friendly behavior at 36 months.
Nonverbal Cognitive Ability and Indiscriminate Friendliness
Indiscriminately friendly behavior was not predicted by 18-month nonverbal cognitive ability, t(96) = 1.13, ns.
Electrophysiological Correlates of Indiscriminate Friendliness
Repeated-measures ANOVAs examined EEG power in relation to indiscriminately friendly behavior. A dichotomous variable for indiscriminately friendly behavior (presence/absence) was the between-subjects factor, and the within-subjects factors were hemisphere and region. Indiscriminately friendly behavior at 36 months was predicted by higher relative theta power at 18 months, F(1, 86) = 4.70, p < .05, partial η2 = .052. Indiscriminately friendly behavior also was associated with lower absolute power in the alpha band, F(1, 86) = 6.71, p < .05, partial η2 = .072.
Delay of Gratification and Group
A one-way ANOVA found that there were no group differences on the z-scored composite delay of gratification variable, F(4,100) = 0.93, ns,for the post-institutionalized (M = −.03), foster care (M = −.21), and non-adopted (M = .08) groups.
Electrophysiological Correlates of Delay of Gratification
Repeated-measures ANOVAs examined EEG power in relation to inhibitory control. A median split of the composite delay of gratification variable was the between-subjects factor, and the within-subjects factors were hemisphere and region. Absolute alpha was not associated with delay of gratification. There was a non-significant trend for higher relative theta power to predict lower scores on the delay of gratification tasks, F(1,90) = 3.61, p = .06, partial η2 = .039. To explore the higher frequency power differences that might be underlying this trend, additional analyses were conducted with relative alpha and relative beta power. Relative alpha power was not related to delay of gratification. Higher relative beta power at 18 months predicted higher scores on the delay of gratification task at 36 months, F(1, 90) = 4.34, p < .05, partial η2 = .046.
Discussion
EEG power distribution was assessed in internationally adopted post-institutionalized 18-month-olds as compared to age-matched non-adopted children and children internationally adopted from foster care. The association of this 18-month power distribution with indiscriminate friendliness and inhibitory control at 36 months was examined. As hypothesized, the post-institutionalized children had a relative concentration of EEG power in lower frequency bands compared to the non-adopted children. That is, the post-institutionalized children had higher relative theta power and lower absolute alpha power than the non-adopted children. This pattern of higher relative theta power and lower absolute alpha power was associated with indiscriminately friendly behavior at 36 months. The relative concentration of power in lower frequency bands also was linked to poorer inhibitory control on delay of gratification tasks at 36 months. Both internationally adopted groups were more likely than the non-adopted group to show indiscriminately friendly behavior. These results were not explained by measures of general deprivation or global cognitive ability. Each of these findings will be discussed, in turn.
Prior to this discussion, it is important to place the present sample of post-institutionalized children within the broader framework of post-institutionalized and institutionalized children whose brain activity patterns have been previously reported in the literature. The studies of neural development reviewed in the introduction included exclusively Eastern European children, often with an extended duration of institutionalization. The post-institutionalized children in this study were adopted at 12 months of age on average, and all of them were 16 months or younger at adoption. They may be at lower risk than children adopted following more prolonged periods of institutional care (Nelson, Zeanah, Fox, Marshall, Smyke, & Guthrie, 2007). The foster care children were even younger, averaging only 8 months old at adoption. About 70% of the current post-institutionalized sample was from China. Eastern European children placed in institutional care are known to be at elevated risk of prenatal alcohol exposure and low birth weight (Johnson, 2000), which are both risk factors for developing attention and behavior problems (e.g. Nanson & Hiscock, 1990). Prenatal alcohol exposure has been linked with EEG abnormalities in human infants (Chernick, Childiaeva, & Ioffe, 1983) and in animals (Cortese, Krahl, Berman, & Hannigan, 1997; Kaneko, Riley, & Ehlers, 1993). For Eastern European samples with a high risk of prenatal alcohol exposure, it is difficult to tell if neural abnormalities are due to prenatal experience, institutional rearing, or some combination. While children adopted from China also could have prenatal risks, this may be less common. In the current sample, prenatal alcohol exposure was suspected by half of the adoptive parents of children from Eastern Europe but by none of the adoptive parents of children from China. Shorter duration of deprivation, lower prenatal risk, and international diversity are strengths of the current sample because they permit characterization of the specific effects of a brief period of deprivation on early neural and behavioral development.
The excess of relative theta power observed in the post-institutionalized children is consistent with the hypoactivation model that has been posited in previous studies of the effects of institutional rearing on neural development (Marshall et al., 2004; Moulson, Westerlund, et al., 2009; Parker et al., 2005). The generalization of this pattern consistent with hypoactivation to this relatively low risk sample strengthens the case that chronic neural hypoactivation may be associated broadly with a history of early deprivation, as opposed to reflecting some idiosyncrasy of the genetic characteristics or prenatal or postnatal experience of children reared in Eastern European institutions. The group differences in relative and absolute power strikingly parallel the group differences Marshall et al. (2004) reported for currently institutionalized children as compared to non-adopted children. The currently institutionalized children in their study and the post-institutionalized children in this study both had higher relative theta power and lower absolute alpha power compared to non-adopted children. Electrical activity in higher frequency bands is associated with a more alert state and with faster and more active processing, so a relative reduction of power in these higher frequency bands suggests the brains of children with a history of deprivation and disruptions in care may be hypoactivated. This hypoactivation may persist for at least some period following adoption into a more stimulating environment.
The post-institutionalized group had been with their families an average of 6 months, so EEG abnormalities persisted for at least 6 months following removal from the institutional rearing environment. This finding is consistent with Marshall et al.’s (2008) follow-up, in which it took 24 to 36 months after removal from the institutional setting for the atypical power distribution to begin to ameliorate compared to still-institutionalized children. The foster care group in the current study, who had been with their families for 10 months on average, were intermediate to the non-adopted and post-institutionalized groups with regard to relative theta power, which may be consistent with a gradual shift in EEG patterns following adoption. It should be noted that the foster care group still had lower absolute alpha power than the non-adopted group. It appears that neural activation patterns have some capacity to adjust following early deprivation, but the course is a protracted one. As Marshall et al.’s follow-up (2008) did not include a never-institutionalized comparison group, it is not known if the group removed from institutional care had reached the point that they approximated the power distribution of never-institutionalized children, or if there were constraints on this plasticity.
While the neurodevelopmental mechanisms underlying the links between early relational deprivation and an atypical power distribution are not yet known, it is possible that the lack of individualized attention from a stable, responsive caregiver delays or derails aspects of neural development. Drawing on evolutionary biology, Shonkoff (2010) proposes a biodevelopmental framework, in which the infant brain expects to develop in the context of certain species-typical environmental characteristics, including ample contingent social interaction with a stable caregiver, and that these experiences are necessary for brain architecture to develop normally (Shonkoff, 2010). EEG becomes concentrated in higher frequency bands with development, reflecting neurodevelopmental processes such as myelination (John et al., 1980). We speculate that for institutionalized children, the lack of social interaction with a primary caregiver may interfere with neurodevelopment, resulting in a relative excess of low frequency power. We further speculate that the partial amelioration of these EEG abnormalities following placement in foster care in Marshall et al.’s (2008) sample could be explained by the increase in one-on-one social interaction that the children experienced, which could have facilitated neurodevelopmental processes that were stalled or derailed while the children were institutionalized.
The current data does not allow differentiation between the maturational lag and sensitive period models. These models are incompatible, as the maturational lag model implies open-ended neural plasticity whereas the sensitive period model specifies limited plasticity to adapt to the post-adoption environment. Either model could fit with cortical hypoactivation in children with a history of institutional rearing: Hypoactivation could be temporary and likely to ameliorate following removal from the institutional environment, or it could be chronic and permanent. Following internationally adopted children over several years after adoption with repeated EEG measurements would allow examination of the developmental course of EEG power distributions. Even if cortical activation eventually rebounds to normal levels, one question to consider in future research is the developmental effect of having experienced an extended period of cortical hypoactivation. It is possible that the neural abnormalities underlying an atypical EEG power distribution might shape the developing child’s brain and behavior in the early years of life in such a way as to contribute to enduring attention problems, even if the power distribution itself eventually returned to a developmentally typical pattern.
In the ADHD literature, a relative excess of low frequency power is associated with attention problems (Barry et al., 2003). The persistence of this same neural abnormality in post-institutionalized children suggests a possible neural basis for the enduring attention problems often observed in these children. In the current study, children with less relative beta at 18 months had poorer inhibitory control at 36 months, and an excess of relative theta power at 18 months predicted the presence of indiscriminately friendly behaviors at 36 months. Thus, a relative excess of slow wave power was associated not only with attention deficits but also with indiscriminate friendliness. This finding provides initial evidence of neurodevelopmental correlates of a persistent socioemotional problem exhibited by some children with a history of early social deprivation. The association of indiscriminate friendliness with cortical hypoactivation, a neural pattern commonly observed in children with ADHD, tallies with reports associating indiscriminate friendliness with attention deficits in internationally adopted children (Bruce et al., 2009; Roy et al., 2004).
The prevalence of attention deficits in the current sample was unknown. Children are unlikely to be diagnosed with ADHD as early as 36 months, and attention regulation abilities are still developing at this age. On the one aspect of attention assessed in the current study, inhibitory control, the post-institutionalized children did not differ from the non-adopted children. While disordered attention was not evident in this sample, which was low risk compared to previously-studied post-institutionalized samples, the association of hypoactivation with indiscriminate friendliness suggests pathways through which disinhibited social behaviors may become organized in development. The observed indiscriminately friendly behaviors were markedly developmentally inappropriate violations of social boundaries. For example, when the stranger entered the room for the first time, one child exclaimed, “I missed you!” Several children touched the stranger’s knee or grabbed her hand. While indiscriminate friendliness was coded as present or absent due to its low incidence (occurring in about 20% of both internationally adopted groups), some children exhibited as many as three inappropriate personal questions or comments and three initiations of physical contact within the 10 minute interaction.
Hypoactivation is not sufficient to result in indiscriminate friendliness. Children with ADHD and no history of early deprivation and disruptions in care typically are not described as exhibiting indiscriminately friendly behaviors. However, early deprivation and early disruptions in care appear to predispose children both to hypoactivation and to indiscriminate friendliness. Further research is needed to determine if cortical hypoactivation predicts individual differences in behavioral outcomes among children who have experienced adverse early care environments.
Accounting for the heterogeneity of outcomes in children who have experienced deprivation, neglect, and disruptions in care is a central challenge as the field moves forward. The measures of preadoption risk in the current study did not support the idea of a risk gradient or dose-response relationship to explain individual differences in relative and absolute power among the internationally adopted children. Parent report of early care risk factors and prenatal risk factors was unrelated to EEG, though this should not be taken as evidence that pre-adoption experiences are unimportant. The dearth of reliable information about children’s preadoption histories is a problem endemic to research on internationally adopted children, and the current study is no exception. The children came from a variety of institutions and foster care homes in several countries, and direct measures of the quality of care in these settings were not available. Adoptive parent report was not based on first-hand observation, was likely incomplete, and could be colored by the parent’s perception of the child’s current functioning. Age at adoption and growth at adoption are more objective, though rough, estimates of exposure to deprivation, and these measures were not related to EEG power. However, the adopted children as a group were not growth delayed and were all adopted before 18 months of age. To determine if EEG power is related to malnourishment or duration of deprivation, a higher risk sample would be needed. While the adopted children had lower nonverbal cognitive ability, this measure was unrelated to EEG power or to indiscriminate friendliness, suggesting that atypical power distribution and indiscriminate friendliness were not simply indicators of global developmental delays. Marshall et al. (2008) reported that EEG measures did not mediate the relation between age at placement and developmental quotient in their sample of post-institutionalized children in foster care.
Progress in accounting for heterogeneity will depend both on acquiring more reliable and detailed information about the pre-adoption environment and on moving toward a consideration of genetic factors. Partnering with adoption agencies or individual institutions in the future may provide better estimates of the quality and characteristics of care in the pre-adoption environments. Work of this sort is beginning (Groark et al., 2005; Zeanah et al., 2003), though this will be challenging to put into practice. Obtaining measures of targeted genetic polymorphisms also will be challenging but potentially very useful. Genetics may play a role in individual differences in the vulnerability of the developing brain to adverse early care environments and the degree of neural plasticity in response to the post-adoption environment (for example see Stevens, Kumsta, Kreppner, Brookes, Rutter, & Sonuga-Barke, 2009).
To discriminate specific effects of institutional rearing from effects that would generalize to other forms of adversity, a comparison group of children internationally adopted from foster care was included. Like the post-institutionalized children, the foster care children had experienced abandonment and early care disruptions, but they had spent little or no time in institutional settings. While the foster care children did not differ from either of the other two groups in the relative power distribution, they had lower absolute alpha power than the non-adopted children. Indeed, the foster care children looked very similar to the post-institutionalized children with regard to absolute alpha power, with both adopted groups having lower absolute alpha power than the non-adopted children in central, parietal, and temporal scalp regions. The presence of EEG abnormalities in the foster care children, who were adopted at an average of 8 months old, is testament to the vulnerability of this developing system. Foster care children were just as likely as post-institutionalized children to exhibit indiscriminately friendly behaviors at 36 months. Indiscriminate friendliness occurred in about one in five of the post-institutionalized and foster care children; in contrast, none of the non-adopted children exhibited indiscriminately friendly behaviors. The presence of indiscriminate friendliness in both internationally adopted groups is consistent with results Bruce et al. (2009) reported for six- and seven-year-old children.
The prevalence of EEG abnormalities and indiscriminate friendliness in the foster care children indicates that these neural and behavioral deficits are not specific to institutional care histories but reflect a broad range of adverse early care experiences. Both groups experienced abandonment and relational disruptions in the early years of life. Little information is available as to the quality of foster care in the various countries, so it is possible that some of the children experienced deprivation or neglect while in foster care. If possible, it would be helpful to obtain better descriptive information about international foster care environments, though there are numerous barriers to this type of research. At a minimum, these children experienced relational disruption in the form of the loss of a stable primary caregiver, the foster parent, at the time of adoption. A pattern of EEG abnormalities quite similar to that seen in the post-institutionalized and foster care groups has been observed in impoverished, high risk Latin American children (Harmony et al., 1988, 1990; Otero, 1994, 1997; Otero et al., 2003), indicating that psychosocial deprivation can have developmental effects on EEG. Documenting indiscriminate friendliness in foster care children, Bruce et al. (2009) emphasized the importance of including comparison groups, such as children internationally adopted from foster care and maltreated/neglected children, in studies of post-institutionalized children’s behavioral development. The current findings underscore this recommendation, and suggest the need for these comparison groups in studies of post-institutionalized neural development, as well. The resulting data would clarify whether all these children are simply on a continuum of deprivation, with corresponding effects on neural and behavioral development, or whether certain abnormalities may be specifically associated with the institutional rearing environment.
The current study has several limitations. As has already been noted, parents may not have been able to provide complete or reliable information about their children’s pre-adoption experiences, so it was not possible to disentangle the influence of specific prenatal and early care factors on neural development. A low-density EEG array was used, which has limited spatial resolution. Use of a high-density array in future studies would allow for fine-grained analyses of regional patterns of EEG power. There was a predominance of females in the postinstitutionalized sample, though there were no gender differences on any of the variables of interest. Finally, the children were too young for most measures of attention regulation at 36 months. Executive functions are only just beginning to emerge at 36 months, and develop extensively throughout the preschool years and beyond (Diamond & Taylor, 1996). The current study did include delay of gratification tasks as age-appropriate measures of one aspect of attention regulation, inhibitory control, but a comprehensive assessment of multiple domains of attention regulation was not developmentally appropriate for this age group.
In sum, the findings from this study provide initial evidence that the relative excess of theta power which has been observed in currently institutionalized children persists in post-institutionalized children several months after adoption, and is not limited to children of Eastern European origin. This atypical power distribution, which also is characteristic of ADHD, predicted both indiscriminate friendliness and poor inhibitory control. These findings complement and extend previous research associating institutional rearing with neural abnormalities consistent with cortical hypoactivation. This study identifies longitudinal electrophysiological correlates of indiscriminate friendliness, and suggests that the same pattern of hypoactivation underlies both poorer inhibitory control and indiscriminate friendliness, consistent with behavioral studies relating indiscriminate friendliness to attention regulation difficulties (Bruce et al., 2009; Roy et al., 2004). The deficit in high-frequency absolute power and the presence of indiscriminate friendliness in children internationally adopted from foster care hint that these neural and behavioral abnormalities are not limited to children reared in institutions, but also may characterize other children with a history of relational deprivation. Replicating these findings and employing more specific neuroimaging methods will be critical to further characterize neurodevelopmental links between early relational deprivation and indiscriminate friendliness. It would be helpful to collect multiple electrophysiological and neuroimaging measures from the same sample, to see whether the various measures which have been interpreted as hypoactivation co-occur and whether they have shared behavioral correlates. It may be worthwhile to examine the EEG power distribution in other populations who have experienced disruptions of care, such as children in the U.S. foster care system. While the current study raises more questions than it answers, results highlight the need for more research on the impact of institutionalization and other disruptions of care on developing neural systems and how those neural systems may in turn shape behavioral development.
Acknowledgments
This research was supported by a National Science Foundation Graduate Student Fellowship and National Institute of Mental Health (NIMH) Grant T32 MH018264 to Amanda R. Tarullo and by NIMH through a grant (MH62601) and a Senior Scientist Award (MH066208) to Megan R. Gunnar. We are grateful to Nathan Fox, Jennifer Martin McDermott, and Bonny Donzella, who generously shared their technical expertise, and to the Center for Neurobehavioral Development at the University of Minnesota, which provided space and electrophysiological equipment for data collection. Most of all, we are indebted to all of the families who participated in the study for their time, enthusiasm, and commitment to this research.
Contributor Information
Amanda R. Tarullo, Department of Psychiatry, Columbia University
Melissa C. Garvin, Childcare Assessment Through Research and Evaluation, San Francisco, CA
Megan R. Gunnar, Institute of Child Development, University of Minnesota
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Barry R, Clarke A, Johnstone S. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology. 2003;114:171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Bradley-Johnson S. Cognitive assessment for the youngest children: a critical review of tests. Journal of Psychoeducational Assessment. 2001;19:19–44. [Google Scholar]
- Bruce J, Tarullo A, Gunnar M. Disinhibited social behavior among internationally adopted children. Development and Psychopathology. 2009;21:157–171. doi: 10.1017/S0954579409000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot R, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biological Psychiatry. 1996;40:951–963. doi: 10.1016/0006-3223(95)00576-5. [DOI] [PubMed] [Google Scholar]
- Chernick V, Childiaeva R, Ioffe S. Effects of maternal alcohol intake and smoking on neonatal electroencephalogram and anthropometric measurements. American Journal of Obstetrics and Gynecology. 1983;146:41–47. doi: 10.1016/0002-9378(83)90924-9. [DOI] [PubMed] [Google Scholar]
- Chisholm K. A three year follow-up of attachment and indiscriminate friendliness in children adopted from Romanian orphanages. Child Development. 1998;69:1090–1104. [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: A study of post-institutionalized Romanian orphans. NeuroImage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: Development of the normal child. Clinical Neurophysiology. 2001;112:806–814. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Cortese B, Krahl S, Berman R, Hannigan J. Effects of prenatal ethanol exposure on hippocampal theta activity in the rat. Alcohol. 1997;14:231–235. doi: 10.1016/s0741-8329(96)00147-4. [DOI] [PubMed] [Google Scholar]
- Diamond A, Taylor C. Development of an aspect of executive control: Development of the abilities to remember what I said and to “Do as I say, not as I do”. Developmental Psychobiology. 1996;29:315–334. doi: 10.1002/(SICI)1098-2302(199605)29:4<315::AID-DEV2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Eluvathingal T, Chugani H, Behen M, Juhasz C, Muzik O, Maqbool M, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Fisher L, Ames EW, Chisholm K, Savoie L. Problems reported by parents of Romanian orphans adopted to British Columbia. International Journal of Behavioral Development. 1997;20:67–82. [Google Scholar]
- Groark C, Muhamedrahimov RJ, Rifkat J, Palmov OI, Nikiforova NV, McCall RB. Improvements in early care in Russian orphanages and their relationship to observed behaviors. Infant Mental Health Journal. 2005;26:96–109. doi: 10.1002/imhj.20041. [DOI] [PubMed] [Google Scholar]
- Groze V, Ileana D. A follow-up study of adopted children from Romania. Child and Adolescent Social Work Journal. 1996;13:541–565. [Google Scholar]
- Gunnar MR, Bruce J, Grotevant H. International adoption of institutionally reared children: Research and Policy. Development and Psychopathology. 2000;12:677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Van Dulmen HM the International Adoption Project Team. Behavior problems in postinstitutionalized internationally adopted children. Development and Psychopathology. 2007;19:129–148. doi: 10.1017/S0954579407070071. [DOI] [PubMed] [Google Scholar]
- Harmony T, Alvarez A, Pascual R, Ramos A, Marosi E, Diaz de Leon A. EEG maturation on children with different economic and psychosocial characteristics. International Journal of Neuroscience. 1988;41:103–113. doi: 10.3109/00207458808985747. [DOI] [PubMed] [Google Scholar]
- Harmony T, Marosi E, Diaz de Leon AE, Becker J, Fernandez T. Effect of sex, psychosocial disadvantages and biological risk factors on EEG maturation. Electroencephalography and Clinical Neurophysiology. 1990;75:482–491. doi: 10.1016/0013-4694(90)90135-7. [DOI] [PubMed] [Google Scholar]
- Hodges J, Tizard B. Social and family relationships of ex-institutional adolescents. Journal of Child Psychology, Psychiatry, and Allied Disciplines. 1989;30:77–97. doi: 10.1111/j.1469-7610.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Hoksbergen RA, Ter Laak J, Van Dijkum C, Rijk K, Stoutjesdijk F. Attention deficit hyperactivity disorder in adopted Romanian children living in the Netherlands. Adoption Quarterly. 2003;6:59–73. [Google Scholar]
- Jasper H, Solomon P, Bradley C. Electroencephalographic analyses of behaviour problem children. American Journal of Psychiatry. 1938;95:641–658. [Google Scholar]
- John E, Ahn H, Prichep L, Trepetin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980;210:1255–1258. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- Johnson DE. Medical and developmental sequelae of early childhood institutionalization in Eastern European adoptees. In: Nelson CA, editor. The effects of early adversity on neurobehavioral development: Minnesota Symposia on Child Psychology. Vol. 31. Mahwah, NJ: Lawrence Erlbaum; 2000. pp. 113–162. [Google Scholar]
- Judge S. The impact of early institutionalization on child and family outcomes. Adoption Quarterly. 2004;7:31–48. [Google Scholar]
- Juffer F, Van IJzendoorn MH. Behavior problems and mental health referrals of international adoptees: A meta-analysis. JAMA – The Journal of the American Medical Association. 2005;293:2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- Kadlec MB, Cermak SA. Activity level, organization, and social-emotional behaviors in post-institutionalized children. Adoption Quarterly. 2002;6:43–57. [Google Scholar]
- Kaneko WM, Riley EP, Ehlers CL. Electrophysiological and behavioral findings in rats prenatally exposed to alcohol. Alcohol. 1993;10:169–178. doi: 10.1016/0741-8329(93)90099-a. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray K, Coy KC. Inhibitory control as a contributor to conscience in childhood: From toddler to early school age. Child Development. 1997;68:263–277. [PubMed] [Google Scholar]
- Kreppner JM, O’Connor TG, Rutter M, Beckett C, Castle J, Croft C. Can inattention/overactivity be an institutional deprivation syndrome? Journal of Abnormal Child Psychology. 2001;29:513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- Lee K. Crying patterns of Korean infants in institutions. Child: Care, Health, and Development. 2000;26:217–228. doi: 10.1046/j.1365-2214.2000.00134.x. [DOI] [PubMed] [Google Scholar]
- Lin SH. Sensory integration, growth, and emotional and behavioral regulation in adopted post-institutionalized children. Dissertation Abstracts International: Section B: The Sciences & Engineering. 2003;64(3-B):1478. [Google Scholar]
- Mann CA, Lubar JF, Zimmerman AW, Miller CA, Muenchen RA. Quantitative analysis of EEG in boys with attention-deficit–hyperactivity disorder: Controlled study with clinical implications. Pediatric Neurology. 1992;8:30–36. doi: 10.1016/0887-8994(92)90049-5. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA the Bucharest Early Intervention Project Core Group. A comparison of the electroencephalogram between institutionalized and community children in Romania. Journal of Cognitive Neuroscience. 2004;16:1327–1338. doi: 10.1162/0898929042304723. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Kenney JW. Biological perspectives on the effects of early psychosocial experience. Developmental Review. 2009;29:96–119. [Google Scholar]
- Marshall PJ, Reeb BC, Fox NA, Nelson CA, Zeanah CH. Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Development and Psychopathology. 2008;20:861–880. doi: 10.1017/S0954579408000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matousek M, Petersen I. Automatic evaluation of EEG background activity by means of age-dependent EEG quotients. Electroencephalography and Clinical Neurophysiology. 1973;35:603–612. doi: 10.1016/0013-4694(73)90213-7. [DOI] [PubMed] [Google Scholar]
- Morison SJ, Ames EW, Chisholm K. The development of children adopted from Romanian orphanages. Merrill-Palmer Quarterly. 1995;41:411–430. [Google Scholar]
- Moulson MC, Fox NA, Zeanah CH, Nelson CA. Early adverse experiences and the neurobiology of facial emotion processing. Developmental Psychology. 2009;45:17–30. doi: 10.1037/a0014035. [DOI] [PubMed] [Google Scholar]
- Moulson MC, Westerlund A, Fox NA, Zeanah CH, Nelson CA. Child Development. 2009;80:1039–1056. doi: 10.1111/j.1467-8624.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, McCarley RW, Kubicki M, Dickey CC, Niznikiewicz MA, Volqmaier MM, et al. Frontotemporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biological Psychiatry. 2005;58:468–478. doi: 10.1016/j.biopsych.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanson JL, Hiscock M. Attention deficits in children exposed to alcohol prenatally. Alcoholism: Clinical and Experimental Research. 1990;14:656–661. doi: 10.1111/j.1530-0277.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Luciana M. Electrophysiological studies II: Evoked potentials and event-related potentials. In: Coffey CE, Brumback RA, editors. Textbook of pediatric neuropsychiatry. Washington, DC: American Psychiatric Press; 1998. pp. 331–356. [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz MA, Frumin M, McCarley RW, et al. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18:629–637. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Bredenkamp D, Rutter M the ERA Study Team. Attachment disturbances and disorders in children exposed to early severe deprivation. Infant Mental Health Journal. 1999;20:10–29. [Google Scholar]
- Otero G. EEG spectral analysis in children with sociocultural handicaps. International Journal of Neuroscience. 1994;79:213–220. doi: 10.3109/00207459408986082. [DOI] [PubMed] [Google Scholar]
- Otero G. Poverty, cultural disadvantage and brain development: a study of preschool children in Mexico. Electroencephalography and Clinical Neurophysiology. 1997;102:512–516. doi: 10.1016/s0013-4694(97)95213-9. [DOI] [PubMed] [Google Scholar]
- Otero G, Pliego-Rivero F, Fernandez T, Ricardo J. EEG development in children with sociocultural disadvantages: A follow-up study. Clinical Neurophysiology. 2003;114:1918–1925. doi: 10.1016/s1388-2457(03)00173-1. [DOI] [PubMed] [Google Scholar]
- Parker SW, Nelson CA Bucharest Early Intervention Project Core Group. The impact of deprivation on the ability to discriminate facial expressions of emotion: An event-related potential study. Child Development. 2005;76:54–72. doi: 10.1111/j.1467-8624.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- Roy P, Rutter M, Pickles A. Institutional care: Risk from family background or pattern of rearing? Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41(2):139–149. [PubMed] [Google Scholar]
- Roy P, Rutter M, Pickles A. Institutional care: Associations between overactivity and lack of selectivity in social relationships. Journal of Child Psychology & Psychiatry. 2004;45:866–873. doi: 10.1111/j.1469-7610.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Colvert C, Kreppner J, Beckett C, Castle J, Groothues C, et al. Early adolescent outcomes for institutionally deprived and non-deprived adoptees. I: Disinhibited attachment. Journal of Child Psychology and Psychiatry. 2007;48:17–30. doi: 10.1111/j.1469-7610.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, O’Connor TG the ERA Study Team. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Developmental Psychology. 2004;40:81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Satterfield JH, Cantwell DP, Satterfield BT. Pathophysiology of the hyperactive child syndrome. Archives of General Psychiatry. 1974;31:839–844. doi: 10.1001/archpsyc.1974.01760180079010. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Development. 2010;81:357–367. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Somsen RJ, van’t Klooster BJ, van der Molen MW, van Leeuwen HM, Licht R. Growth spurts in brain maturation during middle childhood as indexed by Eeg power spectra. Biological Psychology. 1997;44:187–209. doi: 10.1016/s0301-0511(96)05218-0. [DOI] [PubMed] [Google Scholar]
- Stevens SE, Kumsta R, Kreppner JM, Brookes KJ, Rutter M, Sonuga-Barke EJ. Dopamine transporter gene polymorphism moderates the effects of severe deprivation on ADHD symptoms: developmental continuities in gene-environment interplay. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150B:753–761. doi: 10.1002/ajmg.b.31010. [DOI] [PubMed] [Google Scholar]
- Tizard B, Rees J. The effect of early institutional rearing on the behaviour problems and affectional relationships of four-year-old children. Journal of Child Psychology and Psychiatry. 1975;16:61–73. doi: 10.1111/j.1469-7610.1975.tb01872.x. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn M, Juffer M. The Emanuel Miller Memorial Lecture 2006: Adoption as intervention. Meta-analytic evidence for massive catch-up and plasticity in physical, socioemotional, and cognitive development. Journal of Child Psychology and Psychiatry. 2006;47:1228–1245. doi: 10.1111/j.1469-7610.2006.01675.x. [DOI] [PubMed] [Google Scholar]
- Verhulst FC, Althaus M, Versluis-den Bieman HJM. Damaging backgrounds – later adjustment of international adoptees. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:518–524. doi: 10.1097/00004583-199205000-00020. [DOI] [PubMed] [Google Scholar]
- Vorria P, Papaligoura Z, Sarafidou J, Kopakaki M, Dunn J, Van IJzendoorn M, et al. The development of adopted children after institutional care: A follow-up study. Journal of Child Psychology and Psychiatry. 2006;47:1246–1253. doi: 10.1111/j.1469-7610.2006.01666.x. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, et al. Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Koga SF, Simion B, Stanescu A, Tabacaru C, Fox NA, et al. Ethical considerations in international research collaboration: The Bucharest Early Intervention Project. Infant Mental Health Journal. 2006;27:559–576. doi: 10.1002/imhj.20107. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, et al. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology. 2003;15:885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Smyke AT, Dumitrescu A. Attachment disturbances in young children. II: Indiscriminate behavior and institutional care. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:983–989. doi: 10.1097/00004583-200208000-00017. [DOI] [PubMed] [Google Scholar]