Abstract
During growth of the ovarian follicle, the mammalian oocyte becomes surrounded by an acellular coat called the zona pellucida. Whether the zona pellucida originates from the oocyte, surrounding follicle cells, or both has remained an unresolved issue. In experiments described here, denuded and follicle-enclosed mouse oocytes at various stages of growth were isolated and cultured in vitro in the presence of either [35S]methionine or [3H]fucose in order to determine the site of synthesis of the three, recently identified, zona pellucida proteins, ZP1, ZP2, and ZP3 [Bleil, J. D. & Wassarman, P. M. (1980) Dev. Biol., in press]. Approximately 1.5% of the [35S]methionine, and as much as 45% of the [3H]fucose, that was incorporated into trichloroacetic acid-insoluble material by denuded or follicle-enclosed oocytes during a 12-hr culture period was found associated with zonae pellucidae removed from the cultured oocytes. Incorporation of [35S]methionine into zona pellucida proteins was depressed to less than 1/50th when denuded oocytes were cultured in the presence of puromycin, and secretion of zona pellucida proteins by denuded oocytes was demonstrated by pulse-chase experiments. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis of [35S]methionine- and [3H]fucose-labeled proteins present in oocytes, zonae pellucidae, and follicle cells revealed that denuded oocytes synthesize and secrete zona pellucida proteins, whereas no evidence was obtained to suggest that follicle cells synthesize these proteins. Denuded oocytes, ranging in diameter from 48 to 68 μm, incorporated both [35S]methionine and [3H]fucose into zona pellucida proteins during culture in vitro, whereas zonae pellucidae removed from fully-grown oocytes (85 μm) were not radiolabeled to a significant extent. After culture of denuded or follicle-enclosed oocytes for 12 hr, more than 95% of the [3H]fucose incorporated into oocyte proteins was found in ZP1, ZP2, and ZP3, indicating that the zona pellucida proteins are the major class of proteins glycosylated during oocyte growth. These results provide biochemical evidence supporting the idea that the zona pellucida originates from the mammalian oocyte itself, rather than from the surrounding follicle cells.
Keywords: zona pellucida, oogenesis, mouse, [35S]methionine incorporation, [3H]fucose incorporation
Full text
PDF
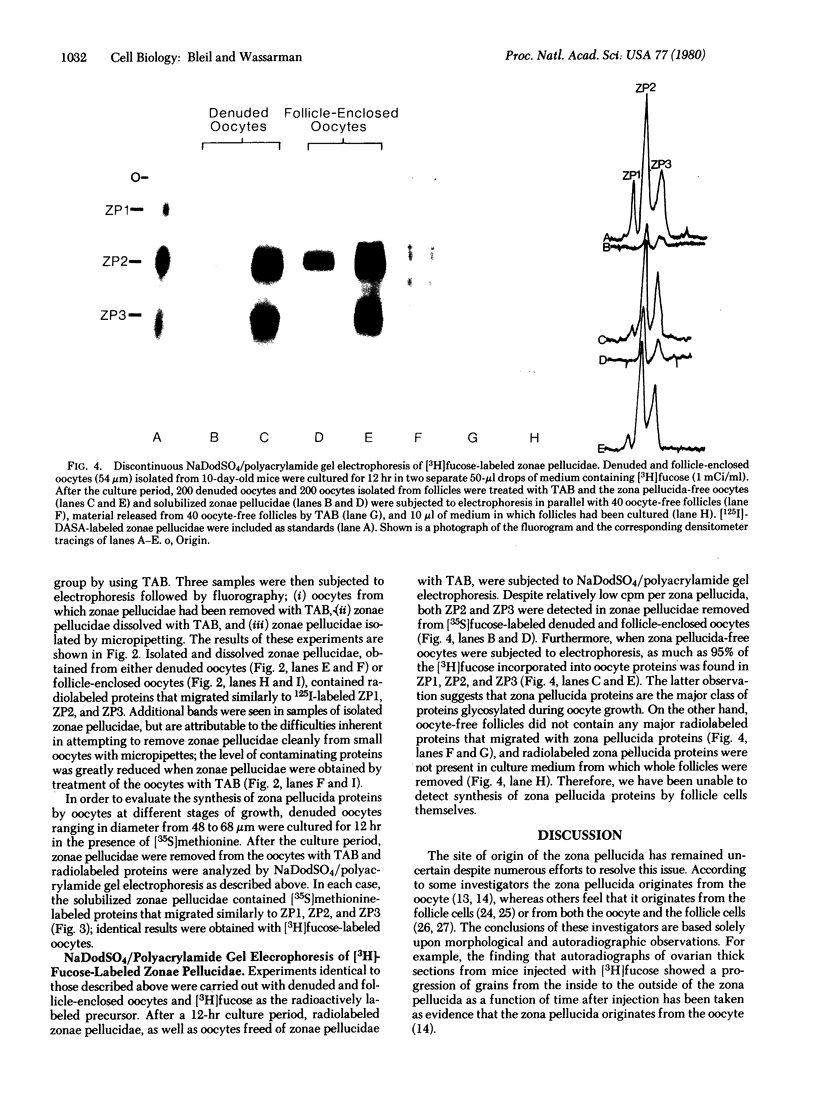

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- CHIQUOINE A. D. The development of the zona pellucida of the mammalian ovum. Am J Anat. 1960 Mar;106:149–169. doi: 10.1002/aja.1001060207. [DOI] [PubMed] [Google Scholar]
- Carraway K. L. Covalent labeling of membranes. Biochim Biophys Acta. 1975 Dec 29;415(4):379–410. doi: 10.1016/0304-4157(75)90005-2. [DOI] [PubMed] [Google Scholar]
- Haddad A., Nagai M. E. Radioautographic study of glycoprotein biosynthesis and renewal in the ovarian follicles of mice and the origin of the zona pellucida. Cell Tissue Res. 1977 Feb 15;177(3):347–369. doi: 10.1007/BF00220310. [DOI] [PubMed] [Google Scholar]
- Kang Y. H. Development of the zona pellucida in the rat oocyte. Am J Anat. 1974 Apr;139(4):535–565. doi: 10.1002/aja.1001390406. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neutra M., Leblond C. P. The Golgi apparatus. Sci Am. 1969 Feb;220(2):100–107. doi: 10.1038/scientificamerican0269-100. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Yanagimachi R., Yanagimachi H. Ultrastructural localization of lectin-binding sites on the zonae pellucidae and plasma membranes of mammalian eggs. J Cell Biol. 1975 Aug;66(2):263–274. doi: 10.1083/jcb.66.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOTELO J. R., PORTER K. R. An electron microscope study of the rat ovum. J Biophys Biochem Cytol. 1959 Mar 25;5(2):327–342. doi: 10.1083/jcb.5.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Bleil J. D., Wassarman P. M. Quantitation of nanogram amounts of protein using [3H]dinitrofluorobenzene. Anal Biochem. 1978 Nov;91(1):354–356. doi: 10.1016/0003-2697(78)90850-3. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Letourneau G. E., Wassarman P. M. Program of early development in the mammal: changes in patterns and absolute rates of tubulin and total protein synthesis during oogenesis and early embryogenesis in the mouse. Dev Biol. 1979 Feb;68(2):341–359. doi: 10.1016/0012-1606(79)90209-4. [DOI] [PubMed] [Google Scholar]
- WARTENBERG H. [Electron microscopic and histochemical studies on the oogenesis of amphibia egg cells]. Z Zellforsch Mikrosk Anat. 1962;58:427–486. [PubMed] [Google Scholar]
- Wassarman P. M., Josefowicz W. J. Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence. J Morphol. 1978 May;156(2):209–235. doi: 10.1002/jmor.1051560206. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Letourneau G. E. RNA synthesis in fully-grown mouse oocytes. Nature. 1976 May 6;261(5555):73–74. doi: 10.1038/261073a0. [DOI] [PubMed] [Google Scholar]